Abstract
Monoamine neuromodulators such as dopamine, norepinephrine, serotonin (5-HT), octopamine, and tyramine are signaling molecules in the nervous system, where they play critical roles in both health and disease. Given the complex spatiotemporal dynamics, similar structural features, and multiple receptors, studying their dynamics has been limited using conventional methods such as microdialysis and electrochemistry. However, recent advances in optics have facilitated the development of imaging-based detection methods. In this review, we summarize current detecting approaches for specific monoamines, emphasizing their design strategies, detection properties, applications, and limitations. We highlight the genetically encoded GPCR–based sensors for DA and NE, which have high signal-to-noise ratio, selectivity and can be used in vivo in different living organisms. Finally, we discuss the potential for using this approach to generate new neuromodulator sensors with nonoverlapping spectra, which will ultimately pave the way for studying the interplay among various neuromodulators and neurotransmitters.
Keywords: Monoamine neuromodulators, Genetically encoded GPCR-based sensor
Introduction
The human brain performs a highly versatile array of functions via the concerted activity of its several billion neurons. The principal messengers for communication among these neurons is a wide variety of neurotransmitters and neuromodulators, which are released from the presynaptic terminal and are detected by receptors expressed by postsynaptic neurons. Monoamine neuromodulators contain an aromatic ring and an amino group connected by a two-carbon chain. They comprise an essential group of neuromodulators that include dopamine (DA), norepinephrine (NE), serotonin (5-HT), octopamine (OA), and tyramine (TA) (Figure 1A). In both vertebrates and invertebrates, these neuromodulators participate in a wide range of physiological processes, including learning, movement, sleep, and memory [1–4]. Impaired monoamine transmission has been correlated with a range of pathological conditions, including Parkinson’s disease, attention deficit hyperactivity disorder, depression, and addiction [5–7].
Figure 1.
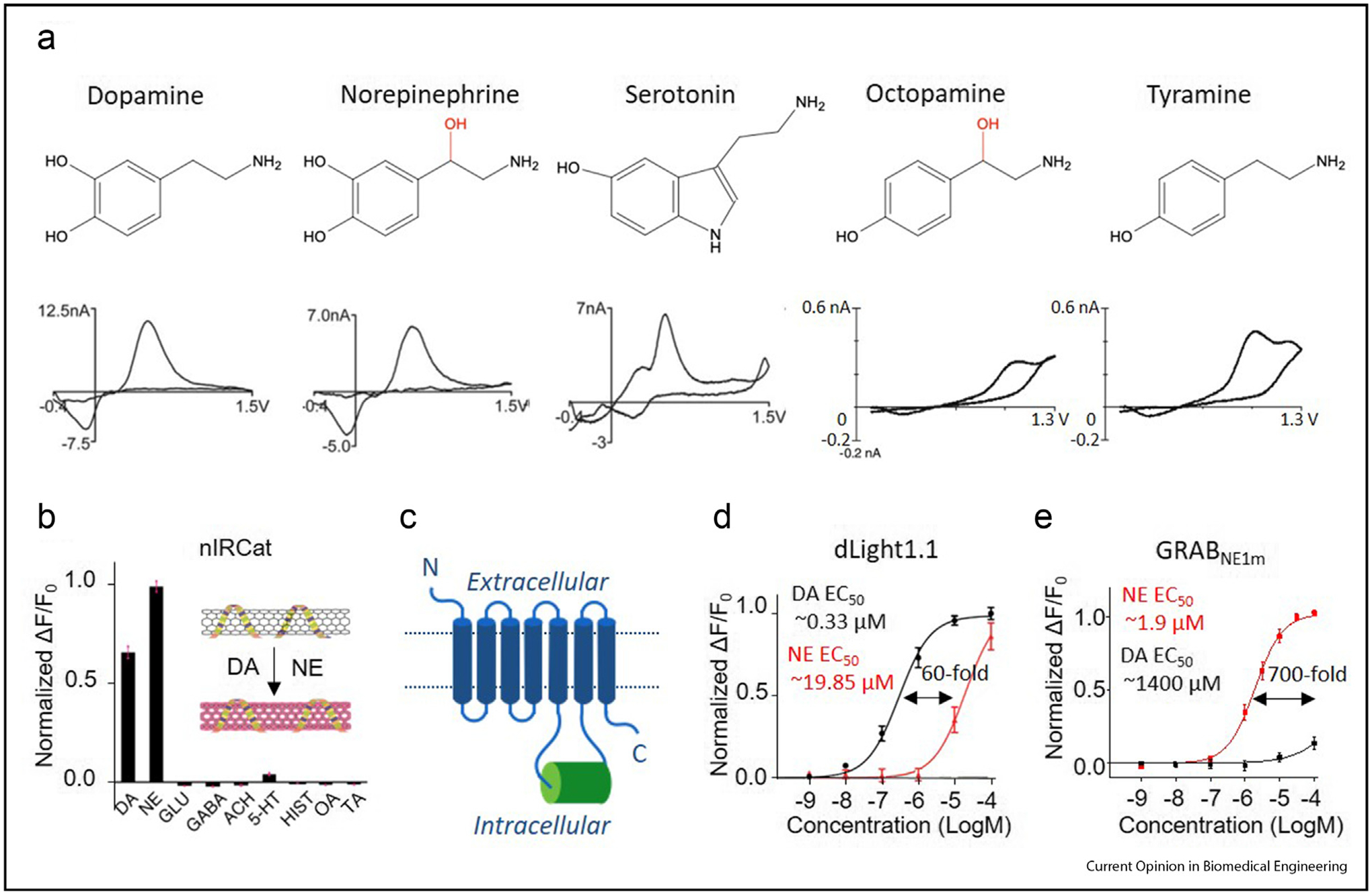
Distinguishing monoamine neuromodulators using fast-scan cyclic voltammetry (FSCV) and GPCR-based single-fluorophore sensors (a) The chemical structures (top) and corresponding FSCV voltammogram traces (bottom) of the indicated monoamines [27,57]. The hydroxyl group that differs between DA and NE and between OA and TA is shown in red (b) Schematic diagram of carbon nanoparticle–based catecholamine sensor nIRCat and its normalized response to 100 mM DA, NE, GLU, GABA, acetylcholine (ACH), 5-HT, histamine (HIST), OA and TA [11] (c) Schematic diagram of a generic GPCR-based single-fluorophore sensor; the fluorophore is inserted in the third intracellular loop and is shown in green (d and e) Dose–response curves for the dLight1.1 expressed in HEK293T cells [9] and GRABNE1m expressed in cultured cortical neurons [8] in response to DA and NE; the difference in EC50 between DA and NE is also shown for each sensor. (Figures are modified from original research paper with permission from the publisher).
Unlike glutamate (GLU) and γ-aminobutyric acid (GABA) that mediate fast synaptic transmission by targeting ionotropic receptors, monoamine neuromodulators primarily target metabotropic G protein–coupled receptors (GPCRs), thereby regulating distinct downstream signaling pathways. Although much is currently known regarding the properties of monoamine neuromodulators, their precise spatiotemporal dynamics during specific brain functions cannot be measured using traditional electrophysiological methods and are therefore poorly understood. To measure monoamines in vivo, several methods have been developed in recent decades, including analytical chemistry–based tools and electrochemical methods. Thanks to the recent advances in fluorescence imaging techniques, both genetically encoded sensors [8–10] and a nongenetically encoded nanosensor [11] have also been developed. In this review, we summarize the recent progress with respect to monitoring various monoamines (Table 1).
Table 1.
Overview of the currently available tools and techniques for detecting monoamine neuromodulators.
| DA | NE | 5-HT | OA &TA | |
|---|---|---|---|---|
| GC/HPLC/MEKC-electrochemistry/MS | Musshoff et al., 2000 [49] Carrera et al., 2007 [50] |
Kuhlenbeck et al., 2000 [51] Carrera et al., 2007 [50] |
Carrera et al., 2007 [50] | Watson et al., 1993 [38] Powell et al., 2005 [40] Hardie et al., 2006 [39] |
| Electrochemistry | Robinson et al., 2008 [15] Dreyer et al., 2016 [14] |
Robinson et al., 2008 [15] Bucher et al., 2015 [26] |
Marcinkiewcz et al., 2016 [32] Saylor et al., 2019 [33] |
Cooper et al., 2009 [27] Fang et al., 2011 [43] Majdi et al., 2015 [42] Pyakurel et al., 2016 [41] |
| Microdialysis-biochemistry | Gu et al., 2015 [12] Nesbitt et al., 2015 [13] |
McReynolds et al., 2010 [25] | Gardier et al., 2013 [31] | N.A. |
| FFNs | Gubernator et al., 2009 [17] Rodriguez et al., 2013 [19] Pereira et al., 2016 [52] |
N.A. | Henke et al., 2018 [52] | N.A. |
| Tango assay | Barnea et al., 2008 [20] Inagaki et al., 2012 [21] Lee et al., 2017 [22] Kim et al., 2017 [23] |
Hanson et al., 2009 [53] Kroeze et al., 2015 [54] |
Hanson et al., 2009 [53] Kroeze et al., 2015 [54] |
N.A. |
| CNiFER | Muller et al., 2014 [16] | Muller et al., 2014 [16] | Yamauchi et al., 2011 [55] | N.A. |
| FRET-based sensors | N.A. | Hoffmann et al., 2005 [29] Vilardaga et al., 2003 [30] |
Candelario et al., 2012 [34] | N.A |
| GRAB/dLight | Sun et al., 2018 [10] Patriarchi et al., 2018 [9] |
Feng et al., 2019 [8] | N.A. | N.A. |
| Nanosensor | Kruss et al., 2017 [56] Beyene et al., 2019 [11] |
N.A. | N.A. | N.A. |
DA, dopamine; NE, norepinephrine; 5-HT, 5-hydroxytryptamine (serotonin); OA, octopamine; TA, tyramine; N.A., not available; GC, gas chromatography; HPLC, high-performance liquid chromatography; MEKC, micellar electrokinetic capillary chromatography; MS, mass spectrometry; FFNs, fluorescent false neurotransmitters; CNiFER, cell-based neurotransmitter fluorescent engineered reporter; FRET, Förster resonance energy transfer; GRAB, GPCR activation-based sensor.
Dopamine
DA plays a key role in modulating motor control, reward signaling, decision making, and attention [1,2]. Impaired dopaminergic transmission has been correlated to several pathological conditions, including Parkinson’s disease, addiction, and various psychiatric disorders [5]. Several approaches have been used to detect DA, including microdialysis [12,13], electrochemical methods [14,15], and cell-based methods [16]; however, these techniques have either low spatiotemporal resolution or insufficient molecular selectivity, as discussed in the following sections.
Fluorescent false neurotransmitters (FFNs) are a family of synthesized neurotransmitter analogs, which can be loaded into synaptic vesicles of mono-aminergic neurons or dopaminergic neurons through vesicular monoamine transporter 2 or dopamine transporter [17–19]. Loaded FFNs are irreversibly released along with synaptic vesicles and this vesicular destaining process is used as a readout of DA transmission. FFNs have been used to monitor electrically or pharmacologically evoked DA release from individual presynaptic terminals in cultured neurons and brain slices. However, the preloading and washing processes have limited cell-type specificity and are not amenable for in vivo studies. In addition, FFNs themselves do not report neurotransmitter identity, and the depletion of loaded FFNs makes it challenging for repetitive measurement over time.
The Tango assay is designed to report GPCR activation through the expression of reporter genes [20]. This assay includes two critical components: (i) a GPCR tandemly attached to a transcription factor via a tobacco etch virus cleavage site and (ii) a β-arrestin fused to tobacco etch virus protease. Activation of the GPCR recruits β-arrestin and triggers the release of the transcription factor, which then drives the expression of a reporter gene. The Tango assay has been used to identify circuit-level dopaminergic modulation in the fly brain [21]. The next-generation iTango2 [22] and SPARK [23] reporters provide temporally precise labeling by similarly introducing a light-gated motif into the system. The interchangeable reporter gene in this system significantly expands its applications with respect to both the reporter protein and the ability to incorporate chemogenetics/optogenetics; thus, this system has been used to monitor and manipulate behaviorally relevant DA-responsive neuronal populations in mice in vivo. On the other hand, the relatively long gene expression time (in the order of hours) precludes its use in detecting rapid dynamic changes and in longitudinal experiments.
Recently, two series of genetically-encoded single-fluorophore DA sensors are developed by introducing an environment-sensitive circularly permutated enhanced green fluorescent protein (cpEGFP) into the third intracellular loop of the DA receptors (Figure 1C) called GRABDA [10] and dLight [9]. A pair of D2 receptor–based sensors with either medium (GRABDA1m, EC50: 130 nM) or high (GRABDA1h, EC50: 10 nM) apparent DA affinity were used to rapidly and specifically detect DA in vivo in several living organisms, including flies, fish, mice, and songbirds [24]. In addition, the family of D1, D2, and D4 receptor–based sensors (dLight1.1 to dLight1.5) has an apparent DA affinity in the nanomolar to micromolar range, and both dLight1.1 and dLight1.2 have been used in vivo in mice. Both the GRABDA and dLight sensors inherit the intrinsic selectivity for DA over NE from the corresponding scaffold DA receptors, with the D2-based GRABDA sensor and the D1-based dLight1.1 sensor (Figure 1D) having 10-fold and 60-fold higher affinity for DA compared with NE. In addition, they have minimal effect on cellular physiology due to the negligible coupling with the downstream signaling pathways of GPCRs.
Recently, a near-infrared catecholamine (nIRCat) carbon nanoparticle–based probe was developed (Figure 1B) [11]. This sensor has an emission spectrum of 1000–1300 nm, which has minimal light scattering, and is therefore suitable for imaging deep tissues. nIRCat has been used to detect striatal DA release in brain slices with temporal resolution comparable with that of electrochemical methods. Comparing with GPCR-based fluorescent sensors, nIRCat has limitations with respect to selectivity for DA over NE and lacks cell-type specificity, but it has unique advantages to be useable in the presence of DA receptor ligands. Given the nongenetically encoded nature, it also provides an additional tool for studying organisms that are currently not amenable to genetic manipulation; moreover, it serves as an orthogonal approach that can be combined with protein-based sensors in several model organisms.
Norepinephrine
NE, a key biogenic monoamine neuromodulator, is generated from dopamine by the enzyme dopamine β-hydroxylase. Consistent with the extensive projections of noradrenergic neurons from the locus coeruleus to the entire brain, NE plays an important role in a wide range of physiological processes throughout the central nervous system, including modulating sensory information, regulating attention, and controlling both sleep and arousal [3]. It is therefore not surprising that impaired noradrenergic signaling has been linked to a wide range of psychiatric disorders and neurodegenerative diseases, including stress and anxiety [6].
In the past few decades, scientists have studied the dynamics of NE in vivo using low-throughput microdialysis coupled with biochemical analysis [25]. Taking advantage of the oxidative nature of monoamine neuromodulators such as NE, electrochemical amperometry can be used to detect these molecules by applying a steady voltage potential and quantifying the oxidative currents. Although amperometry is sensitive and offers high temporal resolution, the relatively high voltage applied can also oxidize many other molecules, thus limiting the molecular selectivity of this approach. With the improved electrochemical method fast-scan cyclic voltammetry (FSCV) monoamines are scanned using a specific voltage wave, and the chemical can be identified by analyzing the specific shape of the voltammogram; nevertheless, FSCV is unable to distinguish between structurally similar monoamines such as NE and DA [15,26] or OA and TA [27] (Figure 1A).
Optical imaging methods have emerged as an ideal alternative, providing high spatial resolution and minimal invasiveness. For example, HEK293 cells stably expressing a cell-based neurotransmitter fluorescent engineered reporter (CNiFER) have been implanted in mice and used to detect NE and DA in real time [16]. The NE reporter α1A-CNiFER is an engineered cell line that expresses the α1A adrenergic receptor (α1AR) as the NE-sensing module, a chimeric Gq protein that couples α1AR activation to stimulation of Phospholipase C and increase of cytosolic Ca2+ concentration, and the Förster resonance energy transfer (FRET)–based Ca2+ sensor TN-XXL as the reporter. The NE reporter a1A -CNiFER responds to NE with an EC50 of approximately 19 nM which is 70-fold more sensitive to NE compared with DA. After the cells are implanted in the mouse’s target brain region, they can be used to detect NE dynamics in vivo. On the other hand, the use of a tumor-derived cell line limits subcellular spatial specificity to approximately 100 μm and has the potential for inducing an undesired immune response.
These limitations can be overcomed using a genetically encoded probe, which provides cell-type specificity for monitoring neuromodulator dynamics [28]. One such example is sensors based on FRET; however, these sensors have a relatively low signal-to-noise ratio, which limits their applicability, particularly in vivo [29,30]. Recently, a single-fluorophore GPCR-based NE sensor called GRABNE was generated by inserting a single cpEGFP into the third intracellular loop of the α2 adrenergic receptor (Figure 1C), yielding a 230% fluorescence increase in response to NE [8], with a 700-fold higher sensitivity to NE compared with DA (Figure 1E). Importantly, GRABNE has been used to rapidly and specifically detect NE with high spatiotemporal resolution in a variety of systems, including cultured cells, awake zebrafish larvae, and freely behaving mice.
Serotonin
Serotonin is highly conserved from invertebrates to humans and plays a key role in a wide range of physiological processes, including mood control, appetite, the sleep–wake cycle, learning, and memory [4]. Malfunction of serotonergic system has been linked to several psychiatric disorders, including depression [7], and many clinically prescribed antidepressants target the serotonergic system (e.g., the selective serotonin reup-take inhibitors).
Combining microdialysis with high-performance liquid chromatography and mass spectrometry is a widely used technique for the continuous in vivo detection of various biomolecules in mammals, including 5-HT [31] and other monoamine neuromodulators. Using this approach, a microdialysis probe of ~200-μm diameter is implanted into the brain region of interest, and biomolecules are collected via passive diffusion through the probe’s semipermeable membrane. Although this technique can achieve nanomolar-level sensitivity, the relatively slow sampling rate of approximately 10 min makes it unsuitable for monitoring the rapid dynamic changes of 5-HT, which occur on the order of seconds or even subseconds.
Another powerful tool for detecting 5-HT is FSCV, which can be used to distinguish between 5-HT and other monoamine neuromodulators with high temporal resolution in mouse brain slices and in freely moving mice during social behaviors [32,33]. However, because the spatial resolution depends on both the size (~70–300 μm in diameter) and position of the implanted probe, it can only report volume-averaged signals without providing cell specificity.
Yet, another tool is the genetically encoded FRET-based 5-HT sensor 5HT-CC, which was engineered by inserting two fluorescent proteins, one in the third intracellular loop and one in the C-terminus of the 5-HT1b receptor [34]. This sensor has good selectivity for 5-HT and produces an approximately 4% change in the FRET ratio in response to a saturated concentration of 5-HT, with an EC50 of approximately 428 nM. To date, the 5HT-CC sensor has not been used in in vivo applications, possibly because of the relatively low signal-to-noise ratio associated with FRET-based sensors.
Octopamine and tyramine
Both OA and TA are key monoamine neuromodulators in invertebrates, serving as counterparts to the adrenergic neuromodulators present in vertebrates [35]. Both OA and TA are derived from tyrosine, and TA is the precursor of OA. The physiological roles of OA in the invertebrate nervous system have been well documented, showing that OA controls a wide range of processes, including the sleep–wake cycle, oviposition, aggression, and associative learning. In contrast, the role of TA is less known; however, studies of mutant Drosophila and Caenorhabditis elegans have revealed that TA plays a role in several processes, including olfaction, courtship, movement, and cocaine sensitization. Both OA and TA are classified as trace amines in the nervous system of vertebrates, as they are approximately 100-fold less abundant than other monoamine neuromodulators [36]. Although the function of trace amines in vertebrates is currently unclear, the identification of their respective GPCRs in the central nervous system supports the notion that they function as neuromodulators [37].
Previously, OA and TA were measured in homogenates prepared from the Drosophila brain using a combination of separation and detection techniques. Initially, gas chromatography was coupled with mass spectrometry and used to detect several monoamines in the Drosophila brain; interestingly, TA was detected, whereas OA was not detectable [38]. Subsequently, high-performance liquid chromatography [39] and micellar electrokinetic capillary chromatography [40] have been used to isolate monoamines from samples, with highly sensitive electrochemical amperometry used for detection. Given the low molecular specificity associated with amperometry, additional genetic and/or pharmacological approaches are needed to confirm the chemical’s identity. Overall, methods used to separate and detect chemicals from homogenates provide a snapshot of the amounts of OA and TA present in the brain; however, the preparation is highly destructive and precludes taking repeated measurement in individual animals.
Recently, researchers used electrochemical techniques to monitor the endogenous release of OA from intact tissues such as the Drosophila larval body wall and ventral nerve cord [41,42]. Importantly, FSCV can be used to distinguish OA and TA from other monoamines, but it cannot distinguish between OA and TA, as — similar to DA and NE — they differ by only one hydroxyl group (Figure 1A) [27,43].
Conclusions and future perspectives
Detecting monoamine neuromodulators in freely behaving animals is essential for understanding the role that these chemicals play in both health and disease. Given their release mode and chemical nature, probing the dynamics of monoamine neuromodulators in the intact nervous system has been challenging. First, monoamine neuromodulators — particularly DA — have two release modes: (i) constitutive tonic release, which is induced by the spontaneous activity of dopaminergic neurons, thereby setting the basal level; and (ii) phasic release, which is triggered by action potentials and typically lasts several seconds [44]. Thus, a rapid and robust detection method is needed to fully capture both release modes. A second challenge is that once released from the nerve terminal, monoamine neuromodulators are not restricted to the synaptic cleft, but can diffuse and reach neighboring cells and/or synapses [45]. Because the dynamics of this volume transmission has not been characterized, a suitable probe should have high spatial resolution, ideally in the subcellular range. Finally, neurons that contain different neuromodulators are intermingled in the same brain area; for example, both DA and NE are released in the prefrontal cortex [46]. To tease apart the interplay between structurally similar monoamine neuromodulators the sensors used should have sufficient molecular selectivity.
Except for aforementioned DA, NE, 5-HT, OA and TA, there are some additional monoamine neuromodulators in the nervous system, including histamine as well as trace amines (e.g. phenethylamine and tryptamine). The dynamics of these monoamines can also be studied with existing detection methods. For example, both electrochemistry [47] and Tango assays [48] have been adopted to monitor histamine dynamics in fly and in mouse. However, classic methods used to detect monoamines, including microdialysis, amperometry/FSCV, CNiFER, FFN, and Tango assay, all lack sufficient spatiotemporal resolution and/or molecular selectivity. Moreover, FRET-based sensors have limited dynamic range and are less suitable for use in tissues and in vivo imaging. On the other hand, the recently developed nongenetically encoded nanosensor nIRCat uses low-scattering infrared fluorescence, but cannot distinguish DA and NE.
A promising new strategy is the development of single-fluorophore GPCR-based sensors, which has been used successfully to generate sensors for detecting both DA and NE, thereby providing a wide dynamic range, rapid kinetics, single-cell resolution, and — most importantly — exquisite selectivity. Given the large variety of neuromodulator/neurotransmitter-sensing GPCRs in the nervous system, this strategy can be used to develop sensors for a wide range of molecules, including 5-HT, OA, and TA. Moreover, by attaching different fluorescent proteins, sensors with nonoverlapping spectra can be developed and then used to study the complex interplay between different neuromodulators. Thus, using simultaneous multicolor imaging to measure the release of different monoamines in real time will provide important insights into how these neuromodulators function in the brain.
Acknowledgements
The authors thank the members of the Li Lab for providing feedback on the manuscript. This work was supported by the grants from Beijing Municipal Science & Technology Commission (Z181100001318002), the National Basic Research Program of China (973 Program; grant 2015CB856402), the General Program of National Natural Science Foundation of China (project 31671118 and project 31871087), the National Institutes of Health Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (1U01NS103558-01), the Peking-Tsinghua Center for Life Sciences, and the State Key Laboratory of Membrane Biology at Peking University School of Life Sciences (all to YL).
Footnotes
Conflict of interest statement
The authors declare the following competing financial interests: JZ, FS, JW, JF, and YL have filed patent applications, the value of which may be affected by this publication.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Nieoullon A: Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002, 67:53–83. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA: Dopamine, learning and motivation. Nat Rev Neurosci 2004, 5:483–494. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Cohen JD: An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005, 28: 403–450. [DOI] [PubMed] [Google Scholar]
- 4.Berger M, Gray JA, Roth BL: The expanded biology of serotonin. Annu Rev Med 2009, 60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missale C, et al. : Dopamine receptors: from structure to function. Physiol Rev 1998, 78:189–225. [DOI] [PubMed] [Google Scholar]
- 6.Chrousos GP: Stress and disorders of the stress system. Nat Rev Endocrinol 2009, 5:374–381. [DOI] [PubMed] [Google Scholar]
- 7.Blier P, Demontigny C: Current advances and trends in the treatment of depression. Trends Pharmacol Sci 1994, 15: 220–226. [DOI] [PubMed] [Google Scholar]
- 8* *.Feng J, et al. : A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 2019, 102:745–761 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; GRABNE (2019). The authors engineered a fluorescent protein cpEGFP into α2AR and developed two genetically encoded single-fluorophore NE sensors with over 700-fold selectivity for NE than DA. They were successfully applied in brain slice, living zebra fish and mouse to detect NE dyanmics.
- 9* *.Patriarchi T, et al. : Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]; dLight (2018). The authors developed a family of DA sensors, while the mostly used versions are dLight1.1/1,2 based on D1 receptor with around 300–700 nM apparent affinity to DA and 60-fold higher selectivity to DA than NE. Their applicability has been demonstrated in mouse.
- 10* *.Sun F, et al. : A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 2018, 174:481–496. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; GRABDA (2018). The authors developed a pair of D2 receptor-based DA sensors with around 10 nM–100 nM apparent affinity to DA and 10-fold higher selectivity to DA than NE. Their applicability has been demonstrated in versatile animals including fruit fly, zebrafish and mouse.
- 11*.Beyene AG, et al. : Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci Adv 2019, 5, eaaw3108. [DOI] [PMC free article] [PubMed] [Google Scholar]; nIRCat (2019). In this paper, the authors developed a non-genetically encoded carbon nanoparticle-based catecholamine probe. It emits infrared fluorescence that is suitable for in vivo deep tissue imaging, and was used in brain slice to detect DA dynamics. Though it has limited selectivity for DA than NE, it provides an orthogonal approach that can be readily combine with other genetically encoded sensors.
- 12.Gu H, et al. : In vivo monitoring of dopamine by microdialysis with 1 min temporal resolution using online capillary liquid chromatography with electrochemical detection. Anal Chem 2015, 87:6088–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesbitt KM, et al. : Microdialysis in the rat striatum: effects of 24 h dexamethasone retrodialysis on evoked dopamine release and penetration injury. ACS Chem Neurosci 2015, 6: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyer JK, et al. : Functionally distinct dopamine signals in nucleus accumbens core and shell in the freely moving rat. J Neurosci 2016, 36:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson DL, et al. : Monitoring rapid chemical communication in the brain. Chem Rev 2008, 108:2554–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller A, et al. : Cell-based reporters reveal in vivo dynamics of dopamine and norepinephrine release in murine cortex. Nat Methods 2014, 11:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubernator NG, et al. : Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science 2009, 324:1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira DB, et al. : Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat Neurosci 2016, 19:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez PC, et al. : Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc Natl Acad Sci U S A 2013, 110:870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnea G, et al. : The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A 2008, 105: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inagaki HK, et al. : Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 2012, 148:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Lee D, et al. : Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain. Nat Methods 2017, 14:495. [DOI] [PubMed] [Google Scholar]; iTango (2017). The authors improved gene-expression based Tango assay by introducing light sensitive domains. They further expand its applicability for not only label but also manipulate neuronal populations in vivo by switching reporter gene from florescent proteins to opsins.
- 23*.Kim MW, et al. : Time-gated detection of protein-protein interactions with transcriptional readout. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; SPARK (2017). The authors introduced light sensitive domain to improve the temporal resolution and decrease the background noise of gene-expression based Tango assay.
- 24.Tanaka M, et al. : A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature 2018, 563: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre C, et al. : Memory-enhancing cortico-sterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem 2010, 93:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucher ES, Wightman RM: Electrochemical analysis of neurotransmitters. Annu Rev Anal Chem 2015, 8:239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper SE, Venton BJ: Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Anal Bioanal Chem 2009, 394:329–336. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Jing M, Li Y: Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Curr Opin Neurobiol 2018, 50: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann C, et al. : A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat Methods 2005, 2:171–176. [DOI] [PubMed] [Google Scholar]
- 30.Vilardaga JP, et al. : Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol 2003, 21:807–812. [DOI] [PubMed] [Google Scholar]
- 31.Gardier AM: Antidepressant activity: contribution of brain microdialysis in knock-out mice to the understanding of BDNF/5-HT transporter/5-HT autoreceptor interactions. Front Pharmacol 2013, 4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcinkiewcz CA, et al. : Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016, 537:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saylor RA, et al. : In vivo hippocampal serotonin dynamics in male and female mice: determining effects of acute escitalopram using fast scan cyclic voltammetry. Front Neurosci 2019, 13:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Candelario J, Chachisvilis M: Mechanical stress stimulates conformational changes in 5-hydroxytryptamine receptor 1B in bone cells. Cell Mol Bioeng 2012, 5:277–286. [Google Scholar]
- 35.Roeder T: Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 2005, 50:447–477. [DOI] [PubMed] [Google Scholar]
- 36.Berry MD: Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem 2004, 90:257–271. [DOI] [PubMed] [Google Scholar]
- 37.Borowsky B, et al. : Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A 2001, 98:8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson DG, et al. : The determination of biogenic amines in four strains of the fruit fly Drosophila melanogaster. J Pharm Biomed Anal 1993, 11:1145–1149. [DOI] [PubMed] [Google Scholar]
- 39.Hardie SL, Hirsh J: An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J Neurosci Methods 2006, 153:243–249. [DOI] [PubMed] [Google Scholar]
- 40.Powell PR, et al. : Analysis of biogenic amine variability among individual fly heads with micellar electrokinetic capillary chromatography-electrochemical detection. Anal Chem 2005, 77:6902–6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyakurel P, Privman Champaloux E, Venton BJ: Fast-scan cyclic voltammetry (FSCV) detection of endogenous octopamine in Drosophila melanogaster ventral nerve cord. ACS Chem Neurosci 2016, 7:1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majdi S, et al. : Electrochemical measurements of optogenetically stimulated quantal amine release from single nerve cell varicosities in Drosophila larvae. Angew Chem Int Ed Engl 2015, 54:13609–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang H, Vickrey TL, Venton BJ: Analysis of biogenic amines in a single Drosophila larva brain by capillary electrophoresis with fast-scan cyclic voltammetry detection. Anal Chem 2011, 83:2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grace AA: Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 1991, 41:1–24. [DOI] [PubMed] [Google Scholar]
- 45.Agnati LF, et al. : Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 1995, 69: 711–726. [DOI] [PubMed] [Google Scholar]
- 46.Finlay JM, Zigmond MJ, Abercrombie ED: Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 1995, 64:619–628. [DOI] [PubMed] [Google Scholar]
- 47.Samaranayake S, et al. : In vivo histamine voltammetry in the mouse premammillary nucleus. Analyst 2015, 140:3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagadish S, et al. : Identifying functional connections of the inner photoreceptors in Drosophila using Tango-Trace. Neuron 2014, 83:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musshoff F, et al. : Determination of dopamine and dopamine-derived (R)-/(S)-salsolinol and norsalsolinol in various human brain areas using solid-phase extraction and gas chromatography/mass spectrometry. Forensic Sci Int 2000, 113:359–366. [DOI] [PubMed] [Google Scholar]
- 50.Carrera V, et al. : A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: application to the secretion of bovine chromaffin cell cultures. J Chromatogr B Analyt Technol Biomed Life Sci 2007, 847:88–94. [DOI] [PubMed] [Google Scholar]
- 51.Kuhlenbeck DL, et al. : Determination of norepinephrine in small volume plasma samples by stable-isotope dilution gas chromatography-tandem mass spectrometry with negative ion chemical ionization. J Chromatogr B Biomed Sci Appl 2000, 738:319–330. [DOI] [PubMed] [Google Scholar]
- 52.Henke A, et al. : Toward serotonin fluorescent false neurotransmitters: development of fluorescent dual serotonin and vesicular monoamine transporter substrates for visualizing serotonin neurons. ACS Chem Neurosci 2018, 9:925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanson BJ, et al. : A homogeneous fluorescent live-cell assay for measuring 7-transmembrane receptor activity and agonist functional selectivity through beta-arrestin Recruitment. J Biomol Screen 2009, 14:798–810. [DOI] [PubMed] [Google Scholar]
- 54.Kroeze WK, et al. : PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 2015, 22:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamauchi JG, et al. : Characterizing ligand-gated ion channel receptors with genetically encoded Ca++ sensors. PLoS One 2011, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kruss S, et al. : High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array. Proc Natl Acad Sci U S A 2017, 114:1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang H, et al. : Quantitation of dopamine, serotonin and adenosine content in a tissue punch from a brain slice using capillary electrophoresis with fast-scan cyclic voltammetry detection. Anal Methods 2013, 5:2704–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]


