Abstract
Post-kala-azar dermal leishmaniasis (PKDL) is a neglected skin disease that has tremendous epidemiological significance as a reservoir of Leishmania parasites. Relapse, drug resistance, non-compliance to prolonged treatment, poor health-seeking behaviour, along with limited therapeutic options pose a significant impact on the management of PKDL. In this study, we aimed to review the efficacy, safety and tolerability data of combination therapies for PKDL in the published literature. We have also described patients’ compliance with treatment and associated co-infections in PKDL. A comprehensive literature search was conducted in PubMed, Scopus and Google Scholar to identify the relevant articles. A total of nine studies were eligible for inclusion in this review. Drug combinations used in India were miltefosine-liposomal amphotericin-B, miltefosine-paromomycin, miltefosine-amphotericin-B, sodium stibogluconate (SSG)-immunotherapy and SSG-rifampicin. However, in Sudan, except one, all studies have used SSG-based combinations viz. SSG-rifampicin, SSG-paromomycin and SSG-immunotherapy. The efficacy and safety of miltefosine in combination with liposomal amphotericin-B as well as conventional amphotericin-B were found to be excellent in a limited number of patients. These combinations are said to have better patient compliance and shorter treatment duration. Another combination of miltefosine and paromomycin was found to be satisfactory with a final cure rate of 83.3%. SSG in combination with paromomycin had a good clinical outcome among severe PKDL patients in Sudan, though pain at the injection site was experienced by all patients. There is a lack of data on combination therapies for PKDL through large-scale randomised controlled trials (RCTs). Therefore, multicentric randomized controlled trials with a sufficiently large sample size are urgently needed to verify the efficacy, safety, and other advantages of combination therapies for PKDL. With the availability of liposomal amphotericin-B, miltefosine and immunotherapy, clinical management of PKDL appears promising.
KEY WORDS: Combination therapy, compliance, leishmaniasis, PKDL
Introduction
Post-kala-azar dermal leishmaniasis (PKDL) is a dermatosis, caused by the protozoan parasite, Leishmania donovani. Infected female phlebotomine sand flies transmit the disease. Lesions of PKDL appear on the skin as hypopigmented macules, papules, nodules or their combinations all over the body including the face.[1] This disease commonly affects poor people living in rural areas. Earlier visceral leishmaniasis (VL) patients treated with sodium antimony gluconate (SSG) used to manifest lesions of PKDL.[2] Nevertheless, recent studies have documented PKDL occurrences with all available antileishmanial including AmBisome®,[3] miltefosine,[4] paromomycin[5] and miltefosine-paromomycin combination.[6] Besides, people with no history of VL have developed PKDL.[7] India and Sudan, where L. donovani remains the predominant leishmanial species, render the highest burden of PKDL in the world. Only a few chemotherapeutic drugs are approved for the treatment of PKDL amidst a growing threat of widespread drug resistance that in turn is limiting the treatment options.
In East Africa, PKDL is generally self-limiting and only severe cases need treatment. The standard treatment regimen for PKDL in East Africa is SSG and an alternative option is liposomal amphotericin-B.[1,8] In India, miltefosine remains the sole oral, first-line drug for the treatment of PKDL. It is used at a daily dose of 2.5mg/kg body weight in children and 100mg in adults for 12 weeks.[9] The efficacy of miltefosine was found as 85% in the treatment of PKDL.[9] However, the efficacy of miltefosine is gradually decreasing over time.[9] A major adverse effect associated with this therapy is gastrointestinal disturbances that limit its widespread acceptability.[10] Conventional amphotericin-B is another treatment option i.e. effective at a dose of 1 mg/kg for 60-80 infusions but, nephrotoxicity remains a major concern.[11] Besides, long treatment duration increases the risk of treatmentnon-compliance. AmBisome® is a liposomal formulation of conventional amphotericin-B shown to be safe and effective in the treatment of PKDL.[12] AmBisome® has added advantage over conventional formulations in terms of nephrotoxicity. A recent study from Bangladesh, reported a complete cure rate of 78% with AmBisome® when administered as a total dose of 15mg/kg divided into 3mg/kg biweekly for 3 weeks.[12] No serious toxicity was reported. Paromomycin is an aminoglycoside antibiotic tried for the treatment of PKDL but did not show a satisfactory response (cure rate 62.5%).[13] SSG was the main drug used for the treatment of leishmaniasis since 1920. The most common side effects of SSG include cardiac arrhythmias and severe pancreatitis, which can be fatal in some situations.[14,15] Because of several toxicities and longer treatment duration, patients face difficulty in tolerating and complying with this treatment.[16] Over the last decade, the use of SSG in the Indian subcontinent has largely decreased because of widespread resistance.[17] Remarkably, the antibacterial drug rifampicin was found effective in leishmaniasis.[18] The reported cure rate ranged from 0% to 80% when rifampicin was used as monotherapy at doses ranging from 600 to 1200 mg/day.[18] Reports of the effectiveness of rifampicin in the treatment of PKDL are rare or incomplete.
Combination therapies in the treatment of different disorders have been widely accepted for many years owing to the enhancement of efficacy. Besides, combination therapy may cover a broader antiparasitic spectrum, elicit synergistic effects and delay the risk of drug resistance that may arise because of selection pressure. It also shortens the duration of treatment; thereby, it may reduce the cost and improve compliance.[19] These attributes of combination therapies may eventually improve treatment outcomes. Combination therapy has been playing a significant role in the treatment of HIV,[20] malaria[21] and tuberculosis.[22] It has been increasingly approved for the treatment of infectious and life-threatening diseases. The use of combination therapy in the treatment of PKDL is advocated by many researchers.[9,23,24] To the best of our knowledge, no exclusive review is available on combination therapies in PKDL. Therefore, we aimed to summarise the available evidence pertaining to the efficacy and safety of combination therapies used in PKDL. This article also describes treatment compliance and co-infection associated with PKDL.
Methodology
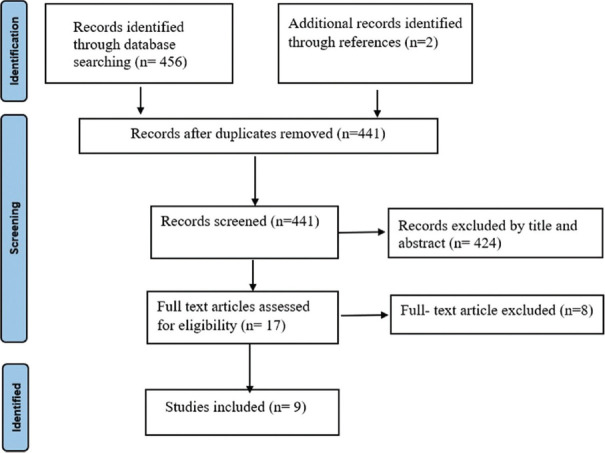
We searched over various platforms such as PubMed, Scopus and Google Scholar. The following keywords were used in electronic search, either alone or in combination: (‘PKDL’ OR ‘Post Kala-azar Dermal Leishmaniasis’) AND (‘Treatment’ OR ‘Management’ OR ‘Antimony Sodium Gluconate’ OR ‘Sodium Stibogluconte’ OR ‘Meglumine Antimoniate OR ‘Amphotericin-B’ OR ‘AmBisome’ OR ‘Liposomal Amphotericin -B’ OR ‘Paromomycin’ OR ‘Miltefosine’ OR ‘Combination therapy’ OR ‘Compliance’ OR ‘Co-infection’). This search was confined to English-language studies published before December 2021. Clinical trials, cohort studies, case reports and case series that reported treatment outcomes with any combination therapy in PKDL patients and were available in full text were included for abstract screening. Monographs, secondary research, commentaries, review articles and editorials were excluded. We evaluated the abstracts of identified papers to determine whether they met our study inclusion criteria. To find other potentially suitable studies, the reference lists of all selected studies were also reviewed. Data on the following variables were collected: first author, year of publication, study design, sample size, drug combinations, dose, treatment duration, adverse events, initial cure and final cure. The detailed search strategy is shown in Figure 1.
Figure 1.
Flow diagram of search strategy
Mechanism of Action of Commonly Used Antileishmanial Drug
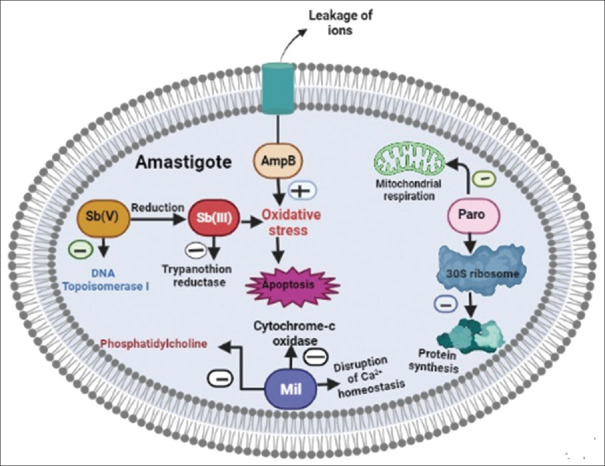
Miltefosine is the first-line drug for the treatment of PKDL in the Indian Subcontinent. The mechanism of antileishmanial action of this drug is not clear, multiple mechanisms have been proposed by different studies. It induces apoptosis-like death in L. donovani promastigotes.[25] Miltefosine inhibits the cytochrome c oxidase in mitochondria thus reducing oxygen consumption and production of ATP in leishmania donovani.[26] This drug also exerts its effect by inhibiting phosphatidylcholine (PC) biosynthesis.[27] Disruption of Ca+2 homeostasis of the parasite was also documented in a recent study.[28] Amphotericin-B is another leishmanicidal drug that acts by binding to ergosterol of the cell membrane and altering the cell membrane permeability. Resulting in, an increase in the leakage of essential ions and small solute molecules followed by cell death. This drug also induces oxidative stress in leishmanial amastigote.[29] The mechanism of the antileishmanial action of sodium stibogluconate is unknown, several mechanisms have been reported, but it is believed to be because of the decrease in available ATP and GTP, which is likely secondary to a blockage of the citric acid cycle and glycolysis.[30] This drug also acts by inhibition of DNA topoisomerase I.[31] Pentavalent antimonial [Sb (V)] converted into more toxic and active Sb (III) and induced apoptosis by oxidative stress, raising intracellular Ca+2.[32,33] Wyllie et al. reported that [Sb (III)] inhibits trypanothione reductase thereby disulfide forms of trypanothione and glutathione accumulate inside the leishmanial amastigote.[34] Paromomycin, an aminoglycoside antibiotic was also found to be effective in leishmaniasis. The clear antileishmanial mechanism of this drug needs to be elucidated. It was proposed that it binds with the ribosomal 30S subunit and inhibits protein synthesis of leishmanial amastigote.[35] Study has also reported that paromomycin inhibits the metabolism and mitochondrial respiration of leishmania.[27] The mechanism of action of commonly used antileishmanial drugs in the treatment of PKDL is presented in Figure 2.
Figure 2.
Mechanism of action of commonly used antileishmanial drugs in PKDL
Studies on Combination Therapy Used in PKDL
Tables 1 and 2 present nine selected studies on PKDL combination therapy including two randomised controlled trials, three cohort studies, two retrospective observational studies, one case report and a case series. Five studies were conducted in India and four in Sudan. Various drug combinations tried in PKDL were miltefosine plus liposomal amphotericin-B,[36] miltefosine plus paromomycin,[37] miltefosine plus amphotericin-B,[38] SSG plus Rifampicin,[39] SSG plus paromomycin,[40,41] SSG plus immunotherapy[42,43] and itraconazole plus terbinafine.[44] The detailed efficacy and safety parameters are presented here.
Table 1.
Characteristics of the included studies
| Author/Ref. | Year | Journal | Country | Study population | Dose/Duration | Age group (year) | Study design |
|---|---|---|---|---|---|---|---|
| Ramesh et al.[36] | 2020 | J. Infect. Dis. | India | LAmB + MIL=16 MIL=16 | 3 injections of LAmB, 5 mg/kg + MIL100 mg/day (45 days) 100mg/day (for 90 days) | 9-60 | Cohort study |
| Pandey et al.[37] | 2017 | Br. J. Dermatol. | India | MF + PM=30 | 2.5mg/kg + 11mg/kg for 30 days | 5-65 | RCT |
| Abongomera et al.[40] | 2016 | PLOS One | Sudan | SSG + PM=77 | 20 mg/kg/day + 11mg/kgfor 17 days | ≥5 | Retrospective observational study |
| Younis et al.[41] | 2015 | Int J Res Med Sci. | Sudan | SSG + PM=9 | 20mg/kg + 11mg/base/kg/day for 30 days | 5-17 | Case series |
| Ramesh et al.[38] | 2014 | Acta DermVenereol | India | AmB + MIL=3 | 50 mg AmB for 20 days + 50 mg oral MIL thrice daily for 40 days | Case1=20 Case2=24 Case3=26 |
Case report |
| Ramesh et al.[42] | 2010 | Indian J Dermatol Venereol Leprol | India | Group I SSG=32 Group II SSG + ALP=10 SSG + RMF=6 SSG + ALP + RMF=5 SSG + MW vaccine=8 | 20 mg/kg/day (total dose 1 gm) 20 mg/kg/day (total dose 1 gm) + 800 mg/day 20 mg/kg/day (total dose 1 gm) + 15 mg/kg varied from 750-900 mg/day. 20 mg/kg/day (total dose 1 gm) + 800 mg/day + 15 mg/kg varied from 750-900 mg/day. 20 mg/kg/day (total dose 1 gm) + 0.1 ml | 18-65 | Retrospective study observational study |
| Musa et al.[43] | 2008 | Trans R Soc Trop Med Hyg | Sudan | ALM + BCG + SSG=15 Placebo + SSG=15(control) | 100 mcg + one-tenth of the 0.1 ml of dose used in TB for 4 weekly doses + 20 mg/kg/day for 40 days 20mg/kg for 40 days | 7-60 | RCT |
| Sharma et al.[39] | 2007 | Indian J Dermatol VenereolLeprol | India | SSG + RMF=8 | 10mg/kg/day + 900mg/daily for 120 days | 18-39 | Cohort study |
| Khalil et al.[44] | 1996 | Trans R Soc Trop Med Hyg | Sudan | TRF + ITZ=9 | 250 mg/day + 200 mg/day for 4 weeks | >18 | Cohort study |
MIL-miltefosine, PM-paramomycin, TRF-terbinafine, ITZ-itraconazole, ALM-alum-precipitatedautoclaved Leishmaniamajorvaccine, BCG-Bacille Calmette-Guerin, SSG-sodium stibogluconate, RMF-rifampicin, LAmB-liposomal amphotericin-B, AmB-amphotericin-B, ALP- Allopurinol, RCT-Randomized controlled trial
Table 2.
Treatment outcome of combination regimen
| Author/Ref | Initial cure (at the end of treatment) | Final cure | Lesion type | Adverse events |
|---|---|---|---|---|
| Ramesh et al.[36] | MIL + LAmB initial cure rate NR 16 (100%) cured in MLF group | 16 (100%) [18 months follow up] 12 (75%) cured and 4 (25%) relapsed [18 months follow up] | 4 macular, 5 papular, 7 nodular. 6 macular, 6 papular, 4 nodular. | Moderate anorexia in all cases, vomiting in some cases. and vertigo in 6 patients. |
| Pandey et al.[37] | 100% | Final cure rate was 83.33% &16.67%relapsed. (1 year follow up) | Macular (46.7%), Papular (6.7%), Nodular (10%), Maculopapular (26.7%) Polymorphic (10%) | Pain at injection site, increased hepatic enzymes, eosinophilia and vomiting, hypokalaemia, gastritis, swelling of legs. |
| Abongomera et al.[40] | 75 cured 2 relapsed | 75 cured 2 relapsed | Grade 3 or grade 2 in all patients | Pain at injection site. |
| Younis et al.[41] | 8 cured 1 relapsed | Follow up period was varied 3 cured [10 months follow up] 1cured [8 months follow up] 1cured [9 months follow up] 1cured [6 months follow up] 3 cured [>12 months follow up] 1 relapsed | Grade 2:1 (5/9 cases) with 2 cases of grade 1:1, one case of grade 1:2, and one had grade 3:3. | Pain at injection site experienced by all patients. |
| Ramesh et al.[38] | all 3 cases (100%) | 100% [2 years follow up] | Case 1- Mix of macular & nodular Case 2- Nodular Case 3- polymorphic | Vomiting |
| Ramesh et al.[42] | Group I 5/32 SSG Group II 3/10 SSG + ALP within 110 days 2/6 SSG + RMF 0/5 SSG + RMF + ALP 2/8 SSG + MW vaccine | 5 (Group I) [follow up 1-2 year] 4/7 (Group II) [follow up 1-2 year] | All the patients had mixed or polymorphic lesions | Postural hypotension, body aches, giddiness, metallic taste, loss of appetite, severe joint pain, vomiting and febrile illness |
| Musa et al.[43] | 13 healed completely and 2 shows improvement within 60 days - ALM + BCG + SSG group 8 healed completely and 1 shows improvement within 60 days- SSG + placebo group | 11 cured [90 days follow up] 9 cured [90 days follow up] | The majority of PKDL was 2:2 grade. | Injection site indurations and ulcers, myalgia. |
| Sharma et al.[39] | 5 cured 5 lost to follow up | 5 cured [mean follow up 1 year] | Maculopapular in all patients and larger nodules in 4 patients. | Myalgia, thrombophlebitis Headache, mild leukopenia, and elevated hepatic enzymes |
| Khalil et al.[44] | 1 patient showed a good clinical response after 2-3 weeks. Other 8 patients relapsed | NR | 4 maculopapular 4 papular 1 papulonodular | Mild albuminuria in one patient |
MIL-miltefosine, PM-paramomycin, TRF-terbinafine, ITZ-itraconazole, ALM-alum-precipitated autoclaved Leishmania major vaccine, BCG-Bacille Calmette-Guerin, SSG-sodiumstibogluconate, RMF-rifampicin, LAmB-liposomal amphotericin-B, AmB-amphotericin-B, ALP-Allopurinol
Miltefosine based combination
Miltefosine-Paromomycin (MF-PM)
Pandey et al. assessed miltefosine plus paromomycin combination in 30 PKDL patients.[37] In that, patients of group-A received a combination of paromomycin injection, daily (10 days) at a dose of 11 mg/kg plus miltefosine capsule orally at 2.5 mg/kg/day (10 days) for two courses at a gap of 15 days; group-B received a combination of paromomycin injection plus miltefosine capsules in the same dose for three courses. Patients of group-A did not improve after completion of two courses hence, all group-A patients were treated with one more course of miltefosine at 2.5mg/kg plus paromomycin 11mg/kg for 10 days. The Initial cure rate after completion of therapy was 100%. The final cure rate was 83.3% and 16.67% of cases relapsed at 12 months of follow-up. A total of 63 adverse events (AEs) were reported in 26 patients. The most common AEs were pain at the injection site followed by increased hepatic enzymes level, eosinophilia, hypokalemia, vomiting, gastritis and swelling of the legs andface. This study also reported an excellent compliance rate as none of the patients were lost to follow-up. The recommended dose of miltefosine is 2.5 mg/kg for 12 weeks (84 days) in PKDL but in combination with paromomycin, it was given for a period of 60 days (approximately 9 weeks) including the interval between each course. This indicates that combination therapy reduces the treatment duration and shortens the period of hospitalization besides being cheaper. MF-PM combination therapy showed excellent efficacy and safety in visceral leishmaniasis with cure rate of 98.7%.[45]
Miltefosine-Amphotericin-B (MIL-AmB)
In a preliminary study, Ramesh et al. treated two PKDL cases with MIL-AmB.[38] The first patient was given a dose of 50 mg MIL thrice daily for 40 days in combination with intravenous infusions of 50 mg amphotericin-B daily for 20 days. However, in the second case, the dose of MIL was reduced to twice daily for 60 days to avoid vomiting. Apart from these two cases, he also treated another PKDL patient with a daily IV infusion of amphotericin-B alone at a dose of 50mg/day, resulting in a total dose of 4.5 g, over 100 days. All three cases were cured, and no sign of relapse was observed at 2 years of follow-up. This indicates that the dose and treatment duration could be significantly reduced when the two drugs are given in combination. When used alone, the risk of nephrotoxicity due to AmB,[11] and the gastrointestinal side-effects of MIL increases drastically.[10] The authors concluded that the MIL AmB combination[38] is the preferred regimen for wide-ranging extensive PKDL cases over AmB or MIL alone. An animal study also reported that the combination of MIL-AmB was safe and effective in VL.[46]
Miltefosine-Liposomal amphotericin -B (MIL-LAmB)
In a study by Ramesh et al., 16 PKDL cases were treated with the combination of MIL-LAmB.[36] In which, miltefosine was used at a dose of 100 mg/day for 45 days and LAmBat 5 mg/kg for 3 injections whereas, in another arm, an equal number of patients received treatment with miltefosine monotherapy at a dose of 100 mg/day for 90 days. This study was based on the fact that LAmB and MIL, both are highly effective in PKDL as a monotherapy[12,23] hence, their synergistic effect in combination may augment the cure rate and reduce the rate of relapse. A rapid decline in parasite load resulted in a 100% cure, without any relapse at 18 months of follow-up in the combination group was observed. Whereas, with MIL monotherapy, clinical cure was seen with a gradual decrease in parasite load and 25% relapsed within 18 months of follow-up. None of the patients in combination therapy developed renal toxicity. Apart from two patients who developed vomiting, no other adverse event was reported with the combination therapy. Concurringly, an RCT with the combination of MIL-LAmB was found to be excellent (cure rate of 97.5%) in the treatment of VL.[45]
SSG based combination
It was observed that PKDL does not respond to the curative dose of SSG for VL, which is 10 mg/kg/day for 21 days. In India, SSG is typically administered intramuscularly or slowly intravenously at a daily dose of 20 mg/kg, up to a maximum of 850 mg/day (equivalent to 8.5 mL of SSG or 10 mL of Meglumine antimoniate), for a minimum of 120 days for the treatment of PKDL.[47] In contrast, the optimum recommended duration for Sudanese PKDL is 2 months.
SSG-Rifampicin
Sharma et al., documented the safety and efficacy of combination therapy with SSG 10 mg/kg/day I.V plus oral rifampicin 900 mg/day for 120 days in eight PKDL patients.[39] Only six patients completed the treatment. Five of them responded well at the end of therapy. The response began within a week, and all five patients responded after three weeks of therapy. Except for one patient, all five patients showed excellent response, and no relapse was observed till the one-year follow-up. A total of five patients experienced adverse events such as myalgia, thrombophlebitis, headache, joint pain, leukopenia and increased hepatic enzymes. Despite the longer treatment duration, compliance with the therapy was good. The study concluded that a 120-day course of SSG plus rifampicin is effective and reasonably safe.
SSG-Paromomycin (SSG-PM)
A retrospective study by Abongomera et al. reported the effectiveness of the SSG-PM combination in PKDL.[40] In this combination, SSG was administered intramuscularly at a dose of 20 mg/kg/day (with a minimum daily dose of 200 mg and no maximum dose), while PM was administered intramuscularly at a dose of 15 mg sulfate/kg/day (equivalent to 11 mg/base/kg/day) for a duration of 17 days (with a minimum daily dose of 50 mg and a maximum dose of 1,150 mg). The reported cure rate for the SSG-PM combination was 97%, whereas the cure rate with SSG monotherapy was 90%. The cure rate was not differentiated as initial and final cure in this study. A total of 77 patients’ data were included in which all the patients had either grade-2 or grade-3 lesions. PKDL lesions are graded as grade-1when macular, papular or nodular lesions appear on the face, with or without the involvement of the upper chest and arms. Grade-2 is characterised by the appearance of dense macular, papular or nodular lesions on most of the face and spreading to the chest, back, upper arms and legs, with few rashes on the forearms and legs. Whereas, in grade-3 dense macular, papular or nodular lesions appear on most of the trunk, including hands and feet, mucosa of the lips and palate with the presence of crusting and scaling. It was concluded that the SSG-PM combination had more favourable outcomes in terms of shorter treatment duration, low cost and lower default rate compared with SSG. Pain at the injection site was a common adverse event experienced by all patients. In VL, the efficacy of SSG-PM combination for 17 days was found as 91.4% with a good safety profile.[14] This combination has been recommended for use in the treatment of VL in East Africa.[14] In another study, Younis et al. treated nine PKDL patients with an SSG-PM combination for a period of 30 days.[41] Five patients were cured and one (1/9; 11.1%) relapsed after completion of 30 days of SSG-PM combination therapy. The remaining three patients (3/9; 33.3%) who failed to cure previously with SSG were completely cured after 40 days of combination therapy with SSG-PM. Similar to the study performed by Abongomera et al. this study also reported pain at the site of injection as one of the common AEs among all patients.[40]
SSG-Immunotherapy and antifungal based combination
Response of immunochemotherapy was also found satisfactory for PKDL in Sudan.[43] An interventional study by Musa et al. reported that a combination of alum-precipitated autoclaved Leishmania major (Alum/ALM) vaccine + BCG with SSG is safe and effective for PKDL treatment.[43] Another SSG-based retrospective study was reported by Ramesh et al., in which SSG was combined with allopurinol, rifampicin and the Mycobacterium W vaccine.[42] No major difference was observed with these combinations when compared with SSG alone. An earlier study by Khalil et al. reported that antifungal drugs itraconazole-terbinafine combination failed to heal PKDL lesions.[44]
Only a few studies on combination therapy for PKDL are available in the existing literature. Most of the studies have limitations of inadequate sample size, non-confirmatory diagnostic method, variable study design and follow-up duration. Among the various combinations tried, miltefosine in combination with liposomal amphotericin-B or conventional amphotericin-B in India and SSG-paromomycin in Sudan showed promising results. Hence, these combinations can be considered for future therapeutic options for PKDL.
PKDL Co-infection
PKDL cases act as a reservoir for Leishmania parasites hence, treatment of PKDL cases is of paramount importance for the elimination of kala-azar (VL). Similar to VL co-infections, PKDL was also found to be complicated by leprosy,[48] tuberculosis[49] and HIV co-infection.[50] In addition, para kala-azar dermal leishmaniasis is a new challenge.[51] Currently, there is no comprehensive treatment guideline available for these emerging problems. Hence, management of leishmaniasis co-infection cases is substantially challenging for the kala-azar elimination program. The available data on the antileishmanial drugs do not support their use in VL co-infection. A study from Ethiopia including HIV-VL co-infected patients used miltefosine at a dose of 100mg/day for 100[52] days. The initial parasitological cure was 64% but all relapsed in a short duration.[52] A cure rate of 85% using liposomal amphotericin-B, at a total dose of 20-25mg/kg/day was achieved in HIV-VL co-infected cases.[53] Combination therapy with liposomal amphotericin-B at 30mg/kg divided into 6 equal doses on alternate days plus miltefosine 50mg twice or once daily showed promising results in HIV-VL patients.[54] In this study, eight patients relapsed out of 100 patients discharged after the initial cure.[42] However, apart from a few case reports, no efficacy and safety assessment of the available drugs and/or their combinations for the treatment of PKDL co-infections has been performed. Co-infected patients are reported to have multiple relapses.[52] Besides, repeated exposure to the same drug increases the risk of emergence of drug-resistant parasites. Miltefosine is the first-line therapy for the treatment of PKDL in the Indian subcontinent and is also used to manage PKDL cases having co-infections. Considering the emergence of miltefosine resistance, combination therapies for co-infections should be evaluated to develop its substitute.
Compliance with Therapy
All available monotherapy of PKDL requires a long-term treatment, which may further get prolonged due to PKDL co-infections. Moreover, all therapies are limited by several toxicities that in turn increase the risk of treatment non-compliance. No study has been conducted to exclusively assess the compliance rate and its determinants for the drugs used in the treatment of PKDL. However, a study from Nepal reported an adherence rate of 83% with miltefosine in VL patients; the adherence rate was better among educated and patients having awareness of the side effecs of the drug.[55] Data from a hospital-based retrospective study by Ramesh et al. reported that approximately 15% of PKDL patients did not complete the course of miltefosine treatment.[23] Non-compliance was also observed in a controlled clinical trial, in which four patients did not complete the treatment including one patient who prematurely interrupted the treatment due to nausea and vomiting.[56] These non-compliant patients may have a high risk of emergence of drug resistance and persistent disease transmission. This may hamper the kala-azar elimination efforts. Therefore, a safe, effective and short-duration treatment regimen is required to improve treatment compliance. Combination formulations shorten the treatment duration and reduce its cost that in turn may improve compliance among PKDL patients. Studies have also recommended implementing ‘directly observed treatment’ with miltefosine for PKDL.[56,57] In addition, clinical pharmacists may be used to educate patients regarding PKDL and its treatment to improve compliance.
Conclusion
Combination therapy has the advantage of reduced dose and duration, and hence, better tolerance and compliance as opposed to monotherapy for PKDL. It would also reduce the chance of the emergence of microbial resistance. Miltefosine in combination with liposomal amphotericin-B or conventional amphotericin-B in India and SSG in combination with paromomycin in Sudan showed good treatment response in limited number of patients. Therefore, a large-scale multicenter study of the above-mentioned drug combination at various doses and frequencies are recommended. Simultaneously, the quest for a short, affordable, safe and effective drug that can be used in ambulatory care with minimal patient monitoring is urgently needed for the elimination of kala-azar from Southeast Asia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zijlstra E, Musa A, Khalil E, El Hassan I, El-Hassan A. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 2.Saha S, Mondal S, Ravindran R, Bhowmick S, Modak D, Mallick S, et al. IL-10-and TGF-?-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: The significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol. 2007;179:5592–5603. doi: 10.4049/jimmunol.179.8.5592. [DOI] [PubMed] [Google Scholar]
- 3.Burza S, Sinha PK, Mahajan R, Sanz MG, Lima MA, Mitra G, et al. Post Kala-Azar dermal leishmaniasis following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) for primary visceral leishmaniasis in Bihar, India. PLoS Negl Trop Dis. 2014;8:e2611. doi: 10.1371/journal.pntd.0002611. doi: 10.1371/journal.pntd.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das V, Pandey K, Verma N, Lal CS, Bimal S, Topno RK, et al. Development of post-kala-azar dermal leishmaniasis (PKDL) in miltefosine-treated visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:336–8. [PubMed] [Google Scholar]
- 5.Pandey K, Das V, Singh D, Das S, Lal C, Verma N, et al. Post-kala-azar dermal leishmaniasis in a patient treated with injectable paromomycin for visceral leishmaniasis in India. J Clin Microbiol. 2012;50:1478–9. doi: 10.1128/JCM.05966-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey K, Goyal V, Das V, Verma N, Rijal S. PKDL development after combination treatment with miltefosine and paromomycin in a case of visceral leishmaniasis: First ever case report. J Med Microbiol Immunol Res. 2018;2:1–3. [Google Scholar]
- 7.Das VNR, Ranjan A, Pandey K, Singh D, Verma N, Das S, et al. Clinical epidemiologic profile of a cohort of post–kala-azar dermal leishmaniasis patients in Bihar, India. The American journal of tropical medicine and hygiene. 2012;86:959. doi: 10.4269/ajtmh.2012.11-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zijlstra EE. The immunology of post-kala-azar dermal leishmaniasis (PKDL) Parasites Vectors. 2016;9:1–9. doi: 10.1186/s13071-016-1721-0. doi: 10.1186/s13071-016-1721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh V, Singh R, Avishek K, Verma A, Deep DK, Verma S, et al. Decline in clinical efficacy of oral miltefosine in treatment of post kala-azar dermal leishmaniasis (PKDL) in India. PLoS Negl Trop Dis. 2015;9:e0004093. doi: 10.1371/journal.pntd.0004093. doi: 10.1371/journal.pntd.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Das NK, Mukherjee S, Mukhopadhyay D, Barbhuiya JN, Hazra A, et al. Inadequacy of 12-week miltefosine treatment for Indian post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 2015;93:767–9. doi: 10.4269/ajtmh.14-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabi Das VN, Siddiqui NA, Pal B, Lal CS, Verma N, Kumar A, et al. To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post kala-azar dermal leishmaniasis (PKDL) PLoS One. 2017;12:e0174497. doi: 10.1371/journal.pone.0174497. doi: 10.1371/journal.pone.0174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Boer M, Das AK, Akhter F, Burza S, Ramesh V, Ahmed B-N, et al. Safety and effectiveness of short-course ambisome in the treatment of post–kala-azar dermal leishmaniasis: A prospective cohort study in Bangladesh. Clin Infect Dis. 2018;67:667–75. doi: 10.1093/cid/ciy172. [DOI] [PubMed] [Google Scholar]
- 13.Sundar S, Singh A, Tiwari A, Shukla S, Chakravarty J, Rai M. Efficacy and safety of paromomycin in treatment of post-kala-azar dermal leishmaniasis. ISRN Parasitol 2014. 2014:548010. doi: 10.1155/2014/548010. doi: 10.1155/2014/548010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, et al. Sodium stibogluconate (SSG) &paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: A randomised controlled trial. PLoS Negl Trop Dis. 2012;6:e1674. doi: 10.1371/journal.pntd.0001674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesur S, Bahar K, Erekul S. Death from cumulative sodium stibogluconate toxicity on Kala-Azar. Clin Microbiol Infect. 2002;8:606. doi: 10.1046/j.1469-0691.2002.00456.x. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury K, Zander D, Kube M, Reinhardt R, Clos J. Identification of a Leishmania infantum gene mediating resistance to 'and SbIII. Int J Parasitol. 2008;38:1411–23. doi: 10.1016/j.ijpara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Vanaerschot M, Decuypere S, Downing T, Imamura H, Stark O, De Doncker S, et al. Genetic markers for SSG resistance in Leishmania donovani and SSG treatment failure in visceral leishmaniasis patients of the Indian subcontinent. J Infect Dis. 2012;206:752–5. doi: 10.1093/infdis/jis424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsankov N, Angelova I. Rifampin in dermatology. Clin Dermatol. 2003;21:50–5. doi: 10.1016/s0738-081x(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 19.Stein K, Rosenberg W, Wong J. Cost effectiveness of combination therapy for hepatitis C: A decision analytic model. Gut. 2002;50:253–8. doi: 10.1136/gut.50.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirrone V, Thakkar N, Jacobson JM, Wigdahl B, Krebs FC. Combinatorial approaches to the prevention and treatment of HIV-1 infection. Antimicrob Agents Chemother. 2011;55:1831–42. doi: 10.1128/AAC.00976-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alven S, Aderibigbe B. Combination therapy strategies for the treatment of malaria. Molecules. 2019;24:3601. doi: 10.3390/molecules24193601. doi: 10.3390/molecules24193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerantzas CA, Jacobs WR., Jr Origins of combination therapy for tuberculosis: Lessons for future antimicrobial development and application. mBio. 2017;8:e01586–16. doi: 10.1128/mBio.01586-16. doi: 10.1128/mbio.01586-01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramesh V, Kaushal H, Mishra AK, Singh R, Salotra P. Clinico-epidemiological analysis of Post kala-azar dermal leishmaniasis (PKDL) cases in India over last two decades: A hospital based retrospective study. BMC Public Health. 2015;15:1–8. doi: 10.1186/s12889-015-2424-8. doi: 10.1186/s12889-015-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondal D, Khan MGM. Recent advances in post-kala-azar dermal leishmaniasis. Curr Opin Infect Dis. 2011;24:418–22. doi: 10.1097/QCO.0b013e32834a8ba1. [DOI] [PubMed] [Google Scholar]
- 25.Paris C, Loiseau PM, Bories C, Bréard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004;48:852–9. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luque-Ortega JRn, Rivas L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2007;51:1327–32. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy JS, Wortmann GW, Kirchhoff LV. Drugs for Protozoal Infections Other Than Malaria. 8th ed. Philadelphia, W. B.: Saunders; 2014. pp. 510–8.e3. [Google Scholar]
- 28.Pinto-Martinez AK, Rodriguez-Durán J, Serrano-Martin X, Hernandez-Rodriguez V, Benaim G. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+channel. Antimicrob Agents Chemother. 2018;62:e01614–17. doi: 10.1128/AAC.01614-17. doi: 10.1128/aac.01614-01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamp-freund MT, Ferreira VF, Schreier S. Mechanism of inactivation of the polyene antibiotic amphotericin B evidence for radical formation in the process of autooxidation. J Antibiot. 1985;38:753–7. doi: 10.7164/antibiotics.38.753. [DOI] [PubMed] [Google Scholar]
- 30.Berman JD, Waddell D, Hanson B. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob Agents Chemother. 1985;27:916–20. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty AK, Majumder HK. Mode of action of pentavalent antimonials: Specific inhibition of type I DNA topoisomerase of Leishmaniadonovani. Biochem Biophys Res Commun. 1988;152:605–11. doi: 10.1016/s0006-291x(88)80081-0. [DOI] [PubMed] [Google Scholar]
- 32.Sudhandiran G, Shaha C. Antimonial-induced increase in intracellular Ca2+through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J Biol Chem. 2003;278:25120–32. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee SB, Das M, Sudhandiran G, Shaha C. Increase in cytosolic Ca2+levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovanipromastigotes. J Biol Chem. 2002;277:24717–27. doi: 10.1074/jbc.M201961200. [DOI] [PubMed] [Google Scholar]
- 34.Wyllie S, Cunningham ML, Fairlamb AH. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J Biol Chem. 2004;279:39925–32. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]
- 35.Sundar S, Chakravarty J. Paromomycin in the treatment of leishmaniasis. Expert Opin Investig Drugs. 2008;17:787–94. doi: 10.1517/13543784.17.5.787. [DOI] [PubMed] [Google Scholar]
- 36.Ramesh V, Dixit KK, Sharma N, Singh R, Salotra P. Assessing the efficacy and safety of liposomal amphotericin B and miltefosine in combination for treatment of post kala-azar dermal leishmaniasis. J Infect Dis. 2020;221:608–17. doi: 10.1093/infdis/jiz486. [DOI] [PubMed] [Google Scholar]
- 37.Pandey K, Pal B, Das V, Murti K, Lal C, Verma N, et al. Safety and efficacy of a combination of paromomycin and miltefosine for two vs. three courses in patients with post-kala-azar dermal leishmaniasis: An observational pilot study. Br J Dermatol. 2017;177:557–9. doi: 10.1111/bjd.15119. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh V, Avishek K, Sharma V, Salotra P. Combination therapy with amphotericin-B and miltefosine for post-kala-azar dermal leishmaniasis: A preliminary report. Acta Derm Venereol. 2014;94:242–3. doi: 10.2340/00015555-1582. [DOI] [PubMed] [Google Scholar]
- 39.Sharma V, Prasad H, Sethuraman G, Khaitan B. Combination of sodium stibogluconate and rifampicin in post kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol. 2007;73:53–4. doi: 10.4103/0378-6323.30657. [DOI] [PubMed] [Google Scholar]
- 40.Abongomera C, Gatluak F, Buyze J, Ritmeijer K. A comparison of the effectiveness of sodium stibogluconate monotherapy to sodium stibogluconate and paromomycin combination for the treatment of severe post kala azar dermal leishmaniasis in South Sudan–A retrospective cohort study. PLoS One. 2016;11:e0163047. doi: 10.1371/journal.pone.0163047. doi: 10.1371/journal.pone.0163047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younis B, Mohammed H, Dafalla M, Adam A, Elamin M, Musa A, et al. Cure of post kala-azar dermal leishmaniasis with paromomycin/sodium stibogluconate combination: A proof of concept. Int J Res Med Sci. 2016;3:16–21. [Google Scholar]
- 42.Ramesh V, Kumar J, Kumar D, Salotra P. A retrospective study of intravenous sodium stibogluconate alone and in combinations with allopurinol, rifampicin, and an immunomodulator in the treatment of Indian post-kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol. 2010;76:138–44. doi: 10.4103/0378-6323.60553. [DOI] [PubMed] [Google Scholar]
- 43.Musa AM, Khalil EAG, Mahgoub FAE, Elgawi SHH, Modabber F, Elkadaru AEMY, et al. Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: A novel approach to treatment. Trans R Soc Trop Med Hyg. 2008;102:58–63. doi: 10.1016/j.trstmh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Khalil E, Nur NM, Zijlstra E, El-Hassan A, Davidson R. Failure of a combination of two antifungal drugs, terbinafine plus itraconazole, in Sudanese post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1996;90:187–8. doi: 10.1016/s0035-9203(96)90134-0. [DOI] [PubMed] [Google Scholar]
- 45.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–86. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 46.Seifert K, Croft SL. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother. 2006;50:73–9. doi: 10.1128/AAC.50.1.73-79.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datta A, Podder I, Das A, Sil A, Das NK. Therapeutic modalities in post kala-azar dermal leishmaniasis: A systematic review of the effectiveness and safety of the treatment options. Indian J Dermatol. 2021;66:34–43. doi: 10.4103/ijd.IJD_264_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bansal S, Goel A, Sardana K, Kumar V, Khurana N. Postkala-azar dermal leishmaniasis coexisting with borderline tuberculoid leprosy. Br J Dermatol. 2007;157:811–3. doi: 10.1111/j.1365-2133.2007.08072.x. [DOI] [PubMed] [Google Scholar]
- 49.Das V, Pandey K, Verma N, Bimal S, Lal C, Singh D, et al. Post-kala-azar dermal leishmaniasis (PKDL), HIV and pulmonary tuberculosis. Natl Med J India. 2010;23:88–9. [PubMed] [Google Scholar]
- 50.Zijlstra EE. PKDL and other dermal lesions in HIV co-infected patients with leishmaniasis: Review of clinical presentation in relation to immune responses. PLoS Negl Trop Dis. 2014;8:e3258. doi: 10.1371/journal.pntd.0003258. doi: 10.1371/journal.pntd.0003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar R, Das VNR, Topno RK, Pal B, Imam A, Agrawal K, et al. Para-kala-azar dermal Leishmaniasis cases in Indian subcontinent–A case series. Pathog Global Health. 2016;110:326–9. doi: 10.1080/20477724.2016.1258163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sindermann H, Engel KR, Fischer C, Bommer W, Program MCU. Oral miltefosine for leishmaniasis in immunocompromised patients: Compassionate use in 39 patients with HIV infection. Clin Infect Dis. 2004;39:1520–3. doi: 10.1086/425359. [DOI] [PubMed] [Google Scholar]
- 53.Sinha PK, van Griensven J, Pandey K, Kumar N, Verma N, Mahajan R, et al. Liposomal amphotericin B for visceral leishmaniasis in human immunodeficiency virus-coinfected patients: 2-year treatment outcomes in Bihar, India. Clin Infect Dis. 2011;53:e91–8. doi: 10.1093/cid/cir521. [DOI] [PubMed] [Google Scholar]
- 54.Mahajan R, Das P, Isaakidis P, Sunyoto T, Sagili KD, Lima MA, et al. Combination treatment for visceral leishmaniasis patients coinfected with human immunodeficiency virus in India. Clin Infect Dis. 2015;61:1255–62. doi: 10.1093/cid/civ530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uranw S, Ostyn B, Dorlo TP, Hasker E, Dujardin B, Dujardin JC, et al. Adherence to miltefosine treatment for visceral leishmaniasis under routine conditions in Nepal. Trop Med Int Health. 2013;18:179–87. doi: 10.1111/tmi.12025. [DOI] [PubMed] [Google Scholar]
- 56.Sundar S, Singh A, Chakravarty J, Rai M. Efficacy and safety of miltefosine in treatment of post-kala-azar dermal leishmaniasis. ScientificWorldJournal 2015. 2015:414378. doi: 10.1155/2015/414378. doi: 10.1155/2015/414378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saurabh S, Roy P, Pandey D, Ray D, Tarak S, Pandey R, et al. Changing clinico-epidemiology of post-kala-azar dermal leishmaniasis (PKDL) in India: Results of a survey in four endemic states. J Vector Borne Dis. 2020;57:161–9. doi: 10.4103/0972-9062.310875. [DOI] [PubMed] [Google Scholar]