Abstract
Background:
Androgenetic Alopecia (AGA) refers to the appearance of common non-scarring progressive loss of terminal hair. Trichoscopy shows a magnified view of the hair shafts and hair follicle openings and helps us to diagnose, prognosticate and determine the disease severity.
Aims:
The aim of the study was to identify the trichoscopic findings in patients with androgenetic alopecia and associate these findings with disease severity.
Methods:
This was a cross-sectional analytical study of both male and female patients with AGA. The Hamilton Norwood staging was used in males and Ludwig’s staging was used in females. The NC 2 Heine Dermoscope was used to assess the scalp and a 64 MP rear camera was used for the photographic assessment. A Chi-square test was performed to find out the association between the stage of alopecia and trichoscopic findings.
Results:
A total of 63 patients were assessed in this study. Trichoscopic examination showed Hair Shaft Heterogeneity (HSTH) in all patients of AGA. Scalp honeycomb pigmentation and brown peripilar signs were seen in more than half of the patients. The white peripilar sign was associated with advanced stages in both male and female patients, (P = 0.015) and (P = 0.038) respectively. Focal atrichia was also associated with advanced stages in both male and female patients, (P = 0.001) and (P = 0.05) respectively.
Conclusion:
HSTH was the characteristic trichoscopic feature of AGA. White peripilar sign and focal atrichia correlated with the severity of the disease. Trichoscopy-based assessment can be used to individualise the treatment options and counsel the patients regarding prognosis.
KEY WORDS: Androgenetic alopecia, correlation, trichoscopy
Introduction
Hair loss (Alopecia) affects women and men of all ages and it often significantly affects the social and psychological wellbeing of the person. Androgenetic alopecia (AGA) is the most common type of progressive hair loss in men and women.[1] The condition is characterised by the progressive loss of terminal hairs on the scalp in a characteristic distribution. A combination of factors including genetic predisposition and the effect of androgens cause progressive miniaturisation of the hair follicles. Irrespective of the age, gender or stage of baldness, AGA can have an immense negative psychological impact on the quality of life of the affected persons. As lost hair will not be recovered in AGA, an early diagnosis could ensure that treatment can be started as early as possible.[2] Dermoscopy of hair and scalp is known as trichoscopy. Trichoscopy shows a magnified view of the hair shafts, hair follicle openings, the perifollicular epidermis, and blood vessels at a 10- to 100-fold magnification.[3] It is particularly used for the diagnosis, prognosis, determining severity and follow-up of various hair disorders. Trichoscopy can help in early diagnosis, staging and assess the severity of AGA which in turn could help to plan treatment and monitor the response to treatment.
Though there are a number of studies on trichoscopy in global literature, reports from India are few. These studies have shown a positive association of trichoscopic findings in androgenetic alopecia with the severity of the disease.[3,4,5] However, the trichoscopic findings associated with disease severity vary between the studies. Hence, this study was performed with a primary objective of identifying the trichoscopic findings in adults with androgenetic alopecia. The secondary objective was to study the association of the trichoscopic findings with disease severity.
Materials and Methods
A cross-sectional analytical study was conducted after getting clearance from the Institutional Research and Ethics committees (IEC Number - RC/2021/33). The study was conducted over a period of 1.5 years from January 2021 to November 2022. All consecutive patients over the age of 18 years presenting to the Dermatology OPD in our tertiary care center with androgenetic alopecia were screened for the study. The diagnosis of AGA was made based on clinical assessment. Patients who refused consent were excluded. The estimated sample size was 63, calculated by using the prevalence of 91.2% according to the study conducted by Ummiti et al.[3] with a precision 7% and a confidence level of 95%.
The patients were subjected to trichoscopic examination using the NC 2 Heine Dermoscope. A 64 MP rear camera was used for the photographic assessment of the patient’s scalp at the time of diagnosis. In males, sites with hair loss including vertex, frontal and temporal hairline, occipital area, were examined. In female patients, the frontal, temporal, crown areas and occipital area were observed. The stage of alopecia was evaluated using the Hamilton- Norwood scale in males and Ludwig’s scale in females.
The following trichoscopic findings were documented in each patient.
Hair shaft thickness heterogeneity (HSTH): >20% in Male AGA (MAGA) and >10% in Female AGA (FAGA).
Miniaturisation of hair.
Brown peripilar sign (BPPS): Brown halo around the emergent hair shaft.
White peripilar sign (WPPS): White halo at the follicular ostium.
White dots: Empty follicular ostia.
Yellow dots: Round or polycyclic best seen under polarised light.
Focal atrichia: Areas of total hair loss on the scalp, usually in the size of a pencil eraser.
Scalp honeycomb pigmentation (SHCP): Which corresponded to melanotic rete ridges.
Data was analysed using SPSS software version 21.0. Descriptive statistics were summarised using mean and standard deviation for continuous variables or median with range. The categorical variables were summarised using percentages and proportions. A Chi-square test was performed to find out the association between the stage of alopecia and trichoscopic findings. P value less than 0.05% was considered to be statistically significant.
Results
The 63 patients in the study included 51 males and 12 females. The majority of the men were in the age group of 18–30 years and women were in the age group of 31–45 years. The mean age of the study population was 31 (±8.9) years. A total of 29 patients in total had hair loss for more than 2 years. The mean duration of hair loss in our study was 32.8 months (±16.7). A total of 64% of the patients had a family history of AGA. Among the males, the most common stage of alopecia was stage 3 which constituted 20 patients (41.1%) which was followed by stage 4 with 12 patients (21.56%) [Figure 1]. The most common stage in females was stage I-2 with six patients (50%) followed by two patients each in stage I-3, I-4 and II-2 [Figure 2].
Figure 1.
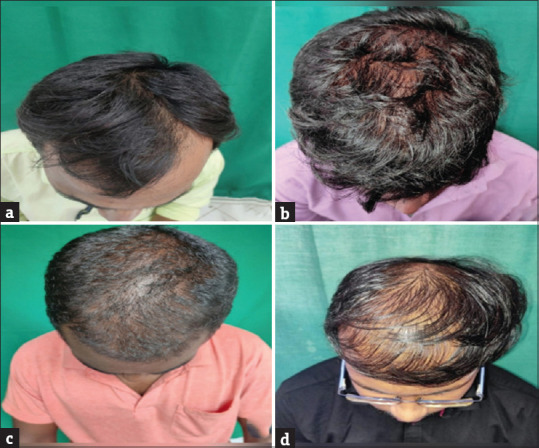
Clinical images of male patients with AGA (Hamilton Norwood staging)- (a)-Stage 2; (b)-Stage 3; (c)-Stage 4; (d)-Stage 5
Figure 2.
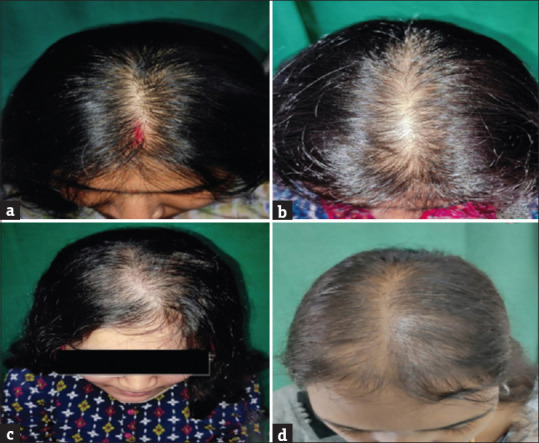
Clinical images of female patients with AGA (Ludwig’s staging)- (a)-Stage I-2; (b)-Stage I-3; (c)-Stage I-4; (d)-Stage II-2
Trichoscopic findings
Tables 1 and 2 show the distribution of trichoscopic findings among the male and female patients respectively. Figure 3 shows some of the trichoscopic images.
Table 1.
Trichoscopy findings in male patients with AGA
| The number of patients in each stage | Stage 2 n=8 | Stage 3 n=20 | Stage 4 n=12 | Stage 5 n=7 | Stage 6 n=4 |
|---|---|---|---|---|---|
| Brown peripilar sign | 4 | 14 | 6 | 1 | 1 |
| White peripilar sign | 3 | 10 | 11 | 6 | 4 |
| Yellow dots | 2 | 8 | 5 | 4 | 3 |
| White dots | 8 | 19 | 12 | 7 | 4 |
| Focal atrichia | 1 | 0 | 4 | 6 | 3 |
| Scalp honeycomb pigmentation | 6 | 17 | 12 | 7 | 4 |
Table 2.
Trichoscopic findings in female patients with AGA
| The number of patients in each stage | Stage I-2 n=6 | Stage I-3 n=2 | Stage I-4 n=2 | Stage II-2 n=2 |
|---|---|---|---|---|
| Brown peripilar sign | 4 | 1 | 1 | 0 |
| White peripilar sign | 1 | 2 | 2 | 2 |
| Yellow dots | 4 | 1 | 1 | 2 |
| White dots | 6 | 2 | 2 | 2 |
| Focal atrichia | 0 | 0 | 1 | 2 |
| Scalp honeycomb pigmentation | 2 | 2 | 2 | 2 |
Figure 3.
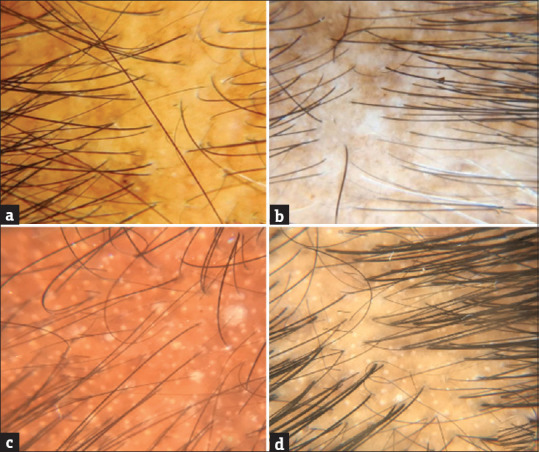
Various trichospoic findings of AGA - (a)-Brown Peripilar sign; (b)-Focal atrichia; (c)-White Peripilar sign; (d)-White dots
Hair shaft heterogeneity (HSTH) was seen in all the patients (100%). The brown peripilar sign among males was noticed in 26 patients (50.98%), more commonly in stage 3 constituting 14 patients followed by 6 in stage 4. Six females (50%) had brown peripilar signs. Out of this 4 belonged to stage I-2. In males, the white peripilar sign was seen in 34 patients (66.66%). Nearly all the patients with stage 4, stage 5 and stage 6 had white peripilar signs. Fisher’s exact test was found to be significant (P = 0.015). The white peripilar sign was found among 7 out of the 12 females (58.33%), among which only one was in stages 1–2.
Almost all the males presented with white dots; 50 out of 51 patients (98.03%) and all 12 female patients had white dots. The yellow dots were seen in 22 out of 51 (43.13%) male patients. It was seen in greater proportion in patients having stage 3 and above disease. Among females, 8 of the 12 females (67%) had yellow dots. The distribution was relatively uniform across the stages. Focal atrichia was seen in 14 of the 51 male patients (27.45%). It was commonly seen in patients with stage 4 and 5 diseases. Focal atrichia was found only in 3 females (25%) with one in stage I-4 and 2 patients in stage II-2. Nearly 90% of the male patients had SHCP, with a maximum number of patients in stage 3 followed by stage 4. Among females, 66.6% had honeycomb pigmentation which was distributed uniformly across all stages.
The white peripilar sign was associated with advanced stages in both male and female patients with a P value of (P = 0.015) and (P = 0.038) respectively. Similarly, focal atrichia was also associated with advanced stages in both male and female patients with a P value of (P = 0.001) and (P = 0.05), respectively.
Discussion
From a demographic point of view, the mean age of the male patients in our study was similar to the mean of the male patients in the study by Ummiti et al.,[3] whereas our female patients had a younger age of onset. The mean duration of hair loss in our study was 32.8 months and this was shorter when compared to the other studies from India where Shah KB et al.,[6] and Ummiti et al.[3] have reported the mean duration of hair loss of 54.54 months and 48 (±3.5) months respectively. Our patients presented earlier when compared to the other two studies. The percentage of family history positivity in our study (64%) was similar to the observations found in the studies conducted by Paik et al.[7] and Shah KB et al.[6] Patients with a positive family history tend to have a higher stage of AGA when compared to those without a positive family history, but this was not statistically significant. A follow-up of these patients would help to identify if patients with a positive family history finally develop a higher stage of AGA.
In this study, the prevalence of AGA is more common in males than in females and the most common stage in males was stage 3 and in females, it was stage I-2. Table 3 gives a comparison of the trichoscopic findings among studies conducted across the world.
Table 3.
Comparison of Trichoscopic findings among various studies
| Study | Yellow dots | White dots | White peri pilar sign | Brown peri pilar sign | Honeycomb pigmentation | Hair shaft thickness heterogeneity | Focal Atrichia |
|---|---|---|---|---|---|---|---|
| MALE AGA | |||||||
| Ummiti A et al.[3] | 92.5% | - | 60.6% | 90% | 88% | 100% | 21.2% |
| Hu R et al.[4] | 20% | 21.3% | 20% | 44% | 33% | 100% | 28% |
| Kibar M et al.[8] | 25% | 28.6% | 14.3% | 46% | 25.4% | 100% | - |
| Inui S et al.[9] | 26% | - | - | 66% | - | 100% | - |
| Presentstudy | 43.1% | 98% | 66.6% | 50.9% | 90.1% | 100% | 27.4% |
| FEMALE AGA | |||||||
| Ummiti A et al.[3] | 88% | - | 68% | 40% | 80% | 96% | 24% |
| Hu R et al.[4] | 24% | 22% | 15% | 44.5% | 30.5% | 100% | 56.5% |
| Ramos LD et al.[2] | 3% | - | - | 64.7% | 41% | 100% | - |
| Inui S et al.[9] | 10% | - | - | 20% | - | 100% | - |
| Present study | 66.6% | 100% | 58.3% | 50% | 66.6% | 100% | 25% |
The most common trichoscopic finding was HSTH which corresponds to vellus hair transformation and is a hallmark feature of AGA. This finding was observed in all the patients. This aligns with the findings of Hu et al.,[4] Kibar et al.[8] and Inui et al.,[9] where 100% of both male and female patients exhibited HSTH. Ummiti et al.[3] also reported 100% prevalence in males and 96% in females. BPPS which histopathologically corresponds to superficial perivascular and interstitial infiltrate of lymphocytes is observed in equal proportions in both male and female patients in our study, in contrast to the findings reported by Inui et al.,[9] and Ummiti et al.,[3] where female patients exhibited a lower incidence of BPPS. Yellow dots which represent the distention of the affected follicular infundibulum with keratinous material and sebum, were more prevalent in females and there was no correlation with the stage of the disease. This difference in the frequency of yellow dot findings may be attributed to variations in genetic predisposition. For instance, in a study by Hu et al.,[4] only 20% of advanced-stage AGA patients exhibited yellow dots. In contrast, the study conducted by Ummiti et al.[3] in India reported a higher prevalence of yellow dots. The higher prevalence of SHCP noted in our study could be attributed to increased sun exposure on the bald scalp.
White peripilar sign corresponds to the affected follicles with the surrounding zone of lamellar perifollicular fibrosis and focal atrichia represents areas of total hair loss on the scalp commonly in the size of a pencil eraser. These findings are consistent with the observations of Hu et al. and Ummiti et al., among all trichoscopic findings, the white peripilar sign and focal atrichia were significantly more prevalent in patients with severe disease. Consequently, the presence of these features in a patient may suggest a reduced likelihood of response to medical treatment. Therefore, surgical intervention might be deemed necessary, as these indicators serve as poor prognostic factors.
The other findings did not have a statistically significant association with the progression of the disease. Their presence, as well as the absence of the characteristic trichoscopic findings of other types of alopecia, are of diagnostic value, helping us to rule out other differential diagnoses.
This study is one of the few studies in India, which has documented the various trichoscopic findings in patients with AGA and associated them with the disease severity.
The trichoscopic findings could help us to tailor the treatment options for individual patients. Certain trichoscopic findings could be used to counsel the patients regarding prognosis. A further step would be to study the changes in the trichoscopic findings with the treatment and correlate them with clinical response to the treatment. The limitation of the present study is that trichoscopic findings were assessed only during the first visit to OPD. If the patients had been further followed up after starting the treatment, it would have enabled us to see if the findings that were previously noted still persisted or regressed. Also, the response to treatment like new hair growth and reversal of HSTH could be assessed.
Conclusion
To conclude, trichoscopy is a non-invasive tool that can be used as an adjunct to the clinical diagnosis of androgenetic alopecia. HSTH is the characteristic trichoscopic feature that is found in all patients with androgenetic alopecia. SHCP was more commonly seen in the advancing stages of alopecia. White peripilar signs and focal atrichia correlate with the severity of the disease. Trichoscopy-based assessment can be used to individualise the treatment options and counsel the patients regarding prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We sincerely thank all the faculty of our department and the residents who have contributed to the manuscript.
References
- 1.Springer K, Brown M, Stulberg DL. Common hair loss disorders. Am Fam Physician. 2003;68:93–102. [PubMed] [Google Scholar]
- 2.Ramos LD, Santili MCN, Bezerra FC, Ruiz M de FMA, Petri V, Patriarca MT. Dermoscopic findings in female androgenetic alopecia. An Bras Dermatol. 2012;87:691–4. doi: 10.1590/s0365-05962012000500003. [DOI] [PubMed] [Google Scholar]
- 3.Ummiti A, Priya PS, Chandravathi PL, Kumar ChS. Correlation of trichoscopic findings in androgenetic alopecia and the disease severity. Int J Trichol. 2019;11:118–22. doi: 10.4103/ijt.ijt_103_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu R, Xu F, Han Y, Sheng Y, Qi S, Miao Y, et al. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol. 2015;42:602–7. doi: 10.1111/1346-8138.12857. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Caulloo S, Zhao Y, Zhang B, Cai Z, Yang J. Female pattern hair loss: Clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichol. 2012;4:23–8. doi: 10.4103/0974-7753.96082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah KB, Shah AN, Solanki RB, Raval RC. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology. 2017;9:14–8. doi: 10.4103/ijt.ijt_75_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–9. doi: 10.1046/j.1365-2133.2001.04289.x. [DOI] [PubMed] [Google Scholar]
- 8.Kibar M, Aktan S, Bilgin M. Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann Dermatol. 2014;26:478–84. doi: 10.5021/ad.2014.26.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36:82–5. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]


