Abstract
Introduction
Lung cancer is the second most common cancer worldwide and the leading cause of cancer deaths; non-small cell lung cancer (NSCLC) constitutes about 85% of lung cancer cases, with ALK fusions representing 3–6% of them. The SQSTM1-ALK fusion is a rare finding in NSCLC, accounting for only 1.1% of ALK rearrangements. We present a case of lung adenocarcinoma with documentation of SQSTM1-ALK fusion that showed a partial response to alectinib.
Case description
This case details the clinical course of a 71-year-old, non-smoking woman with no significant medical history who presented with confusion, aphasia and multiple cerebral lesions detected on imaging. Further investigations revealed a stage IV lung adenocarcinoma with metastases to the brain and adrenal gland. Molecular profiling identified a rare SQSTM1-ALK fusion mutation alongside other genetic abnormalities, including low programmed death-ligand 1 expression and ROS1 kinase protein presence. Treatment with alectinib, initiated based on the identified ALK fusion, resulted in significant tumour regression in the lungs and complete resolution of the adrenal mass, as evidenced by follow-up imaging and clinical assessments.
Conclusion
This case highlights the efficacy of alectinib in treating rare ALK fusion variants in and underscores the importance of comprehensive molecular profiling in guiding targeted therapy decisions.
LEARNING POINTS
Recognition of rare ALK fusions This case highlights the importance of identifying rare ALK fusions, such as SQSTM1-ALK, in non-small cell lung cancer (NSCLC), which can guide personalised treatment strategies.
Utility of advanced molecular diagnostics The use of next-generation sequencing alongside immunohistochemistry is crucial for accurately detecting ALK and ROS1 rearrangements, avoiding false positives and enabling the identification of druggable mutations.
Impact of personalised medicine This case reinforces the value of personalised medicine in NSCLC, where molecular profiling can uncover unique genetic alterations, allowing for more tailored and potentially more effective treatments.
Keywords: ALK fusion, non-small cell lung carcinoma, alectinib, next-generation sequencing
INTRODUCTION
Lung cancer is the second most commonly diagnosed malignancy worldwide and remains the leading cause of cancer-related deaths, with 1.8 million fatalities reported in 2020. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of cases, with adenocarcinoma being the most prevalent histological subtype. Significant advancements in treatment have been made due to the discovery of driver mutations, such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) gene alterations, which have led to the development of targeted therapies. Despite these advancements, the 5-year survival rate remains poor at 10–20%. ALK fusions, including the EML4-ALK fusion, are present in 3–6% of NSCLC cases. However, rare ALK fusion variants – such as SQSTM1(sequestosome 1)-ALK – are less understood, and their response to standard therapies remains under investigation. In this report, we present a case of a patient with NSCLC harbouring the rare SQSTM1-ALK fusion, who responded to the second-generation ALK inhibitor alectinib. This case underscores the importance of molecular studies, including next-generation sequencing (NGS), in guiding treatment decisions for patients with uncommon ALK alterations.
CASE DESCRIPTION
The patient is a 71-year-old woman, a non-smoker with no relevant medical history, who experienced confusion, non-coherent speech and aphasia. A computed tomography (CT) and magnetic resonance imaging (MRI) of the head revealed multiple space-occupying cerebral lesions in both hemispheres, brainstem and cerebellum. Extension studies showed a solid lesion in the anterior segment of the upper left pulmonary lobe. Abdomen CT revealed a mass in the left adrenal gland. Breast and pelvic ultrasounds were unremarkable. A CT-guided lung biopsy was performed, and rapid on-site evaluation was positive for malignancy and suggested the presence of a lung adenocarcinoma. Histological findings confirmed an invasive adenocarcinoma, histologic grade 3, with predominantly solid and secondary micropapillary pattern. EGFR exons 18,19, 20 and 21 real-time polymerase chain reaction (q-PCR) showed no mutations, and an immunohistochemistry (IHC) test revealed low programmed death-ligand 1 expression (tumour proportion score 20%), the presence of the ROS1 kinase protein (mutation SP384) and the echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion gene (mutation D5F3).
A stage IV lung adenocarcinoma with cerebral and adrenal involvement was diagnosed. Alectinib 600 mg twice a day was started, and genomic studies were ordered looking for further druggable mutations. An NGS-based assay (FoundationOne® CDx) was conducted on the tumour tissue and revealed the presence of a SQSTM1-ALK fusion. Microsatellite (MS) status was MS-stable and tumour mutational burden was 2 mutations per megabase.
During treatment with alectinib the patient complained of lower limb oedema, insomnia, generalised pruritus and constipation, which resolved with diet intervention. None of these symptoms led to treatment discontinuation. Complete blood count showed mild anaemia, and liver function tests were remarkable for mild elevation of bilirubin levels, with normal transaminases.
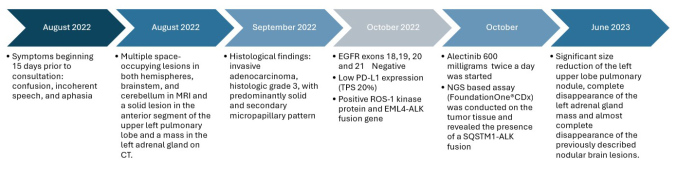
Follow-up imaging at 7 months of treatment showed a significant size reduction of the left upper lobe pulmonary nodule (29 × 20 vs 16 × 8 mm), consistent with partial response (based on the Response Evaluation Criteria in Solid Tumours (RECIST) Version 1.1) (Fig. 1). Abdominal and pelvic MRI revealed complete disappearance of the left adrenal gland mass. A bone scintigraphy was negative for lesions suggestive of metastases but showed the presence of a recent T12 fracture, there was also complete disappearance of the previously described nodular lesions in brain image, with persistence of the frontal lesion with ring enhancement which shows a reduction in size of more than 50%, findings compatible with partial response at 9 months of treatment (Fig. 2). The timeline of the presented case is shown in Figure 3.
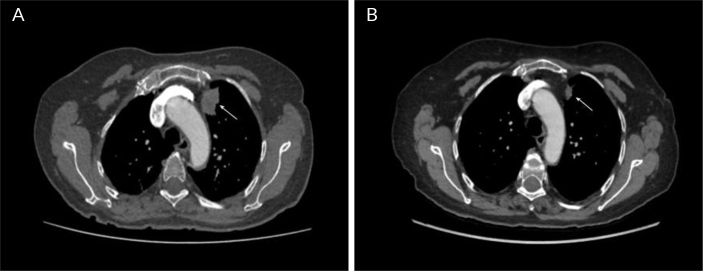
Figure 1.
Contrast-enhanced thoracic tomography, axial cut and mediastinal window. A) Solid subpleural mass, irregular contours and heterogeneous density in the anterior segment of the left upper lobe (arrow); B) Control image 8 months later, showing a reduction of more than 50% of the previously described mass (arrow), compatible with partial response.
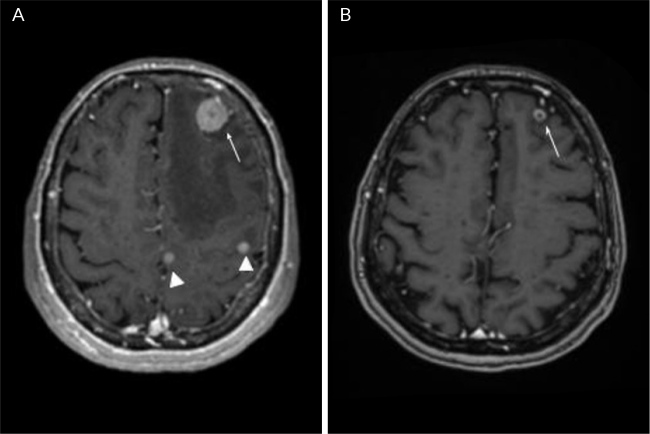
Figure 2.
MRI of the brain, axial sequences enhanced in Figure 1 post-contrast. A) Multiple supra and infratentorial nodular lesions (infratentorial lesions are not shown), the image shows solid nodules in the left frontal and parietal lobes, the smallest ones with homogeneous enhancement (arrowheads) and the largest lesion with ring enhancement (arrow), compatible with metastases with adjacent vasogenic oedema; B) Control image 9 months later, showing almost complete disappearance of the previously described nodular lesions, with persistence of the frontal lesion with ring enhancement (arrow) which shows a reduction in size of more than 50%, findings compatible with partial response.
Figure 3.
Timeline of the presented case. Abbreviations: MRI, magnetic resonance imaging; TC, computed tomography; NGS, next-generation sequencing.
DISCUSSION
Lung cancer is the second most commonly diagnosed tumour worldwide, considering both sexes, and represents the leading cause of cancer-related death with 1.8 million deaths in 2020. Despite recent therapeutic advances due to the introduction of target therapy and immunotherapy, 5-year survival remains poor (10–20%)[1]. NSCLC represents about 85% of all lung cancer diagnoses in the last ten years. An increasing number of activating oncogene mutations have been discovered in this setting. The most frequent are Kirsten rat sarcoma virus (20–30%), EGFR (10–15% of Caucasian patients and up to 40% of Asian patients) and ALK fusions in approximately 3–6% of NSCLC, the most common involving the EML4 gene[1].
SQMST1-ALK fusion was first described in 2011 in a case of ALK-positive large B-cell lymphoma[2]. In NSCLC, it was first described in 2015[3]. SQSTM1 encodes for the sequestosome-1, a protein regulating multiple pathways involving cell survival and death[2]. It mediates activation of the NF-kB, apoptosis and Nrf2 pathways, and modulates oxidative stress response and ubiquitin-binding autophagy[2,3].
The ROS1 rearrangement detection in metastatic and advanced lung adenocarcinoma has allowed the development of targeted treatment strategies with tyrosine kinase inhibitors (TKI), achieving favourable progression-free survival and overall survival of patients. According to the different guidelines of management in this scenario, detection of ROS1 rearrangement in all these patients is mandatory[4]. The immunohistochemistry method for ROS1 rearrangements detection is preferred due to its low cost and high sensitivity, but its diagnostic performance depends on different anti-ROS1 antibodies requiring confirmation techniques. Other tests such as fluorescence in situ hybridisation and nucleic acid detection by techniques that use polymerase chain reaction analysis are also available. As mentioned, ROS1 detection by IHC is an effective detection tool. However, it is necessary to consider its variable performance depending on the antibodies, which requires the use of a confirmation strategy. The fluorescence in situ hybridisation technique can be used, which is considered the gold standard to determine ROS1 positivity[4]. In this case, ROS1 IHC was recommended as a first-line screening test. An NGS study was selected as a diagnostic tool given simultaneous identification of ROS1 and ALK impairments by IHC. NGS can precisely rule out a false positive ROS1 rearrangement and identify low-frequency ALK variants such as occurred here[4].
SQSTM1-ALK fusion is a very atypical finding in patients with NSCLC. Using the AACR Project GENIE Cohort v8.1 database, through the analysis of 11,107 samples from 10,082 patients with lung adenocarcinoma, SQSTM1-ALK fusions accounted for just 1.1% of all identified ALK rearrangements[5]. The SQSTM1 gene is located on chromosome 5q35 and encodes a ubiquitin-binding SQSTM1 protein implicated in processes of autophagy, cell signalling and differentiation[6].
This is one of the first reports in the literature of a patient with SQSTM1-ALK fusion NSCLC responding to alectinib. It is a second-generation ALK-TKI, which showed much better first-line efficacy than the first-generation ALK-TKI, crizotinib, reaching a median progression-free survival of 34 months in untreated ALK-positive patients not previously treated. ALK status in the alectinib pivotal clinical trial (ALEX and J-ALEX) was achieved by IHC[7]. The clinical response of less frequent ALK fusions to standard treatment remains unknown. Patients with different ALK fusions have exhibited varying responses to ALK-TKIs. It is postulated that the type of ALK rearrangement influences treatment response, yet available evidence indicates lower response rates in infrequent ALK fusions[8]. A therapeutic response to alectinib was described in two cases of inflammatory myofibroblastic tumour with metastatic involvement, where SQSTM1 ALK fusion was identified during a three-year follow-up. Given that data for NSCLC is uncertain, extrapolation was used, and the decision was to maintain treatment with alectinib[9]. The SQSTM1-ALK fusion has been reported in two cases of diffuse large B-cell lymphoma that were positive for ALK[10].
CONCLUSION
This is a case of lung adenocarcinoma with documentation of a rare ALK fusion variant that showed partial response to alectinib as previously documented in other neoplasms. Molecular studies such as NGS are important to rule out false positives of ROS1 detection by immunohistochemistry. At the same time, an adequate typing of ALK alterations is necessary, allowing management options to be proposed.
Footnotes
Conflicts of Interests: The authors declare that there are no conflicts of interest associated with the publication of this case report. All authors have no financial or personal relationships that could influence or bias the content of this manuscript. This case report received no specific funding from any funding agency, commercial entity or other external sources.
Patient Consent: Informed consent was obtained from the patient for publication of their medical information, images and histopathological findings.
REFERENCES
- 1.Cognigni V, Pecci F, Lupi A, Pinterpe G, De Filippis C, Felicetti C, et al. The landscape of ALK-rearranged non-small cell lung cancer: a comprehensive review of clinicopathologic, genomic characteristics, and therapeutic perspectives. Cancers (Basel) 2022;14:4765. doi: 10.3390/cancers14194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi K, Soda M, Togashi Y, Ota Y, Sekiguchi Y, Hatano S, et al. Identification of a novel fusion, SQSTM1-ALK, in ALK-positive large B-cell lymphoma. Haematologica. 2011;96:464–467. doi: 10.3324/haematol.2010.033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyevleva AG, Raskin GA, Tiurin VI, Sokolenko AP, Mitiushkina NV, Aleksakhina SN, et al. Novel ALK fusion partners in lung cancer. Cancer Lett. 2015;362:116–121. doi: 10.1016/j.canlet.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40:611–625. doi: 10.1200/JCO.21.01626. [DOI] [PubMed] [Google Scholar]
- 5.Desai A, Mohammed T, Rakshit S, Krull J. 21P The landscape of ALK alterations in non-small cell lung cancer. J Thorac Oncol. 2021;16:S707. [Google Scholar]
- 6.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Lee ATM, Ou SI. ALESIA 5-year update: alectinib at 600 mg twice daily gives lorlatinib a run for its money in Asia. Lung Cancer (Auckl) 2023;14:71–78. doi: 10.2147/LCTT.S419395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang Y, Zhang S, Fang X, Jiang Y, Fang T, Liu J, et al. Therapeutic advances of rare ALK fusions in non-small cell lung cancer. Curr Oncol. 2022;29:7816–7831. doi: 10.3390/curroncol29100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunga CGG, Higgins MS, Ricciotti RW, Liu YJ, Cranmer LD. Inflammatory myofibroblastic tumor of the mesentery with a SQSTM1::ALK fusion responding to alectinib. Cancer Rep (Hoboken) 2023;6:e1792. doi: 10.1002/cnr2.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.d’Amore ES, Visco C, Menin A, Famengo B, Bonvini P, Lazzari E. STAT3 pathway is activated in ALK-positive large B-cell lymphoma carrying SQSTM1-ALK rearrangement and provides a possible therapeutic target. Am J Surg Pathol. 2013;37:780–786. doi: 10.1097/PAS.0b013e318287791f. [DOI] [PubMed] [Google Scholar]