Abstract
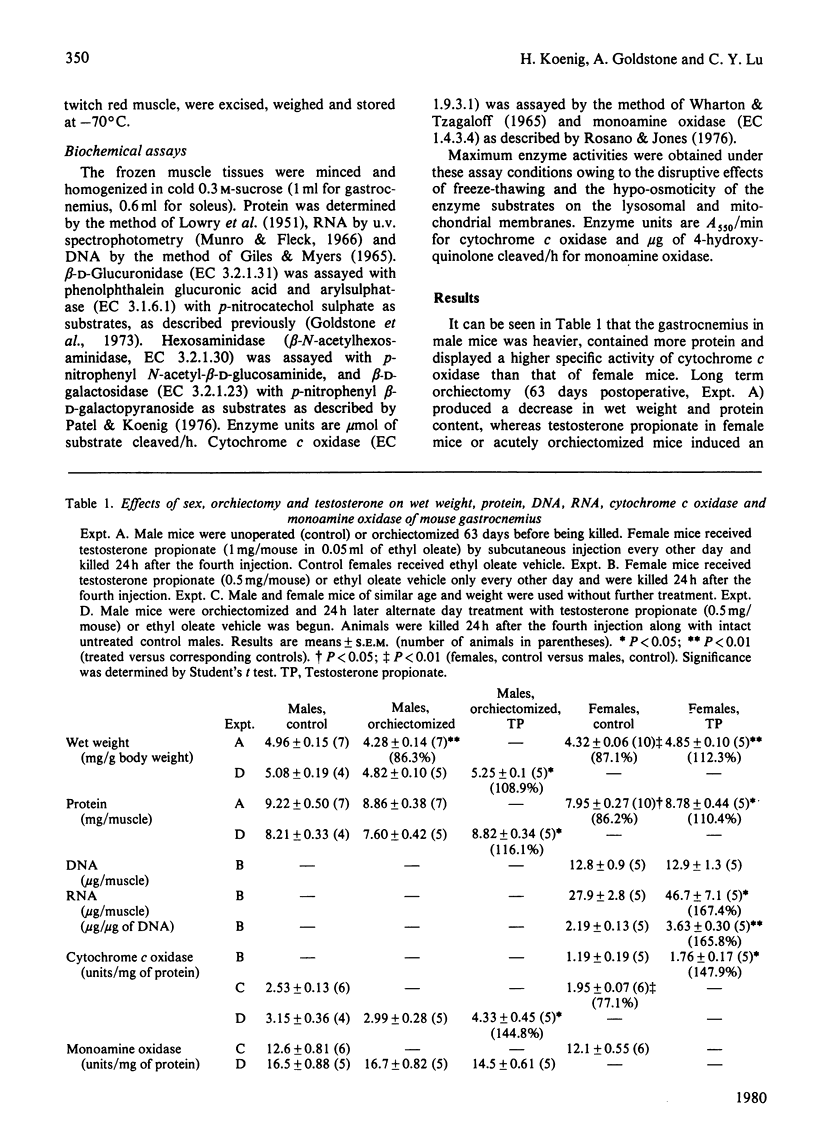
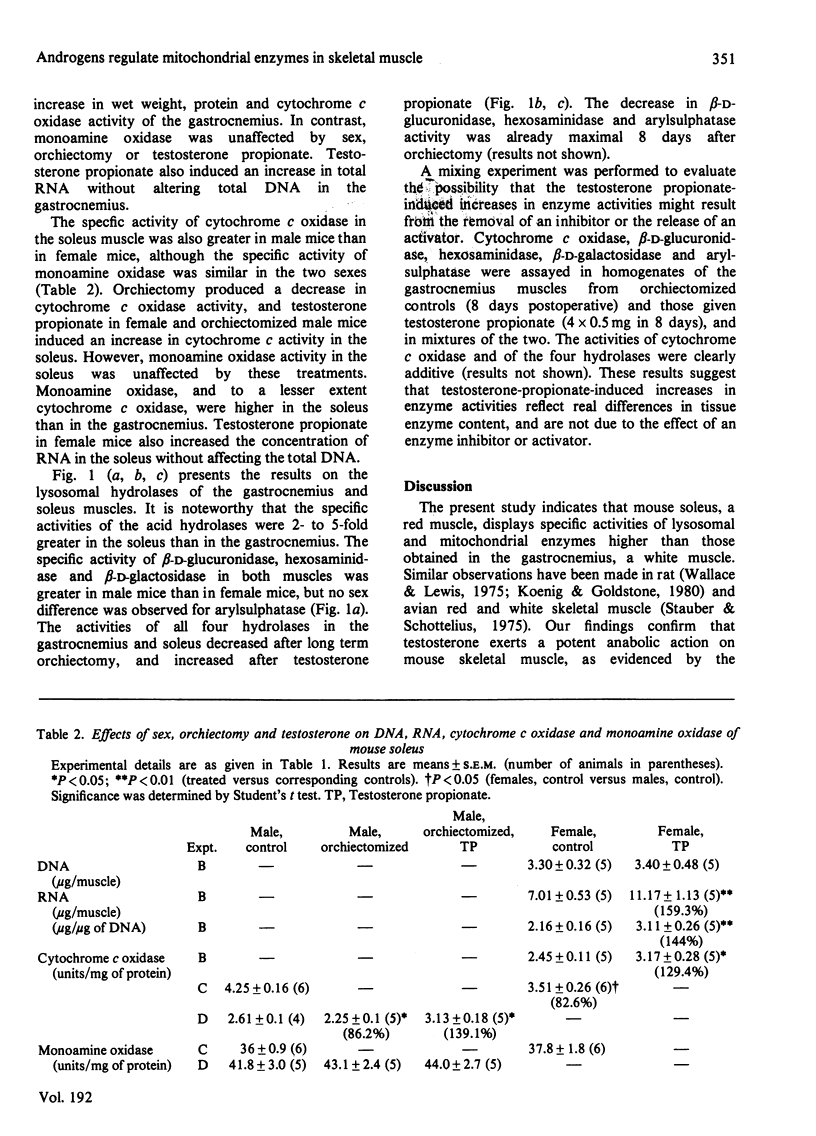
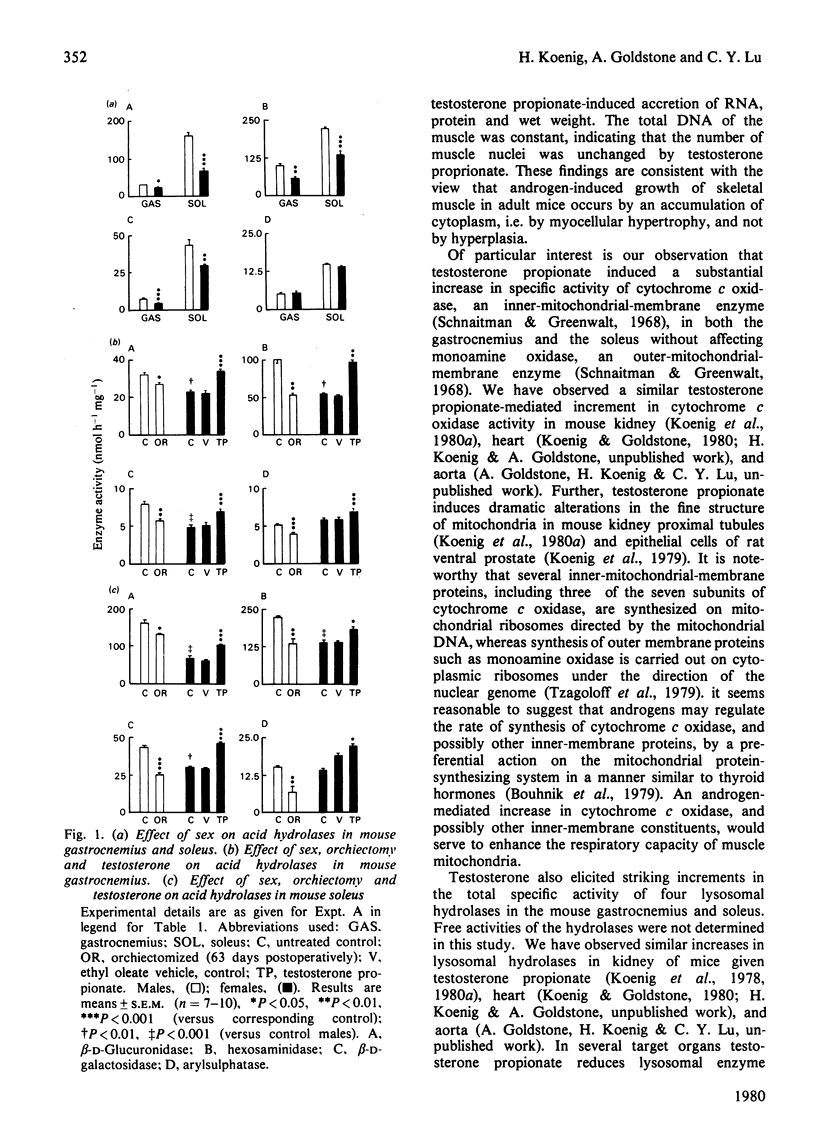
The gastrocnemius, a fast-twitch white muscle, and the soleus, a slow-twitch red muscle, were studied in A/J mice. The specific activities of the lysosomal hydrolases, beta-D-glucuronidase, hexosaminidase, beta-D-galactosidase and arylsulphatase, the inner-mitochondrial-membrane enzyme cytochrome c oxidase, and the outer-mitochondrial-membrane enzyme monoamine oxidase, were greater in the soleus than in the gastrocnemius. The specific activities of the lysosomal hydrolases and cytochrome c oxidase in the gastrocnemius and soleus were substantially higher in male mice than in female mice. Orchiectomy abolished this sex difference. Testosterone increased the activities of the lysosomal hydrolases and cytochrome c oxidase and coincidentally induced muscle hypertrophy and an accretion of protein and RNA, but total DNA remained constant. Monoamine oxidase was unaffected by sex, orchiectomy and testosterone. These findings indicate that endogenous androgens regulate the activity of enzymes associated with lysosomes and the inner mitochondrial membrane, as well as muscle fibre growth in mouse skeletal muscle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouhnik J., Clot J. P., Baudry M., Michel R. Early effects of thyroidectomy and triiodothyronine administration on rat-liver mitochondria. Mol Cell Endocrinol. 1979 Jul;15(1):1–12. doi: 10.1016/0303-7207(79)90065-0. [DOI] [PubMed] [Google Scholar]
- Breuer C. B., Florini J. R. Amino acid incorporation into protein by cell-free systems from rat skeletal muscle. IV. Effects of animal age, androgens, and anabolic agents on activity of muscle ribosomes. Biochemistry. 1965 Aug;4(8):1544–1550. doi: 10.1021/bi00884a013. [DOI] [PubMed] [Google Scholar]
- Dubé J. Y., Lesage R., Tremblay R. R. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976 Jan;54(1):50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Florini J. R. Effects of testosterone on qualitative pattern of protein synthesis in skeletal muscle. Biochemistry. 1970 Feb 17;9(4):909–912. doi: 10.1021/bi00806a027. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H., Nayyar R., Hughes C., Lu C. Y. Isolation and characterization of a rough microsomal fraction from rat kidney that is enriched in lysosomal enzymes. Biochem J. 1973 Feb;132(2):259–266. doi: 10.1042/bj1320259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCHAKIAN C. D. Mechanisms of androgen actions. Lab Invest. 1959 Mar-Apr;8(2):538–556. [PubMed] [Google Scholar]
- Kochakian C. D. Definition of androgens and protein anabolic steroids. Pharmacol Ther B. 1975;1(2):149–177. doi: 10.1016/0306-039x(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Koenig H., Goldstone A., Hughes C. Lysosomal enzymuria in the testosterone-treated mouse. A manifestation of cell defecation of residual bodies. Lab Invest. 1978 Oct;39(4):329–341. [PubMed] [Google Scholar]
- Krieg M. Characterization of the androgen receptor in the skeletal muscle of the rat. Steroids. 1976 Aug;28(2):261–274. doi: 10.1016/0039-128x(76)90114-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mayer M., Rosen F. Interaction of glucocorticoids and androgens with skeletal muscle. Metabolism. 1977 Aug;26(8):937–962. doi: 10.1016/0026-0495(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Michel G., Baulieu E. E. Récepteur cytosoluble des androgènes dans un muscle strié squelettique. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jul 29;279(5):421–424. [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Rosano T. G., Jones D. H. Developmental changes in mitochondria during the transition into lactation in the mouse mammary gland. J Cell Biol. 1976 Jun;69(3):573–580. doi: 10.1083/jcb.69.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumate J. B., Brooke M. H., Carroll J. E., Davis J. E. Increased serum creatine kinase after exercise: a sex-linked phenomenon. Neurology. 1979 Jun;29(6):902–904. doi: 10.1212/wnl.29.6.902. [DOI] [PubMed] [Google Scholar]
- Stauber W. T., Schottelius B. A. Enzyme activities and distributions following denervation of anterior and posterior latissimus dorsi muscles. Exp Neurol. 1975 Sep;48(3 Pt 1):524–533. doi: 10.1016/0014-4886(75)90010-2. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Macino G., Sebald W. Mitochondrial genes and translation products. Annu Rev Biochem. 1979;48:419–441. doi: 10.1146/annurev.bi.48.070179.002223. [DOI] [PubMed] [Google Scholar]
- Venable J. H. Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat. 1966 Sep;119(2):271–301. doi: 10.1002/aja.1001190206. [DOI] [PubMed] [Google Scholar]


