Abstract
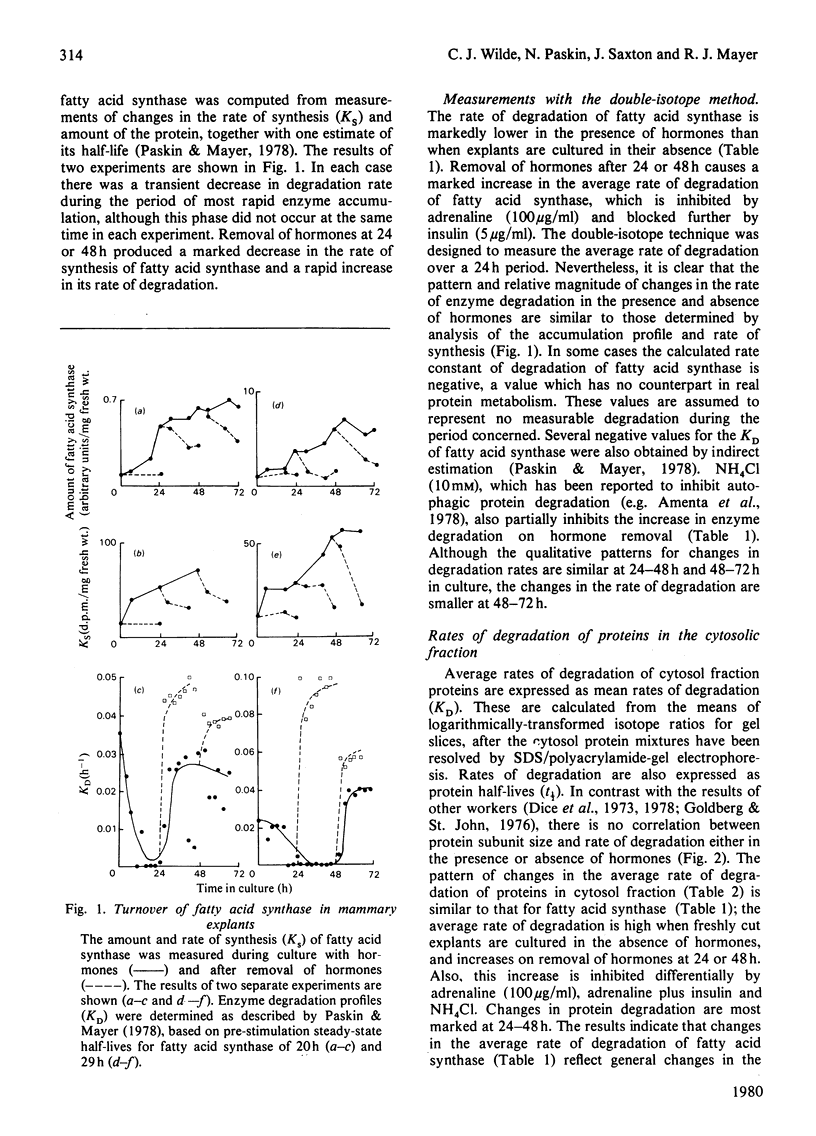
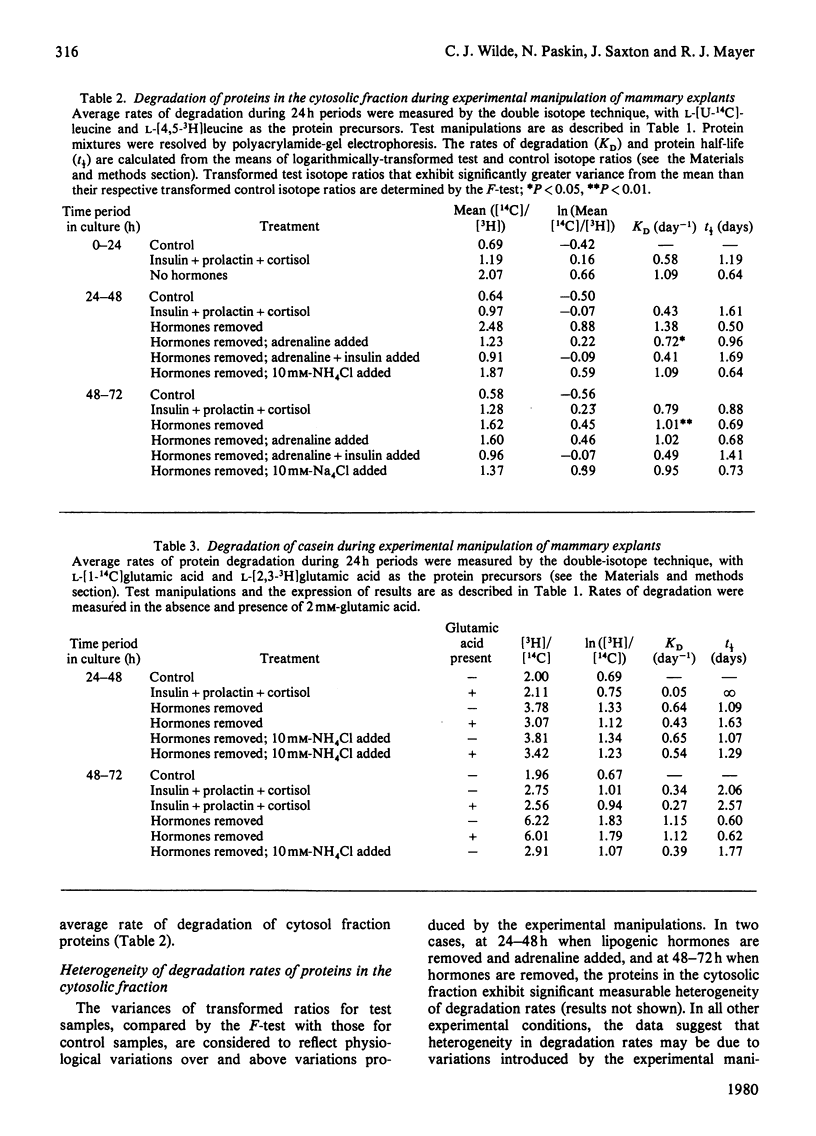
1. In mammary gland explants subjected to experimental manipulation, average rates (during 24 h periods) of degradation of fatty acid synthase, casein and cytosol-fraction proteins were measured by a double-isotope method. Rates of degradation of fatty acid synthase were also computed from measurements of changing enzyme amount and rate of synthesis. 2. During the period of most rapid enzyme accumulation there is a transient decrease in the computed rate of degradation of fatty acid synthase. Removal of hormones produces a rapid increase in the computed rate of degradation of the enzyme. 3. The average rate of degradation of fatty acid synthase measured by the double-isotope method is low in the presence of hormones, and increases on hormone removal. This increase in degradation rate is inhibited by adrenaline and further blocked by insulin. NH4Cl (10 mM) also partially inhibits the increase in protein degradation on hormone removal. 4. The pattern of changes in the average rate of degradation of cytosol-fraction proteins is similar to that for fatty acid synthase alone. There is no relationship between subunit molecular weight and rate of degradation under all experimental conditions. 5. Isotope ratios for resolved cytosol protein mixtures are transformed logarithmically to make the standard deviations an estimate of heterogeneity of degradation rates. By this analysis, in some conditions there appears to be significant measureable heterogeneity of degradation rates. 6. Little degradation of casein is measured in the presence of hormones, but a marked increase in the rate of degradation can be measured when hormones are removed. Whereas at 24-48h NH4Cl (10 mM) has little effect on this enhanced rate of degradation, at 48-72h it causes a large decrease in degradation rate. 7. Results are discussed in terms of a two-component degradation system in mammary gland explants.
Full text
PDF


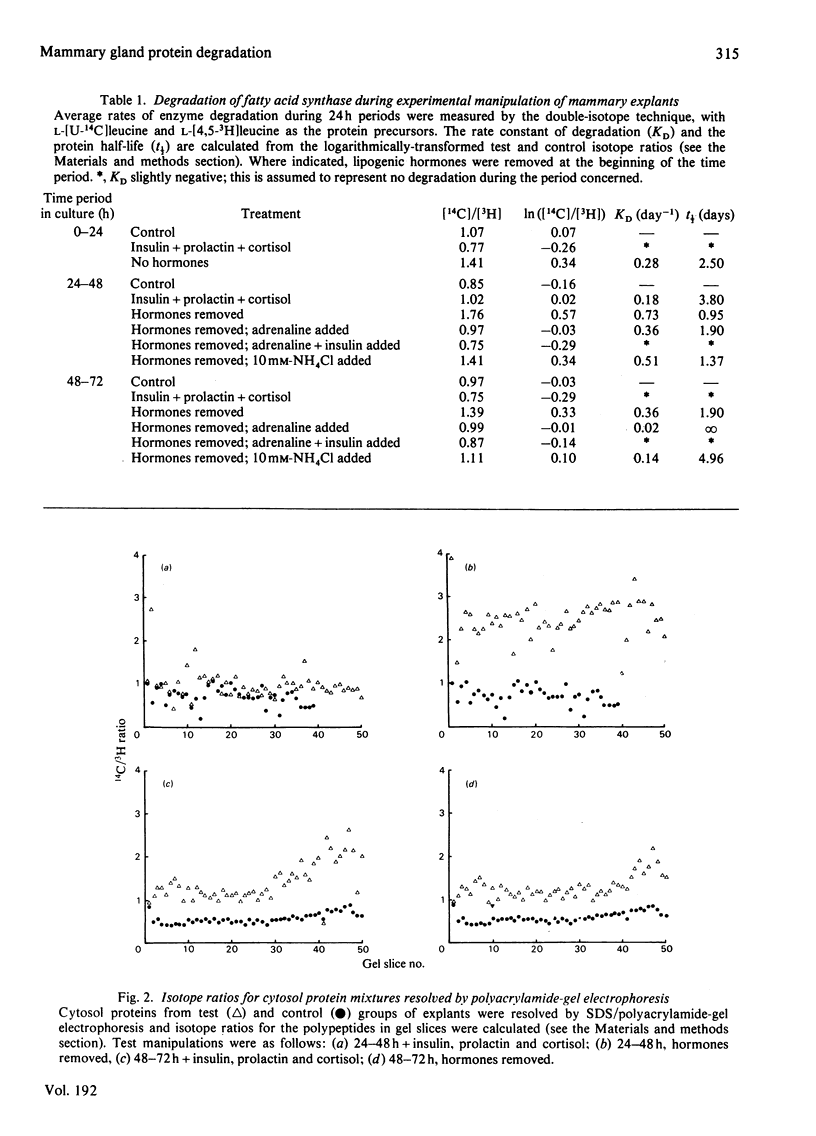




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Sarraj K., Newbury J., White D. A., Mayer R. J. Casein turnover in rabbit mammary explants in organ culture. Biochem J. 1979 Sep 15;182(3):837–845. doi: 10.1042/bj1820837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sarraj K., White D. A., Mayer R. J. Immunochemical characterization of casein from rabbit mammary gland. Biochem J. 1978 Sep 1;173(3):877–883. doi: 10.1042/bj1730877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Arstila A. U., Shelburne J. D., Trump B. F. Studies on cellular autophagocytosis. A histochemical study on sequential alterations of mitochondria in the glucagon-induced autophagic vacuoles of rat liver. Lab Invest. 1972 Sep;27(3):317–323. [PubMed] [Google Scholar]
- Ayuso-Parrilla M. S., Martín-Requero A., Pérez-Días J., Parrilla R. Role of glucagon on the control of hepatic protein synthesis and degradation in the rat in vivo. J Biol Chem. 1976 Dec 25;251(24):7785–7790. [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Brunner S., Joseph X., Levey I. L. Protein degradation in the mouse blastocyst. J Biol Chem. 1979 Mar 25;254(6):1927–1931. [PubMed] [Google Scholar]
- Burgess R. J., Walker J. H., Mayer R. J. Choice of precursors for the measurement of protein turnover by the double-isotope method. Application to the study of mitochondrial proteins. Biochem J. 1978 Dec 15;176(3):919–926. doi: 10.1042/bj1760919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär H. P. Epinephrine- and prostaglandin-sensitive adenyl cyclase in mammary gland. Biochim Biophys Acta. 1973 Sep 15;321(1):397–406. doi: 10.1016/0005-2744(73)90094-6. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Campbell P. N. Intracellular mechanisms involved in the biosynthesis of milk proteins. Biochem Soc Trans. 1978;6(3):501–505. doi: 10.1042/bst0060501. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Subunit constitution of proteins: a table. Arch Biochem Biophys. 1975 Feb;166(2):651–682. doi: 10.1016/0003-9861(75)90432-4. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975 Sep;170(1):213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigel W. N., Hofmann C. J., Chibber B. A., Tomich J. M., Keenan T. W., Mertz E. T. Plasmin-mediated proteolysis of casein in bovine milk. Proc Natl Acad Sci U S A. 1979 May;76(5):2244–2248. doi: 10.1073/pnas.76.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every D., Ashworth J. M. Rates of degradation and synthesis of glycosidases de novo during growth and differentiation of Dictyostelium discoideum. Biochem J. 1975 May;148(2):169–177. doi: 10.1042/bj1480169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth I. A., Myres R. P. Human prolactin. Evidence obtained by the bioassay of human plasma. J Endocrinol. 1971 Sep;51(1):157–168. doi: 10.1677/joe.0.0510157. [DOI] [PubMed] [Google Scholar]
- Glass R. D., Doyle D. On the measurement of protein turnover in animal cells. J Biol Chem. 1972 Aug 25;247(16):5234–5242. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hardwick D. C. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochem J. 1966 Apr;99(1):228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. Aspartate aminotransferase in fat tissues: changes with growth and hormones. Biochim Biophys Acta. 1971 Apr 20;237(1):88–98. doi: 10.1016/0304-4165(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Horst M. N., Roberts R. M. Analysis of polypeptide turnover rates in Chinese hamster ovary cell plasma membranes using two-dimensional electrophoresis. J Biol Chem. 1979 Jun 25;254(12):5000–5007. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J. Hormonal factors in lipogenesis in mammary gland. Vitam Horm. 1978;36:101–163. doi: 10.1016/s0083-6729(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Ovtracht L. Dynamics of the Golgi apparatus: membrane differentiation and membrane flow. Int Rev Cytol Suppl. 1977;(5):61–188. [PubMed] [Google Scholar]
- Novikoff A. B., Shin W. Y. Endoplasmic reticulum and autophagy in rat hepatocytes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5039–5042. doi: 10.1073/pnas.75.10.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. A method for the analysis of protein turnover characteristics. Indirect estimation of rates of protein degradation. Biochem J. 1978 Jul 15;174(1):153–161. doi: 10.1042/bj1740153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. Molecular weight and subunit size of fatty acid synthetase from rabbit mammary gland. Biochem J. 1976 Oct 1;159(1):181–184. doi: 10.1042/bj1590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. The role of enzyme degradation in enzyme turnover during tissue differentiation. Biochim Biophys Acta. 1977 Jan 3;474(1):1–10. doi: 10.1016/0005-2787(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol. 1978 Jul;78(1):152–167. doi: 10.1083/jcb.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- Roberts R. M., Yuan B. O. Radiolabeling of mammalian cells in tissue culture by use of acetic anhydride. Potential value for studying the dynamics of protein turnover in living cells. Arch Biochem Biophys. 1975 Nov;171(1):226–233. doi: 10.1016/0003-9861(75)90027-2. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Burgess R. J., Mayer R. J. Protein degradation in rat liver during post-natal development. Biochem J. 1980 Oct 15;192(1):321–330. doi: 10.1042/bj1920321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Segal H. L., Rothstein D. M., Winkler J. R. A correlation between turnover rates and lipophilic affinities of soluble rat liver proteins. Biochem Biophys Res Commun. 1976 Nov 8;73(1):79–84. doi: 10.1016/0006-291x(76)90499-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976 Jul;100(2):276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differentiation. Interactions of insulin, prolactin and cortisol in controlling the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1976 Feb 15;154(2):359–370. doi: 10.1042/bj1540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tauber R., Reutter W. Protein degradation in the plasma membrane of regenerating liver and Morris hepatomas. Eur J Biochem. 1978 Feb 1;83(1):37–45. doi: 10.1111/j.1432-1033.1978.tb12065.x. [DOI] [PubMed] [Google Scholar]
- Tweto J., Doyle D. Turnover of the plasma membrane proteins of hepatoma tissue culture cells. J Biol Chem. 1976 Feb 10;251(3):872–882. [PubMed] [Google Scholar]
- Walker J. H., Burgess R. J., Mayer R. J. Relative rates of turnover of subunits of mitochondrial proteins. Biochem J. 1978 Dec 15;176(3):927–932. doi: 10.1042/bj1760927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. F., Chua B. L., Li J. B., Morgan H. E., Mortimore G. E. Inhibition of basal and deprivation-induced proteolysis by leupeptin and pepstatin in perfused rat liver and heart. Biochem Biophys Res Commun. 1979 Mar 15;87(1):92–98. doi: 10.1016/0006-291x(79)91651-6. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Blough R. Assessment of protein turnover by use of radioisotopic tracers. Physiol Rev. 1979 Apr;59(2):407–447. doi: 10.1152/physrev.1979.59.2.407. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G., Rabinowitz M. Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977 May 25;252(10):3430–3435. [PubMed] [Google Scholar]


