Abstract
The gut microbiome plays a crucial role in human health by influencing various physiological functions through complex interactions with the endocrine system. These interactions involve the production of metabolites, signaling molecules, and direct communication with endocrine cells, which modulate hormone secretion and activity. As a result, the microbiome can exert neuroendocrine effects and contribute to metabolic regulation, adiposity, and appetite control. Additionally, the gut microbiome influences reproductive health by altering levels of sex hormones such as estrogen and testosterone, potentially contributing to conditions like polycystic ovary syndrome (PCOS) and hypogonadism. Given these roles, targeting the gut microbiome offers researchers and clinicians novel opportunities to improve overall health and well-being. Probiotics, such as Lactobacillus and Bifidobacterium, are live beneficial microbes that help maintain gut health by balancing the microbiota. Prebiotics, non-digestible fibers, nourish these beneficial bacteria, promoting their growth and activity. When combined, probiotics and prebiotics form synbiotics, which work synergistically to enhance the gut microbiota balance and improve metabolic, immune, and hormonal health. This integrated approach shows promising potential for managing conditions related to hormonal imbalances, though further research is needed to fully understand their specific mechanisms and therapeutic potential.
Keywords: dysbiosis, probiotics, prebiotics, synbiotics, hormonal disorders
1. Introduction
The human body is home to a vast array of microorganisms that reside in various areas, such as the gut, skin, mouth, and vagina, with the gastrointestinal (GI) tract being the primary site [1]. This collection of microorganisms forms the microbiome, which is composed of various microbial species known collectively as the microbiota [2]. The gut microbiota plays a critical role in human health, interacting closely with the endocrine system through mechanisms that involve hormones regulating host behavior, metabolism, immunity, insulin signaling, and other functions [3-5]. The gut microbiota can influence host behavior by modulating neurohormones like serotonin, dopamine, and gamma-aminobutyric acid (GABA), as well as stress hormones like cortisol [6].
The gut microbiota is increasingly being shown to influence the host immune net-work [7,8]. This relationship begins at birth, with the microbiota playing a crucial role in shaping immune system development, which in turn impacts the composition of the microbiome [7]. The gut microbiota also significantly affects the host’s metabolic status through multiple mechanisms. It produces short-chain fatty acids (SCFAs) like butyrate, propionate, and acetate. SCFAs via G protein-coupled receptor 43 (GPR43) activation can reduce inflammation and lipolysis, increase adipogenesis and leptin release, and ultimately lead to lower fat accumulation [9]. Additionally, SCFAs activate AMP kinase in muscles, which reduces lipid accumulation and improves insulin sensitivity [9], aiding in appetite regulation and weight management [9,10]. Furthermore, the gut microbiota produces metabolites that act as signaling molecules, influencing the release of key metabolically active hormones such as serotonin, glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and cholecystokinin (CKK) from enteroendocrine cells (EC) in the gut [3]. These hormones regulate important metabolic processes, including glucose metabolism, insulin sensitivity, adiposity, and appetite [3]. SCFAs, in particular, are known to modulate the secretion of these gut peptides [11]. GLP-1 and PYY, secreted by L-cells located primarily in the ileum and colon, play essential roles in regulating food intake and satiety. The gut microbiota’s influence on GLP-1 and PYY secretion highlights its significant implications for the development of metabolic diseases [3]. CCK, secreted from “I cells” predominantly found in the upper small intestine [12], is released in response to dietary fat and protein intake. However, the regulation of CCK by gut microbes is less well understood due to the limited exposure of CCK-containing cells to the microbiota in the small intestine [3].
Recent evidence highlights a possible significant role of the gut microbiota in regulating sex steroid levels. It affects estrogen metabolism through the estrobolome, a collection of bacterial genes encoding enzymes such as β-glucuronidase [13,14]. These enzymes deconjugate estrogens, impacting their bioavailability and circulating levels [14]. Additionally, the gut microbiota has been identified as a major regulator of androgen metabolism in the intestines, leading to relatively high levels of free dihydrotestosterone (DHT), the most potent androgen, in the colonic contents of young and healthy mice and men [15].
A disrupted gut microbiome, in turn, can have detrimental effects on reproductive and metabolic health through hormonal fluctuations and inflammation. This imbalance in the gut microbiota can lead to altered sex hormone levels and metabolic dysfunctions, contributing to conditions such as PCOS, infertility, and various metabolic disorders [13,16-18].
2. Dysbiosis as a Possible Trigger of Hormonal Disorders
Gut dysbiosis refers to an imbalance or disruption in the composition and function of the gut microbiome. Dysbiosis can be triggered by various factors, including xenobiotics (such as prolonged antibiotic use), lifestyle habits (such as an unhealthy diet, smoking, and alcohol use), health status (such as chronic stress, infections, and chronic conditions like inflammatory bowel disease; IBD and irritable bowel syndrome; IBS), as well as environmental toxins, age, ethnicity, and genetic background [13,19,20] (Figure 1).
Figure 1.
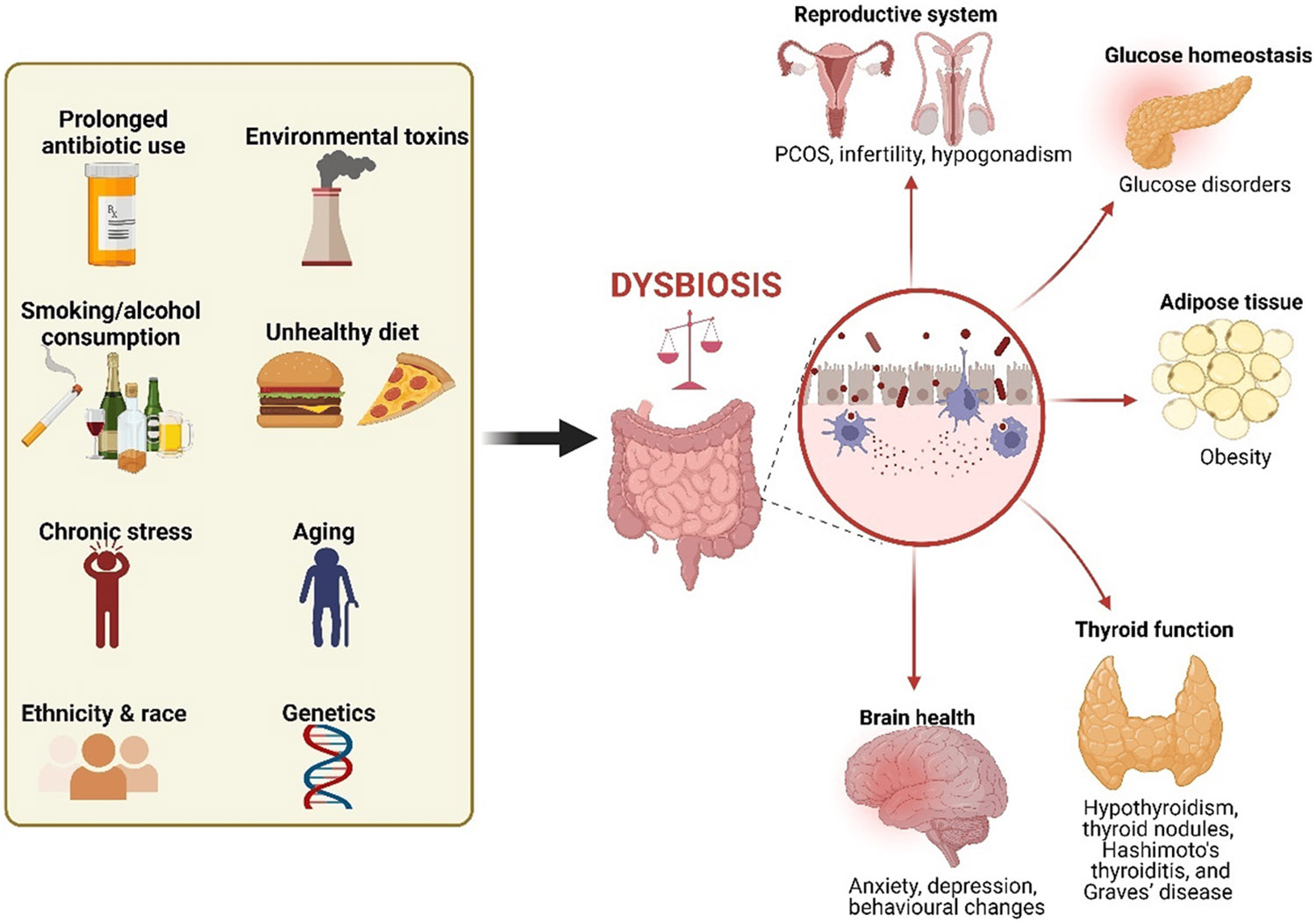
Factors that can induce dysbiosis and the link to hormonal disorders. Dysbiosis can result from factors such as xenobiotics, poor lifestyle habits, chronic stress, environmental toxins, age, ethnicity, and genetics. This imbalance can lead to hormonal fluctuations and inflammation, contributing to several reproductive and metabolic disorders. Created in BioRender.
Gut dysbiosis can lead to alterations in the production and signaling of neurotransmitters and hormones, such as serotonin, dopamine, and cortisol [21]. These imbalances can have profound effects on mood, cognition, and overall brain function [22]. Individuals with gut dysbiosis are more likely to experience symptoms of depression and anxiety [23]. A study in germ-free (GF) mice and specific pathogen-free (SPF) mice showed that altering microbial colonization can affect behavioral responses to chronic stress by modulating hormones and hormone receptors in the hypothalamic–pituitary–adrenal (HPA) axis under stress [24]. Additionally, the altering gut microbiome can influence the production of neurotrophic factors, which are essential for brain development and repair [25].
Recent studies suggest a possible link between gut dysbiosis and female hormonal disorders [20]. Dysbiosis can lead to fluctuations in circulating estrogens by altering β-glucuronidase activity, which may contribute to metabolic complications, PCOS, and female infertility [13,16]. Additionally, dysbiosis may promote PCOS development, the most common reproductive endocrine disorder in females, by increasing gut permeability, leading to systemic inflammation and insulin resistance (IR) [26]. Dysbiosis-induced hypoestrogenemia could also influence the progression of endometriosis and its potential malignant transformation [27]. Hypoandrogenism in males and hyperandrogenism in females are both types of androgen disorders. Gut microbial imbalance can contribute to androgen synthesis dysfunction, which may lead to androgen-driven diseases such as obesity, metabolic syndrome, PCOS in females, and male hypogonadism [15,28].
Intestinal dysbiosis may affect the secretion of multiple hormones and vitamins, including vitamin D, thyroid hormones, and insulin [29,30]. Recent evidence indicates that primary hypothyroidism is associated with altered bacterial diversity and reduced SCFA production, which may contribute to thyroid dysfunction by lowering thyroxine levels [31]. There is also an established link between the gut microbiome and other thyroid disorders, such as thyroid nodules, Hashimoto’s thyroiditis, and Graves’ disease [32-35].
A healthy gut microbiome is essential for maintaining glucose homeostasis. Evidence from basic and clinical studies indicates that gut dysbiosis can be a causal or contributing factor in the pathogenesis of various glucose metabolism disorders, including obesity, IR, and Type-1 and Type-2 Diabetes [36]. Turnbaugh and colleagues demonstrated that gut microbiota dysbiosis can cause metabolic disease in mice independent of genetic background [37]. Their study showed that microbiota transplantation from mice with diet-induced obesity to lean germ-free mice recipients resulted in more fat deposition than transplants from lean mice donors. Likewise, another study reported that the transplant of microbiota from lean and obese human twins into germ-free mice lacking a native gut microbiome resulted in the conveyance of the metabolic phenotype of the host [38]. These findings highlight the potential of targeting the gut microbiome as a strategy for preventing and treating disorders related to hormonal imbalances. Common approaches include the use of probiotics, prebiotics, and synbiotics.
3. Probiotics: Definition, Mechanisms of Action, and Impact
Probiotics are living, non-pathogenic microorganisms that offer benefits to human health when consumed in adequate amounts [2]. Multiple microorganisms that belong to the genera Propionibacterium, Lactococcus, Enterococcus, Pediococcus, and Bacillus are considered to be probiotics [39]. Still, the most important probiotic strains are the Lactobacillus and Bifidobacterium, which are commonly used in functional foods and dietary supplements [40,41]. While microbiota refers to the natural population of microorganisms in the body, probiotics are beneficial microbes that are taken to support or enhance the microbiota.
Probiotics have been found to play a supportive role in the treatment and prevention of various conditions, including IBD, IBS, lactose intolerance, cancer, diarrhea, and allergic diseases [39]. The major therapeutic effects of probiotics are primarily attributed to their direct or indirect effect on the GI tract [42]. These beneficial effects are attributed to several key mechanisms by which probiotics eradicate pathogens and maintain a healthy balance of gut flora. These mechanisms include competing with pathogens for nutrients and adhesion sites in the gut, enhancing intestinal barrier functions, improving the immune system, and producing neurotransmitters [39], which makes it difficult for harmful pathogens to thrive. Probiotics also function as antimicrobial agents by producing substances, such as organic acids and hydrogen peroxide, which combat the pathogenic bacteria in the gut [43]. In addition, probiotics increase the production of mucin proteins, which strengthen the function of the intestinal barrier [44].
Apart from the direct effect on the GI tract, the gut microbiome interacts with the body’s endocrine system via several complex mechanisms. One important pathway is the gut–brain axis [45]. Probiotics influence the production and release of a number of neurotransmitters and hormones, such as dopamine, serotonin, and norepinephrine [46]. Additionally, probiotics reduce the level of stress hormones such as cortisol [47,48]. Accordingly, probiotics have a role in regulating depression, anxiety, and other central nervous system (CNS)-related disorders [48,49]. While existing studies highlighted the impact of probiotics on neurotransmitter production, stress hormone levels, and CNS-related disorders, further research is warranted to determine the strain-specific effects of probiotics for targeting specific CNS disorders.
The synthesis of GI hormones such as leptin, ghrelin, and GLP-1 is influenced by specific strains of microbiota, indicating a role for the microbiota in appetite regulation [3,50]. Probiotics contribute to the fermentation of dietary fibers, producing SCFAs that positively affect metabolism and enhance the release of hormones involved in appetite control and insulin secretion [41,51]. While the impact of probiotics on GI hormones is well documented, the precise mechanisms through which different probiotic strains modulate these hormones remain unclear and warrant further research.
In a recent observational study, probiotic use was associated with higher estradiol levels in premenopausal women and lower total testosterone levels among pre- and postmenopausal women [52]. In ovariectomized mice, probiotics were found to influence estrogen levels by modulating the gut microbiota, enhancing SCFA production, and up-regulating estrogen receptors in adipose tissue [53]. While the gut microbiota’s role as a major regulator of colonic androgen content in young and healthy mice and men is well documented [15], there is a lack of studies investigating the impact of probiotics on androgen regulation in both males and females. Moreover, despite the potential benefits of probiotics for overall health in both men and women, their role in preventing or treating sex hormone-related disorders, such as hypogonadism in men and PCOS in women, remains understudied.
4. Prebiotics: Definition, Mechanisms of Action, and Impact
While probiotics are live beneficial bacteria that support gut health, prebiotics are non-digestible fiber compounds that selectively nourish the gut microbiota, stimulating their growth and activity. This selective stimulation of the microbiota ultimately confers health benefits to the host [54]. Importantly, a prebiotic must be resistant to stomach acid, remain unabsorbed in the GI tract, be fermented by microbiota, and selectively stimulate the growth and activity of beneficial intestinal bacteria [55]. Prebiotics include diverse carbohydrates, including fructans, β-glucans, galacto-oligosaccharides, inulin, starch, guar gum, lactulose, maltodextrin, xylo-oligosaccharides, and arabino-oligosaccharides [54,56].
By promoting a healthy gut microbiome, prebiotics contribute to improving physical health. Several studies have reported the positive role of prebiotics on the GI tract. For instance, prebiotics can help manage conditions like bloating and constipation [57]. In a randomized controlled trial involving patients with functional bowel disorders, the administration of fructo-oligosaccharides (FOSs) over a six-week period was found to improve the symptoms of IBS [58]. FOS supplementation was also shown to decrease Crohn’s disease activity in patients in a clinical trial [59]. Prebiotic fermentation products have also demonstrated protective effects against the development and progression of colorectal cancer [60,61]. In addition, prebiotics have been found to aid in weight management in both adults and children [62,63]. Because the GI tract is connected to the brain via the gut–brain axis, prebiotics have positive effects on the nervous system, such as improved cognition and memory [64].
Prebiotics offer numerous health benefits by selectively stimulating the growth and activity of beneficial bacteria in the gut. Their primary mechanism of action involves promoting the growth of beneficial bacterial strains like Lactobacilli and Bifidobacteria, which outcompete pathogenic microbes for resources and attachment sites, thereby enhancing gut health [65]. Additionally, the fermentation of prebiotics produces SCFAs, which diffuse through gut enterocytes and enter blood circulation, affecting not only the GI tract but also distant organs and systems [66]. The acids produced from prebiotic fermentation alter the gut environment by decreasing its pH, leading to changes in the composition and population of gut microbiota [67]. Prebiotics also improve gut barrier function by increasing mucin production and strengthening the tight junctions between intestinal cells, which helps prevent harmful substances from entering the bloodstream [68,69]. Furthermore, prebiotics stimulate the immune system by increasing the population of beneficial microbes in the gut and altering cytokine expression [70].
The prebiotic, inulin, has been shown to increase plasma levels of GLP-1 and reduce levels of ghrelin [71,72]. This suggests that prebiotics can influence GI hormone production, likely through the production of SCFAs, thereby affecting appetite regulation. As a result, prebiotics could serve as new targets for managing obesity and other eating disorders. In addition, prebiotics may help people cope with stress and mild anxiety by lowering cortisol levels, a stress hormone [73]. Some prebiotics have also been reported to increase estrogen metabolism in the intestine by suppressing β-glucuronidase activity [74], which could potentially reduce the risk of estrogen-mediated cancers. However, data on the role of prebiotics in hormone regulation are still limited. More research is needed to fully understand their impact on hormonal regulation and their potential therapeutic uses.
5. Synergistic Effects of Probiotics and Prebiotics (Synbiotics)
Synbiotics are a specific combination of probiotics, microorganisms that provide health benefits when consumed, and prebiotics, compounds that promote its growth, having a synergistic effect when paired together [75,76]. In May 2019, the International Scientific Association for Probiotics and Prebiotics (ISAPP) updated the definition of a synbiotic to “a mixture of live microorganisms and substrate(s) that confer health benefits to the host” [77]. Synbiotics are classified mainly into two groups: (a) complementary synbiotics and (b) synergistic synbiotics [75]. The complementary synbiotics are composed of probiotics and prebiotics that provide health benefits independently of each other, without requiring any mutual function. In contrast, synergistic synbiotics include a substrate that is specifically utilized by the co-administered live microbial populations, enhancing their effectiveness [75,77].
The use of synbiotics is an efficient and promising approach for maintaining gut microbiota homeostasis, promoting the restoration and maintenance of beneficial gut bacteria [78]. A randomized controlled trial has demonstrated that synbiotics can significantly improve metabolic health in individuals with metabolic syndrome and prediabetes [79]. Synbiotic supplementation under high-fat diet conditions has been found to alleviate metabolic disturbances and improve intestinal barrier integrity by increasing gut hormones and SCFAs [80]. Some potential benefits of synbiotic consumption in humans include the following: (a) increasing the populations of Lactobacilli and Bifidobacterial, which helps maintain gut microbiota balance; (b) boosting the production of SCFAs; (c) improving metabolic processes such as bile acid deconjugation and mineral absorption; (d) strengthening the modulation of the host immune system; and (e) enhancing liver function in individuals with cirrhosis and other [75,81-83]. Overall, the synbiotic approach has proven to be more effective than using prebiotics or probiotics alone in modulating gut microbiota and alleviating metabolic disorders associated with an imbalanced gut microbiota in humans [84].
6. Probiotics and Prebiotics in the Management of Endocrine Disorders
The potential role of probiotics in hormonal regulation and the management of endocrine disorders is suggested by recent findings from both basic and clinical research (Table 1). A recent observational cohort study among 2699 women, comprising a nationally representative sample of adults who participated in the National Health and Nutrition Examination Survey between 2013 and 2016, suggests the potential beneficial effect of probiotics. Probiotic ingestion was considered when a subject reported yogurt or probiotic supplement consumption. The data revealed that premenopausal women who consumed probiotics had higher estradiol levels, and postmenopausal women who consumed probiotics had lower total testosterone levels than women who did not consume probiotics [52]. Whether these findings could be extrapolated to other clinical conditions is unclear. For example, among patients with type 2 diabetes mellitus (T2DM), women have a higher level of circulating testosterone [85] and could be a population that could greatly benefit from probiotics. Another condition where excess of androgen is present in women is PCOS. About 80% of women with PCOS have hyperandrogenemia, and the level of testosterone is about 1.5-fold higher compared to women with normal cycling. Interestingly, women with PCOS and elevated androgen levels have a worse cardiometabolic profile compared to women with PCOS with normal levels of androgens [86]. Thereby, it can be speculated that a simple intervention such as yogurt or probiotics intake may result in beneficial hormonal changes that will decrease cardiovascular risk in those populations. However, this hypothesis remains to be tested. In contrast, in men with hypogonadism, probiotics administration failed to increase the plasma level of testosterone [87]. Whether and how probiotics, in a sexually dimorphic manner, regulate the levels of sex steroids remains to be elucidated.
Table 1.
Role of gut microbiome in hormonal regulation.
| Hormone of Interest | Main Findings | Proposed Mechanisms | Reference |
|---|---|---|---|
| Cortisol, adrenocorticotropic hormone, (ACTH), aldosterone |
|
Tendency to ↑ Cortisol ↑ ACTH ↑ Aldosterone ↑ Corticotropin-releasing hormone receptor 1 (Crhr1) mRNA levels ↓ Mineralocorticoid receptor (MR) mRNA levels |
[24] |
|
↓ Urinary free cortisol in tested subjects | [47] | |
|
↓ Salivary cortisol | [96] | |
|
↓ Salivary cortisol | [73] | |
| Estrogen | Gut bacterial species containing β-glucuronidases and β-glucuronides enzymes are capable of metabolizing estrogens. | The deconjugation and conjugation of estrogen by the estrobolome modulate the enterohepatic circulation of estrogens, thereby affecting circulating and excreted estrogen levels | [97] |
| In men and postmenopausal women, the level of total urinary estrogens was strongly and directly associated with fecal microbiome richness. | Altering β-glucuronidase activity | [98] | |
| Dysbiosis may influence the progression of endometriosis in females. | ↓ Estrogen level | [27] | |
|
↑ Serum estradiol, upregulate estrogen Receptor a (ERα) in adipose tissue. ↑ SCFA production |
[53] | |
|
- | [52] | |
| Androgens |
|
De-glucuronidation of DHT and testosterone | [52] |
|
- | [52] | |
| Insulin |
|
Disrupting insulin signaling | [99] |
|
Altering host gut microbiota composition | [38] | |
|
Increased intestinal permeability, lipopolysaccharide absorption, and inflammatory pathway activation | [100] | |
|
↓ Circulating inflammatory markers and insulin Improves the lipid profile and decreases the atherogenic index | [101] | |
|
↓ Bifidobacterium spp. ↓ Endotoxemia and plasma and adipose tissue proinflammatory cytokines ↑ Colonic mRNA levels of the GLP-1 precursor proglucagon |
[102] | |
| Leptin, Ghrelin, GLP-1 |
|
SCFAs modulate leptin release via activating GPR41 receptor SCFAs induce GLP-1 release through interacting with enteroendocrine cells SCFAs attenuate ghrelin-mediated signaling via the growth hormone secretagogue receptor-1a lipopolysaccharide (LPS) modulates GLP-1 release via TLR4 | [3,50] |
|
↑ GLP-1 production ↓ Serum ghrelin levels |
[71] | |
| Thyroid |
|
↓ SCFA production ↓ Thyroxine levels |
[31] |
Early puberty is defined by the development of secondary sexual characteristics and menses before eight years of age in girls and nine years in boys. Early puberty has been extensively linked to adverse health outcomes, such as metabolic syndrome. Recent data suggest that probiotic drinks or yogurt have a protective effect against early puberty [88]. Thereby, probiotics administration could constitute an effective intervention to modulate sex steroids in a variety range of clinical conditions.
Besides sex steroids, other steroids, such as cortisol, could also be impacted by prebiotics. Cortisol, or the stress hormone, has several functions in the human body, such as mediating the stress response, regulating metabolism, the inflammatory response, and immune function [89]. Data from a small randomized clinical trial demonstrated that a three-week consumption of two types of prebiotic supplements in healthy human volunteers was associated with decreased waking salivary cortisol reactivity (a stress biomarker) and improvement in anxiety [90].
The thyroid hormones are well known for controlling metabolism, growth, and many other critical functions. Recent data have suggested that microbes influence thyroid hormone levels by regulating iodine uptake, degradation, and enterohepatic cycling [35]. A recent meta-analysis of eight randomized clinical trials has shown that although probiotics and prebiotics did not change the level of thyroid hormones, they may modestly reduce thyroid-stimulating hormone receptor antibody levels in patients with hyperthyroidism [91].
T2DM, a metabolic disorder characterized by elevated glucose levels, has emerged as a major public health problem. Its prevalence is increasing, and it is estimated that by 2045, 700 million individuals worldwide are expected to have diabetes mellitus. A recent meta-analysis of 22 randomized clinical trials, including a total of 2218 patients, suggested that probiotics may lower baseline levels of HbA1c, fasting glucose, and IR in patients with T2DM. Similar findings were observed in women with gestational diabetes [92]. Metformin is an antihyperglycemic medication approved for the management of T2DM when glycemic control cannot be accomplished by lifestyle modification alone. Metformin is also recommended for diabetes prevention in patients age < 60 years and/or BMI ≥ 35 kg/m2, or HbA1c of 5.7% to 6.4%, in whom lifestyle modifications failed to reduce hyperglycemia Metformin is the initial therapy of choice in T2DM due to its efficacy, weight-neutral effect, general tolerability, favorable cost, and protection from cardiovascular events [93]. Recent data demonstrated that the co-administration of oral probiotic interventions along with metformin treatment was found to significantly improve glycemic control in T2DM patients [94].
Dyslipidemias, or abnormal levels of cholesterol and/or triglycerides, are frequently associated with T2DM. The administration of probiotics is associated with improvement in the lipid profile of patients with dyslipidemias [95]. However, whether this improvement of glycemic parameters or lipids results in an improvement in cardiovascular morbidity or mortality remains unknown.
7. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) refers to administering stool bacteria into the intestinal tract of a patient, a clinically relevant example is the treatment of recurrent Clostridioides difficile infection (CDI) [103]. Patients with recurrent CDI have a reduced diversity and number of the intestinal microbiome compared to healthy individuals [104]. This infection can be observed in up to 20% of antibiotic users. The mechanism is not entirely understood, but it is related to changes in the homeostatic balance of the GI mucosa. The alteration of the colonic microbiota following FMT appears to be long-term, with a high cure rate after FMT [105]. Limited data suggest that FMT can also be beneficial in CDI-associated bloodstream infections [106]. Additionally, FMT has demonstrated potential in reducing dysbiosis, decreasing hospitalizations, and improving disease severity in patients with hepatic encephalopathy and liver cirrhosis. It has also been shown to enhance metabolic outcomes in patients with non-alcoholic fatty liver disease [107].
The impact of FMT on the endocrine system has been suggested in recent findings in cross-sex fecal transplants in Wistar rats. Male rats that received FMT from female donors displayed lower plasma concentrations of testosterone compared to the male recipients that received same-sex FMT without changes in other hormones such as cortisol [108]. It is very early to fully understand the clinical relevance of these findings and the mechanisms underlying this change. Recent findings suggest the existence of local testosterone synthesis and metabolism in the colon, with higher concentrations than those in plasma in male rats [109]. This finding is not novel per se; several other organs have the full machinery necessary to synthesize or activate sex steroids [110-112]. Although the sex of the donor is accounted for in some transplants, such as the heart [113], it is not usually the case for FMT. Clinical evidence also suggests that FMT may help preserve endogenous insulin production in patients recently diagnosed with type 1 diabetes [114]. However, further research is needed to fully understand the effects of FMT on the endocrine system.
8. Limitations, Future Directions, and Research Gaps
Despite their proven benefits to improve gut health overall, the efficacy of probiotics is limited by several factors. For example, patients who have long-term dysbiosis as a consequence of chronic gut inflammatory conditions, such as Crohn’s disease or ulcerative colitis, may be resistant to new colonization introduced by probiotics, reducing their efficacy [115]. Similarly, the concurrent use of antibiotics can also limit probiotic efficacy [116]. Since probiotics rely on fibers as substrates [117], a diet high in sugar and low in fiber creates a poor environment, further reducing their effectiveness. However, this limitation can be mitigated by the use of probiotics or synbiotics. Although generally considered safe for healthy populations, the use of certain probiotic species in immunocompromised, very young, or elderly patients carries the risk of adverse effects, such as fungemia, fungal septicemia, endocarditis, probiotic-associated pneumonia, allergic responses, and abdominal or liver abscesses [118]. Therefore, the effectiveness of probiotics, prebiotics, and synbiotics depends heavily on the specific strains used and the individual’s unique microbiome profile. Using non-personalized or generic strains that do not address specific microbial imbalances may result in suboptimal outcomes. The personalization of treatment, proper microbial balance, and careful consideration of underlying health conditions are crucial for optimizing the benefits of these interventions.
The use of probiotics is not fully established in clinical practice. This is mainly due to the sizeable, significant heterogeneity in the studies and variability in results. Large-scale randomized clinical trials with clear and predefined endpoints are necessary to fully determine the efficacy and safety of probiotics in humans. Also, clear protocols and dose-dependent effects are required to untangle the complex impact of probiotics in chronic conditions. The findings from basic research could be beneficial in describing novel mechanisms and informing about efficacy and safety that could be translated to humans.
9. Conclusions
A disrupted gut microbiome can negatively impact reproductive and metabolic health by affecting the hormonal system. Due to its relatively safe profile, probiotic and prebiotic supplementation has drawn considerable interest recently as potential strategies to improve gut health. However, stronger evidence, such as data from large randomized clinical trials in different groups, is needed to better estimate its efficacy and safety. Other caveats are the wide-ranging variations in the composition of the probiotics administered, the dosage and duration of the probiotic interventions, and limited endpoints, which could explain the inconsistent findings across studies. Regardless of the gaps in our knowledge, probiotics and prebiotics are emerging as novel co-adjuvant therapies in treating several endocrine disorders.
Funding:
This work was supported by National Institutes of Health National Institute of General Medical Sciences grants P20GM121334 (L.L.Y.C., S.R. and D.G.R.), P50MD017338 (L.L.Y.C.), and P30GM149404 (S.R.), and National Heart, Lung, and Blood Institute (NHLBI) grants R01HL171494 (L.L.Y.C.) and R01HL144847 (D.G.R.). This work was also supported by the American Heart Association Predoctoral Fellowship 24PRE1200831 (J.B.). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Data Availability Statement:
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
- 1.Group, N.H.W.; Peterson J; Garges S; Giovanni M; McInnes P; Wang L; Schloss JA; Bonazzi V; McEwen JE; Wetterstrand KA; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fijan S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AM; Sun EW; Rogers GB; Keating DJ The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol 2019, 10, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu PJ Microbiome: Insulin signaling shapes gut community composition. Curr. Biol 2021, 31, R803–R806. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z; Tian E; Chen Y; Dong Z; Peng Q Gut microbiota and its roles in the pathogenesis and therapy of endocrine system diseases. Microbiol. Res 2023, 268, 127291. [DOI] [PubMed] [Google Scholar]
- 6.Neuman H; Debelius JW; Knight R; Koren O Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev 2015, 39, 509–521. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson JK; Holmes E; Kinross J; Burcelin R; Gibson G; Jia W; Pettersson S Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [DOI] [PubMed] [Google Scholar]
- 8.Shoaie S; Nielsen J Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front. Genet 2014, 5, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfora EE; Jocken JW; Blaak EE Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol 2015, 11, 577–591. [DOI] [PubMed] [Google Scholar]
- 10.Dockray GJ Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol 2014, 592, 2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rastelli M; Cani PD; Knauf C The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev 2019, 40, 1271–1284. [DOI] [PubMed] [Google Scholar]
- 12.Dockray GJ Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes 2012, 19, 8–12. [DOI] [PubMed] [Google Scholar]
- 13.Baker JM; Al-Nakkash L; Herbst-Kralovetz MM Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [DOI] [PubMed] [Google Scholar]
- 14.Kumari N; Kumari R; Dua A; Singh M; Kumar R; Singh P; Duyar-Ayerdi S; Pradeep S; Ojesina AI; Kumar R From Gut to Hormones: Unraveling the Role of Gut Microbiota in (Phyto)Estrogen Modulation in Health and Disease. Mol. Nutr. Food Res 2024, 68, e2300688. [DOI] [PubMed] [Google Scholar]
- 15.Collden H; Landin A; Wallenius V; Elebring E; Fandriks L; Nilsson ME; Ryberg H; Poutanen M; Sjogren K; Vandenput L; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab 2019, 317, E1182–E1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadchan SB; Singh V; Kommagani R Female reproductive dysfunctions and the gut microbiota. J. Mol. Endocrinol 2022, 69, R81–R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X; Yun C; Pang Y; Qiao J The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y; Zeng W; Hou T; Yang H; Wu B; Pan R; Huang L Gut microbiome and reproductive endocrine diseases: A Mendelian randomization study. Front. Endocrinol 2023, 14, 1164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter SE; Baumler AJ Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkafas H; Walls M; Al-Hendy A; Ismail N Gut and genital tract microbiomes: Dysbiosis and link to gynecological disorders. Front. Cell Infect. Microbiol 2022, 12, 1059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dash S; Syed YA; Khan MR Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol 2022, 10, 880544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frohlich EE; Farzi A; Mayerhofer R; Reichmann F; Jacan A; Wagner B; Zinser E; Bordag N; Magnes C; Frohlich E; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun 2016, 56, 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L; Wang H; Chen X; Zhang Y; Zhang H; Xie P Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo R; Zeng B; Zeng L; Cheng K; Li B; Luo Y; Wang H; Zhou C; Fang L; Li W; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell Infect. Microbiol 2017, 7, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bercik P; Denou E; Collins J; Jackson W; Lu J; Jury J; Deng Y; Blennerhassett P; Macri J; McCoy KD; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609, 609e1–609e3. [DOI] [PubMed] [Google Scholar]
- 26.Tremellen K; Pearce K Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [DOI] [PubMed] [Google Scholar]
- 27.Ukrainets RV; Korneva YS; Dorosevich AE Abnormal gut microbiota-induced hypoestrogenemia as a possible risk factor for malignancy in endometrioid heterotopia. Arkhiv Patol. 2020, 82, 57–61. [DOI] [PubMed] [Google Scholar]
- 28.Markle JG; Frank DN; Mortin-Toth S; Robertson CE; Feazel LM; Rolle-Kampczyk U; von Bergen M; McCoy KD; Macpherson AJ; Danska JS Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [DOI] [PubMed] [Google Scholar]
- 29.Tomasello G; Mazzola M; Bosco V; Tomasello G; Damiani P; Sinagra E; Carini F Intestinal Dysbiosis and Hormonal Neuroendocrine Secretion in the Fibromyalgic Patient: Relationship and Correlations. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2018, 162, 258–262. [DOI] [PubMed] [Google Scholar]
- 30.Kreznar JH; Keller MP; Traeger LL; Rabaglia ME; Schueler KL; Stapleton DS; Zhao W; Vivas EI; Yandell BS; Broman AT; et al. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Rep. 2017, 18, 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su X; Zhao Y; Li Y; Ma S; Wang Z Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin. Sci 2020, 134, 1521–1535. [DOI] [PubMed] [Google Scholar]
- 32.Zhao F; Feng J; Li J; Zhao L; Liu Y; Chen H; Jin Y; Zhu B; Wei Y Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [DOI] [PubMed] [Google Scholar]
- 33.Ishaq HM; Mohammad IS; Shahzad M; Ma C; Raza MA; Wu X; Guo H; Shi P; Xu J Molecular Alteration Analysis of Human Gut Microbial Composition in Graves’ disease Patients. Int. J. Biol. Sci 2018, 14, 1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J; Zhang F; Zhao C; Xu Q; Liang C; Yang Y; Wang H; Shang Y; Wang Y; Mu X; et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574. [DOI] [PubMed] [Google Scholar]
- 35.Frohlich E; Wahl R Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab 2019, 30, 479–490. [DOI] [PubMed] [Google Scholar]
- 36.Stefanaki C; Peppa M; Mastorakos G; Chrousos GP Examining the gut bacteriome, virome, and mycobiome in glucose metabolism disorders: Are we on the right track? Metabolism 2017, 73, 52–66. [DOI] [PubMed] [Google Scholar]
- 37.Turnbaugh PJ; Backhed F; Fulton L; Gordon JI Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridaura VK; Faith JJ; Rey FE; Cheng J; Duncan AE; Kau AL; Griffin NW; Lombard V; Henrissat B; Bain JR; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latif A; Shehzad A; Niazi S; Zahid A; Ashraf W; Iqbal MW; Rehman A; Riaz T; Aadil RM; Khan IM; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol 2023, 14, 1216674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingenito E; Solway J; Lafleur J; Lombardo A; Drazen JM; Pichurko B Dissociation of temperature-gradient and evaporative heat loss during cold gas hyperventilation in cold-induced asthma. Am. Rev. Respir. Dis 1988, 138, 540–546. [DOI] [PubMed] [Google Scholar]
- 41.Plaza-Diaz J; Ruiz-Ojeda FJ; Gil-Campos M; Gil A Mechanisms of Action of Probiotics. Adv. Nutr 2019, 10 (Suppl. S1), S49–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tegegne BA; Kebede B Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahire JJ; Jakkamsetty C; Kashikar MS; Lakshmi SG; Madempudi RS In Vitro Evaluation of Probiotic Properties of Lactobacillus plantarum UBLP40 Isolated from Traditional Indigenous Fermented Food. Probiotics Antimicrob. Proteins 2021, 13, 1413–1424. [DOI] [PubMed] [Google Scholar]
- 44.Chang YH; Jeong CH; Cheng WN; Choi Y; Shin DM; Lee S; Han SG Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci 2021, 104, 7415–7425. [DOI] [PubMed] [Google Scholar]
- 45.Accettulli A; Corbo MR; Sinigaglia M; Speranza B; Campaniello D; Racioppo A; Altieri C; Bevilacqua A Psycho-Microbiology, a New Frontier for Probiotics: An Exploratory Overview. Microorganisms 2022, 10, 2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [DOI] [PubMed] [Google Scholar]
- 47.Messaoudi M; Lalonde R; Violle N; Javelot H; Desor D; Nejdi A; Bisson JF; Rougeot C; Pichelin M; Cazaubiel M; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr 2011, 105, 755–764. [DOI] [PubMed] [Google Scholar]
- 48.Anker-Ladefoged C; Langkamp T; Mueller-Alcazar A The Potential Impact of Selected Bacterial Strains on the Stress Response. Healthcare 2021, 9, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinan TG; Stanton C; Cryan JF Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [DOI] [PubMed] [Google Scholar]
- 50.van Son J; Koekkoek LL; La Fleur SE; Serlie MJ; Nieuwdorp M The Role of the Gut Microbiota in the Gut-Brain Axis in Obesity: Mechanisms and Future Implications. Int. J. Mol. Sci 2021, 22, 2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rooks MG; Garrett WS Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 2016, 16, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou S; Yang X; Li N; Wang H; Gui J; Li J Association of probiotic ingestion with serum sex steroid hormones among pre- and postmenopausal women from the NHANES, 2013–2016. PLoS ONE 2023, 18, e0294436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q; Wang B; Wang S; Qian X; Li X; Zhao J; Zhang H; Chen W; Wang G Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davani-Davari D; Negahdaripour M; Karimzadeh I; Seifan M; Mohkam M; Masoumi SJ; Berenjian A; Ghasemi Y Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuo SM The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr 2013, 4, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson JL; Erickson JM; Lloyd BB; Slavin JL Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr 2018, 2, nzy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naseer M; Poola S; Uraz S; Tahan V Therapeutic Effects of Prebiotics on Constipation: A Schematic Review. Curr. Clin. Pharmacol 2020, 15, 207–215. [DOI] [PubMed] [Google Scholar]
- 58.Paineau D; Payen F; Panserieu S; Coulombier G; Sobaszek A; Lartigau I; Brabet M; Galmiche JP; Tripodi D; Sacher-Huvelin S; et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br. J. Nutr 2008, 99, 311–318. [DOI] [PubMed] [Google Scholar]
- 59.Lindsay JO; Whelan K; Stagg AJ; Gobin P; Al-Hassi HO; Rayment N; Kamm MA; Knight SC; Forbes A Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis CD; Milner JA Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem 2009, 20, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pool-Zobel BL Inulin-type fructans and reduction in colon cancer risk: Review of experimental and human data. Br. J. Nutr 2005, 93 (Suppl. S1), S73–S90. [DOI] [PubMed] [Google Scholar]
- 62.Mollard RC; Senechal M; Macintosh AC; Hay J; Wicklow BA; Wittmeier KD; Sellers EA; Dean HJ; Ryner L; Berard L; et al. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am. J. Clin. Nutr 2014, 99, 804–812. [DOI] [PubMed] [Google Scholar]
- 63.Hume MP; Nicolucci AC; Reimer RA Prebiotic supplementation improves appetite control in children with overweight and obesity: A randomized controlled trial. Am. J. Clin. Nutr 2017, 105, 790–799. [DOI] [PubMed] [Google Scholar]
- 64.Best T; Kemps E; Bryan J Saccharide effects on cognition and well-being in middle-aged adults: A randomized controlled trial. Dev. Neuropsychol 2010, 35, 66–80. [DOI] [PubMed] [Google Scholar]
- 65.Jeurink PV; van Esch BC; Rijnierse A; Garssen J; Knippels LM Mechanisms underlying immune effects of dietary oligosaccharides. Am. J. Clin. Nutr 2013, 98, 572S–577S. [DOI] [PubMed] [Google Scholar]
- 66.den Besten G; van Eunen K; Groen AK; Venema K; Reijngoud DJ; Bakker BM The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res 2013, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan SH; Louis P; Thomson JM; Flint HJ The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol 2009, 11, 2112–2122. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh SS; Wang J; Yannie PJ; Ghosh S Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc 2020, 4, bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rose EC; Odle J; Blikslager AT; Ziegler AL Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci 2021, 22, 6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shokryazdan P; Faseleh Jahromi M; Navidshad B; Liang JB Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol 2017, 206, 1–9. [DOI] [PubMed] [Google Scholar]
- 71.Delzenne NM; Cani PD; Neyrinck AM Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: Experimental data. J. Nutr 2007, 137, 2547S–2551S. [DOI] [PubMed] [Google Scholar]
- 72.Tarini J; Wolever TM The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab 2010, 35, 9–16. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt K; Cowen PJ; Harmer CJ; Tzortzis G; Errington S; Burnet PW Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Preter V; Raemen H; Cloetens L; Houben E; Rutgeerts P; Verbeke K Effect of dietary intervention with different pre- and probiotics on intestinal bacterial enzyme activities. Eur. J. Clin. Nutr 2008, 62, 225–231. [DOI] [PubMed] [Google Scholar]
- 75.Yadav MK; Kumari I; Singh B; Sharma KK; Tiwari SK Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol 2022, 106, 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kearney SM; Gibbons SM Designing synbiotics for improved human health. Microb. Biotechnol 2018, 11, 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swanson KS; Gibson GR; Hutkins R; Reimer RA; Reid G; Verbeke K; Scott KP; Holscher HD; Azad MB; Delzenne NM; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol 2020, 17, 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh M; Kumar S; Banakar PS; Vinay VV; Das A; Tyagi N; Tyagi AK Synbiotic formulation of Cichorium intybus root powder with Lactobacillus acidophilus NCDC15 and Lactobacillus reuteri BFE7 improves growth performance in Murrah buffalo calves via altering selective gut health indices. Trop. Anim. Health Prod 2021, 53, 291. [DOI] [PubMed] [Google Scholar]
- 79.Kassaian N; Feizi A; Aminorroaya A; Amini M Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: A randomized controlled trial. Diabetes Metab. Syndr 2019, 13, 2991–2996. [DOI] [PubMed] [Google Scholar]
- 80.Kafoury ME; Ebrahim AT; Abd-El Hamid Ali MS; Shaker Mehanna N; Ibrahim Ramadan GE; Ezzat Morsy W Short chain fatty acids and GIT hormones mitigate gut barrier disruption in high fat diet fed rats supplemented by synbiotics. Mediterr. J. Nutr. Metab 2023, 16, 139–163. [Google Scholar]
- 81.Asemi Z; Aarabi MH; Hajijafari M; Alizadeh SA; Razzaghi R; Mazoochi M; Esmaillzadeh A Effects of Synbiotic Food Consumption on Serum Minerals, Liver Enzymes, and Blood Pressure in Patients with Type 2 Diabetes: A Double-blind Randomized Cross-over Controlled Clinical Trial. Int. J. Prev. Med 2017, 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markowiak P; Slizewska K Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gurry T. Synbiotic approaches to human health and well-being. Microb. Biotechnol 2017, 10, 1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saulnier DM; Gibson GR; Kolida S In vitro effects of selected synbiotics on the human faecal microbiota composition. FEMS Microbiol. Ecol 2008, 66, 516–527. [DOI] [PubMed] [Google Scholar]
- 85.Zietz B; Cuk A; Hugl S; Buttner R; Straub RH; Bauer B; Daffner P; Scholmerich J; Palitzsch K Association of increased C-peptide serum levels and testosterone in type 2 diabetes. Eur. J. Intern. Med 2000, 11, 322–328. [DOI] [PubMed] [Google Scholar]
- 86.Tsuchida S; Kimura Y; Sugawara H; Kato Y; Sekino H Effectiveness of pyrodifenium bromide (Padrin) on diseases of the urinary tract. Hinyokika Kiyo 1967, 13, 699–701. [PubMed] [Google Scholar]
- 87.Ljunggren L; Butler E; Axelsson J; Astrom M; Ohlsson L Effects of probiotic supplementation on testosterone levels in healthy ageing men: A 12-week double-blind, placebo-controlled randomized clinical trial. Contemp. Clin. Trials Commun 2024, 39, 101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai MC; Lee YL; Chen YC Association of the consumption of common drinks with early puberty in both sexes. Front. Public Health 2022, 10, 854477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oakley RH; Cidlowski JA The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol 2013, 132, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Julich H; Klink K; Heberling HJ; Burkmann I Methods of cardiopulmonary functional diagnosis for the expert evaluation of heart patients. Z. Gesamte Inn. Med 1974, 29, 191–198. [PubMed] [Google Scholar]
- 91.Skjerve E; Wasteson Y The situation from Norway’s point of view ecological and health consequences of spreading of pathogenes and genes through an increasing trade in foods. Acta Vet. Scand. Suppl 1999, 91, 33–39. [PubMed] [Google Scholar]
- 92.Lagowska K; Malinowska AM; Zawieja B; Zawieja E Improvement of glucose metabolism in pregnant women through probiotic supplementation depends on gestational diabetes status: Meta-analysis. Sci. Rep 2020, 10, 17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kooy A; de Jager J; Lehert P; Bets D; Wulffele MG; Donker AJ; Stehouwer CD Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med 2009, 169, 616–625. [DOI] [PubMed] [Google Scholar]
- 94.White SA; Doughman T; Hayes P; Nicholson ML The deep circumflex iliac vein for secondary central venous access and haemodialysis. Nephrol. Dial. Transplant 2000, 15, 244–245. [DOI] [PubMed] [Google Scholar]
- 95.Kenyon CJ; Saccoccio NA; Morris DJ Aldosterone effects on water and electrolyte metabolism. J. Endocrinol 1984, 100, 93–100. [DOI] [PubMed] [Google Scholar]
- 96.Allen AP; Hutch W; Borre YE; Kennedy PJ; Temko A; Boylan G; Murphy E; Cryan JF; Dinan TG; Clarke G Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plottel CS; Blaser MJ Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flores R; Shi J; Fuhrman B; Xu X; Veenstra TD; Gail MH; Gajer P; Ravel J; Goedert JJ Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med 2012, 10, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vijay-Kumar M; Aitken JD; Carvalho FA; Cullender TC; Mwangi S; Srinivasan S; Sitaraman SV; Knight R; Ley RE; Gewirtz AT Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caricilli AM; Saad MJ The role of gut microbiota on insulin resistance. Nutrients 2013, 5, 829–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajkumar H; Mahmood N; Kumar M; Varikuti SR; Challa HR; Myakala SP Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014, 2014, 348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cani PD; Neyrinck AM; Fava F; Knauf C; Burcelin RG; Tuohy KM; Gibson GR; Delzenne NM Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [DOI] [PubMed] [Google Scholar]
- 103.Johnson S; Lavergne V; Skinner AM; Gonzales-Luna AJ; Garey KW; Kelly CP; Wilcox MH Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis 2021, 73, e1029–e1044. [DOI] [PubMed] [Google Scholar]
- 104.Tvede M; Rask-Madsen J Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989, 1, 1156–1160. [DOI] [PubMed] [Google Scholar]
- 105.Mamo Y; Woodworth MH; Wang T; Dhere T; Kraft CS Durability and Long-term Clinical Outcomes of Fecal Microbiota Transplant Treatment in Patients With Recurrent Clostridium difficile Infection. Clin. Infect. Dis 2018, 66, 1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ianiro G; Murri R; Sciume GD; Impagnatiello M; Masucci L; Ford AC; Law GR; Tilg H; Sanguinetti M; Cauda R; et al. Incidence of Bloodstream Infections, Length of Hospital Stay, and Survival in Patients With Recurrent Clostridioides difficile Infection Treated With Fecal Microbiota Transplantation or Antibiotics: A Prospective Cohort Study. Ann. Intern. Med 2019, 171, 695–702. [DOI] [PubMed] [Google Scholar]
- 107.Boicean A; Birlutiu V; Ichim C; Brusnic O; Onisor DM Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnsen RC; Jones SJ; Rose AM Mutational accessibility of essential genes on chromosome I(left) in Caenorhabditis elegans. Mol. Gen. Genet 2000, 263, 239–252. [DOI] [PubMed] [Google Scholar]
- 109.Diviccaro S; Giatti S; Borgo F; Falvo E; Caruso D; Garcia-Segura LM; Melcangi RC Steroidogenic machinery in the adult rat colon. J. Steroid Biochem. Mol. Biol 2020, 203, 105732. [DOI] [PubMed] [Google Scholar]
- 110.Quinkler M; Bumke-Vogt C; Meyer B; Bahr V; Oelkers W; Diederich S The human kidney is a progesterone-metabolizing and androgen-producing organ. J. Clin. Endocrinol. Metab 2003, 88, 2803–2809. [DOI] [PubMed] [Google Scholar]
- 111.Le Goascogne C; Sananes N; Eychenne B; Gouezou M; Baulieu EE; Robel P Androgen biosynthesis in the stomach: Expression of cytochrome P450 17 alpha-hydroxylase/17,20-lyase messenger ribonucleic acid and protein, and metabolism of pregnenolone and progesterone by parietal cells of the rat gastric mucosa. Endocrinology 1995, 136, 1744–1752. [DOI] [PubMed] [Google Scholar]
- 112.Quinkler M; Sinha B; Tomlinson JW; Bujalska IJ; Stewart PM; Arlt W Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J. Endocrinol 2004, 183, 331–342. [DOI] [PubMed] [Google Scholar]
- 113.Reed RM; Netzer G; Hunsicker L; Mitchell BD; Rajagopal K; Scharf S; Eberlein M Cardiac size and sex-matching in heart transplantation: Size matters in matters of sex and the heart. JACC Heart Fail. 2014, 2, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boicean A; Ichim C; Todor SB; Anderco P; Popa ML The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santana PT; Rosas SLB; Ribeiro BE; Marinho Y; de Souza HSP Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci 2022, 23, 3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elias AJ; Barna V; Patoni C; Demeter D; Veres DS; Bunduc S; Eross B; Hegyi P; Foldvari-Nagy L; Lenti K Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: A systematic review and meta-analysis. BMC Med. 2023, 21, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sonnenburg ED; Sonnenburg JL Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kothari D; Patel S; Kim SK Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother 2019, 111, 537–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.


