Abstract
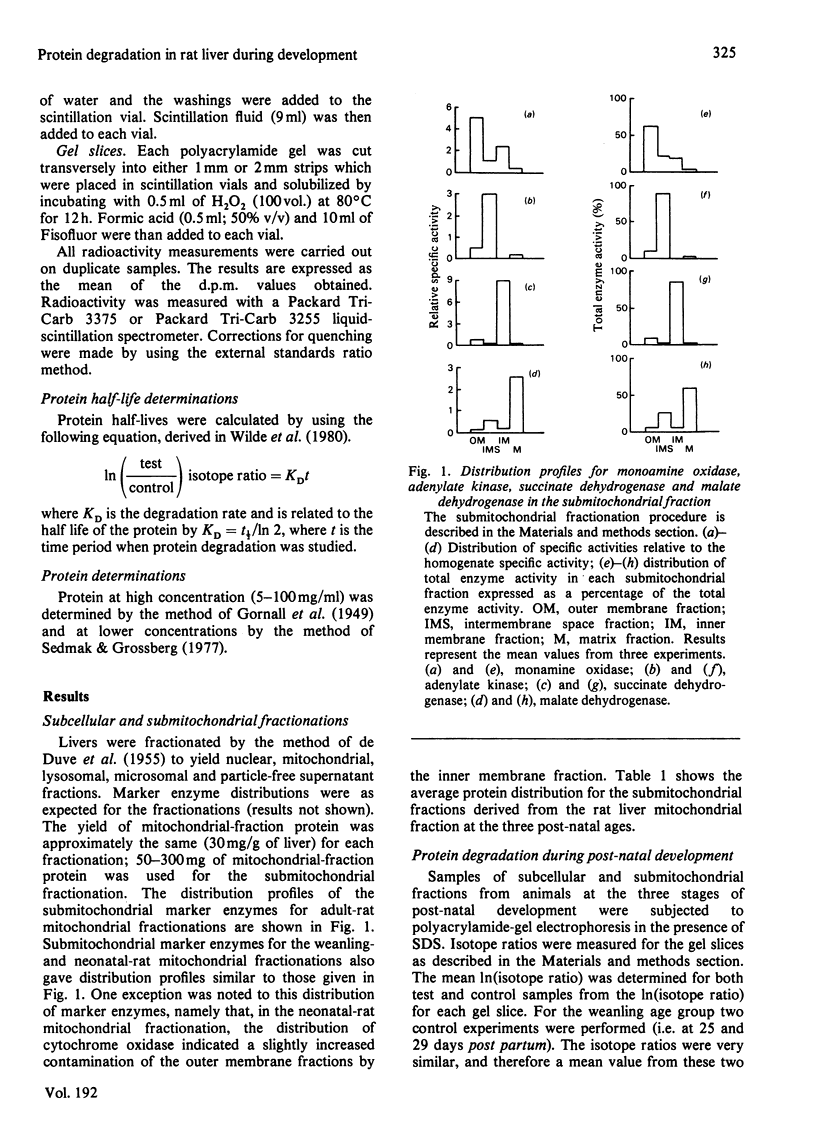
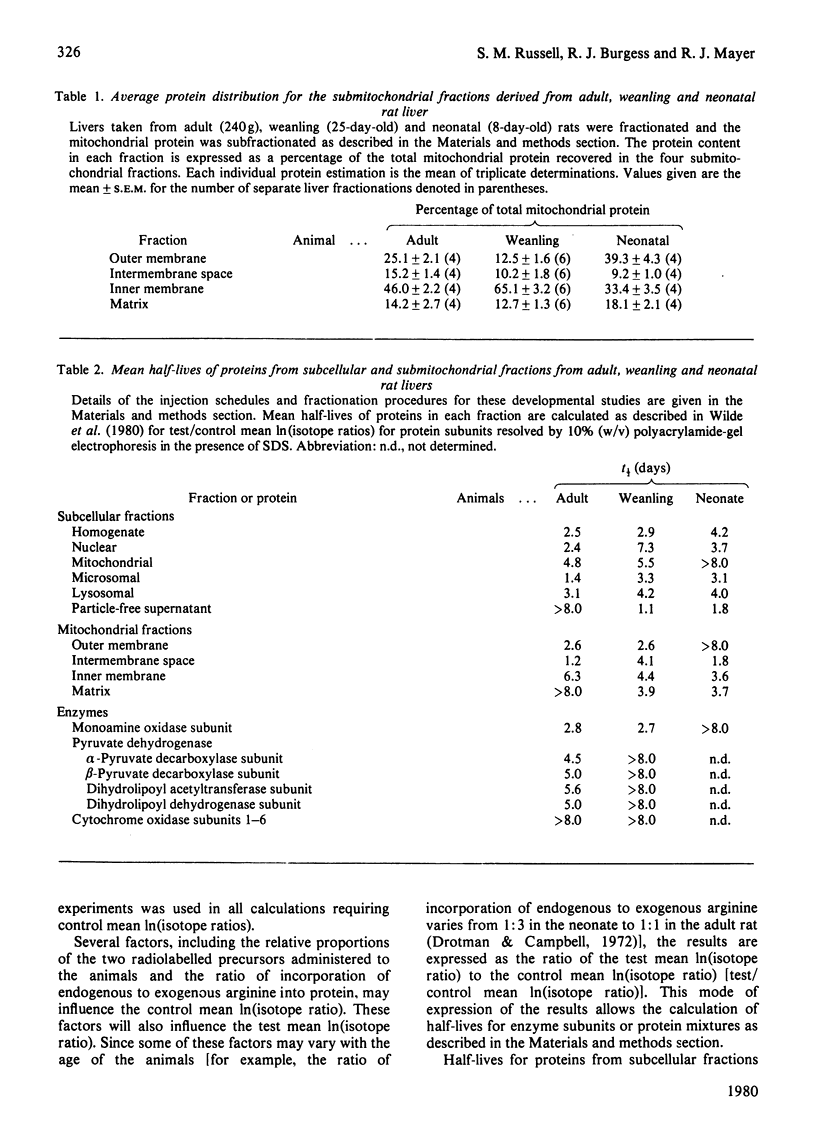
Protein degradation rates for liver subcellular and submitochondrial fractions from neonatal (8-day), weanling (25-day) and adult rats were estimated by the double-isotope method with NaH14CO3 and [3H] arginine as the radiolabelled precursors [Dice, Walker, Byrne & Cardiel (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 2093-2097]. Decreased protein degradation rates were found during post-natal development for homogenate, nuclear, mitochondrial, lysosomal and microsomal proteins. A decrease in degradation rates for the immunoisolated subunits of monoamine oxidase and pyruvate dehydrogenase was also observed in neonatal and weanling rats respectively. The results suggest coordinate degradation of the subunits of the multi-subunit enzyme pyruvate dehydrogenase. Pyruvate dehydrogenase has a faster rate of degradation in adult rat liver than does cytochrome oxidase. Data analysis suggests heterogeneity of protein degradation rates in the mitochondrial outer membrane and intermembrane space fractions at each developmental stage but not in the mitochondrial inner membrane or matrix fractions. Results obtained for protein degradation rates in adult rat liver by the method of Burgess, Walker & Mayer [(1978) Biochem. J. 176, 919-926] in general confirmed the results obtained for the adult rat liver by the above method. No evidence of a subunit-size relationship for protein degradation was found for proteins in any subcellular or submitochondrial fraction.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Aschenbrenner B., Druyan R., Albin R., Rabinowitz M. Haem a, cytochrome c and total protein turnover in mitochondria from rat heart and liver. Biochem J. 1970 Sep;119(2):157–160. doi: 10.1042/bj1190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M., Work T. S. The biogenesis of mitochondria. Annu Rev Biochem. 1970;39:251–290. doi: 10.1146/annurev.bi.39.070170.001343. [DOI] [PubMed] [Google Scholar]
- Brunner G., Neupert W. Turnover of outer and inner membrane proteins of rat liver mitochondria. FEBS Lett. 1968 Aug;1(3):153–155. doi: 10.1016/0014-5793(68)80045-6. [DOI] [PubMed] [Google Scholar]
- Burgess R. J., Walker J. H., Mayer R. J. Choice of precursors for the measurement of protein turnover by the double-isotope method. Application to the study of mitochondrial proteins. Biochem J. 1978 Dec 15;176(3):919–926. doi: 10.1042/bj1760919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canick J. A., Villee D. B. The effect of adrenocorticotrophin on protein degradation in rat adrenal gland and liver. Biochem J. 1974 Nov;144(2):397–402. doi: 10.1042/bj1440397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde R. D., Scornik O. A. Faster synthesis and slower degradation of liver protein during developmental growth. Biochem J. 1977 Jul 15;166(1):115–121. doi: 10.1042/bj1660115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Weibel V. J. Lower rates of protein degradation in developing rat brain. Biochem J. 1979 May 15;180(2):423–426. doi: 10.1042/bj1800423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bernard B., Getz G. S., Rabinowitz M. The turnover of the protein of the inner and outer mitochondrial membrane of rat liver. Biochim Biophys Acta. 1969 Oct 14;193(1):58–63. doi: 10.1016/0005-2736(69)90058-3. [DOI] [PubMed] [Google Scholar]
- Dennick R. G., Mayer R. J. Purification and immunochemical characterization of monoamine oxidase from rat and human liver. Biochem J. 1977 Jan 1;161(1):167–174. doi: 10.1042/bj1610167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Hess E. J., Goldberg A. L. Studies on the relationship between the degradative rates of proteins in vivo and their isoelectric points. Biochem J. 1979 Feb 15;178(2):305–312. doi: 10.1042/bj1780305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotman R. B., Campbell J. W. Protein arginine biosynthesis by mammalian liver tissue during postnatal development. Am J Physiol. 1972 May;222(5):1204–1212. doi: 10.1152/ajplegacy.1972.222.5.1204. [DOI] [PubMed] [Google Scholar]
- Druyan R., DeBernard B., Rabinowitz M. Turnover of cytochromes labeled with delta-aminolevulinic acid-3H in rat liver. J Biol Chem. 1969 Nov 10;244(21):5874–5878. [PubMed] [Google Scholar]
- Dunlop D. S., Elden W. V., Lajtha A. Protein degradation rates in regions of the central nervous system in vivo during development. Biochem J. 1978 Mar 15;170(3):637–642. doi: 10.1042/bj1700637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear A. R. Inner- and outer-membrane enzymes of mitochondria during liver regeneration. Biochem J. 1970 Dec;120(3):577–587. doi: 10.1042/bj1200577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- Horst M. N., Roberts R. M. Analysis of polypeptide turnover rates in Chinese hamster ovary cell plasma membranes using two-dimensional electrophoresis. J Biol Chem. 1979 Jun 25;254(12):5000–5007. [PubMed] [Google Scholar]
- Höchli L., Hackenbrock C. R. Cytochrome c oxidase from rat liver mitochondria: purification and characterization. Biochemistry. 1978 Sep 5;17(18):3712–3719. doi: 10.1021/bi00611a006. [DOI] [PubMed] [Google Scholar]
- Ip M. M., Chee P. Y., Swick R. W. Turnover of hepatic mitochondrial ornithine aminotransferase and cytochrome oxidase using (14C)carbonate as tracer. Biochim Biophys Acta. 1974 Jun 20;354(1):29–38. doi: 10.1016/0304-4165(74)90049-x. [DOI] [PubMed] [Google Scholar]
- MCFARLANE A. S. MEASUREMENT OF SYNTHESIS RATES OF LIVER-PRODUCED PLASMA PROTEINS. Biochem J. 1963 Nov;89:277–290. doi: 10.1042/bj0890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti M., Guerri C., Grisolia S. Turnover of carbamyl-phosphate synthase, of other mitochondrial enzymes and of rat tissues. Effect of diet and of thyroidectomy. Eur J Biochem. 1977 May 16;75(2):583–592. doi: 10.1111/j.1432-1033.1977.tb11558.x. [DOI] [PubMed] [Google Scholar]
- Paskin N., Mayer R. J. A method for the analysis of protein turnover characteristics. Indirect estimation of rates of protein degradation. Biochem J. 1978 Jul 15;174(1):153–161. doi: 10.1042/bj1740153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J. K. Mitochondrial heterogeneity in foetal and suckling rat liver: differential leucine incorporation into the proteins of two mitochondrial populations. Biochem Biophys Res Commun. 1976 Apr 5;69(3):823–829. doi: 10.1016/0006-291x(76)90949-9. [DOI] [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Yuan B. O. Chemical modification of the plasma membrane polypeptides of cultured mammalian cells as an aid to studying protein turnover. Biochemistry. 1974 Nov 5;13(23):4846–4856. doi: 10.1021/bi00720a025. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Davey J., Mayer R. J. Immunochemical characterization of monoamine oxidase from human liver, placenta, platelets and brain cortex. Biochem J. 1979 Jul 1;181(1):15–20. doi: 10.1042/bj1810015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWICK R. W., HANDA D. T. The distribution of fixed carbon in amino acids. J Biol Chem. 1956 Feb;218(2):577–585. [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Satav J. G., Katyare S. S., Fatterpaker P., Sreenivasan A. Regulation of mitochondrial membrane protein turnover by thyroid hormone(s). Biochim Biophys Acta. 1976 Nov 18;451(1):92–95. doi: 10.1016/0304-4165(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith P. A., Vernon R. G., Mayer R. J. Lipogenic enzymes in rat maternal adipose tissue in the perinatal period. Biochem J. 1980 Mar 15;186(3):937–944. doi: 10.1042/bj1860937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differentiation. Interactions of insulin, prolactin and cortisol in controlling the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1976 Feb 15;154(2):359–370. doi: 10.1042/bj1540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differention. Studies on the effects of hormones on the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1975 May;148(2):309–320. doi: 10.1042/bj1480309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Tauber R., Reutter W. Protein degradation in the plasma membrane of regenerating liver and Morris hepatomas. Eur J Biochem. 1978 Feb 1;83(1):37–45. doi: 10.1111/j.1432-1033.1978.tb12065.x. [DOI] [PubMed] [Google Scholar]
- Walker J. H., Burgess R. J., Mayer R. J. Relative rates of turnover of subunits of mitochondrial proteins. Biochem J. 1978 Dec 15;176(3):927–932. doi: 10.1042/bj1760927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. H., Mayer R. J. Kinetics of binding of cytochrome c oxidase from rat liver to an immunoadsorbent [proceedings]. Biochem Soc Trans. 1977;5(4):1101–1103. doi: 10.1042/bst0051101. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Paskin N., Saxton J., Mayer R. J. Protein degradation during terminal cytodifferentiation. Studies on mammary gland in organ culture. Biochem J. 1980 Oct 15;192(1):311–320. doi: 10.1042/bj1920311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Blough R. Assessment of protein turnover by use of radioisotopic tracers. Physiol Rev. 1979 Apr;59(2):407–447. doi: 10.1152/physrev.1979.59.2.407. [DOI] [PubMed] [Google Scholar]


