Abstract
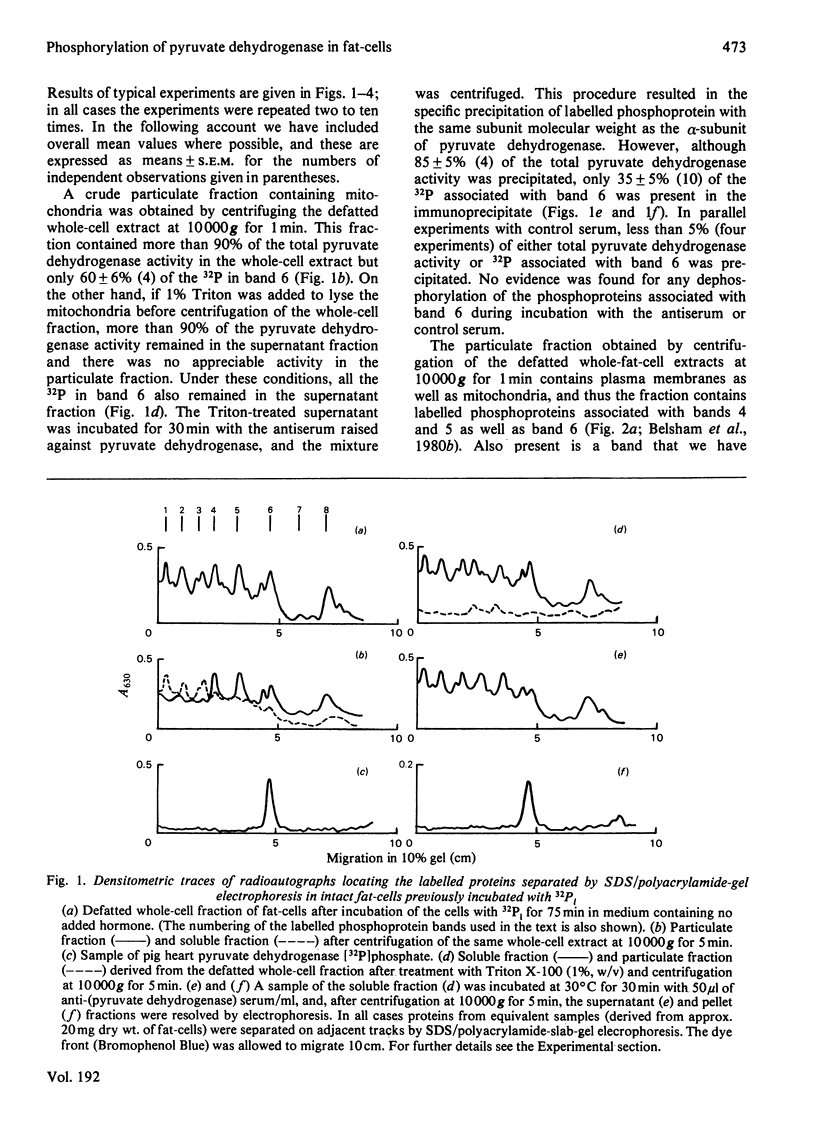
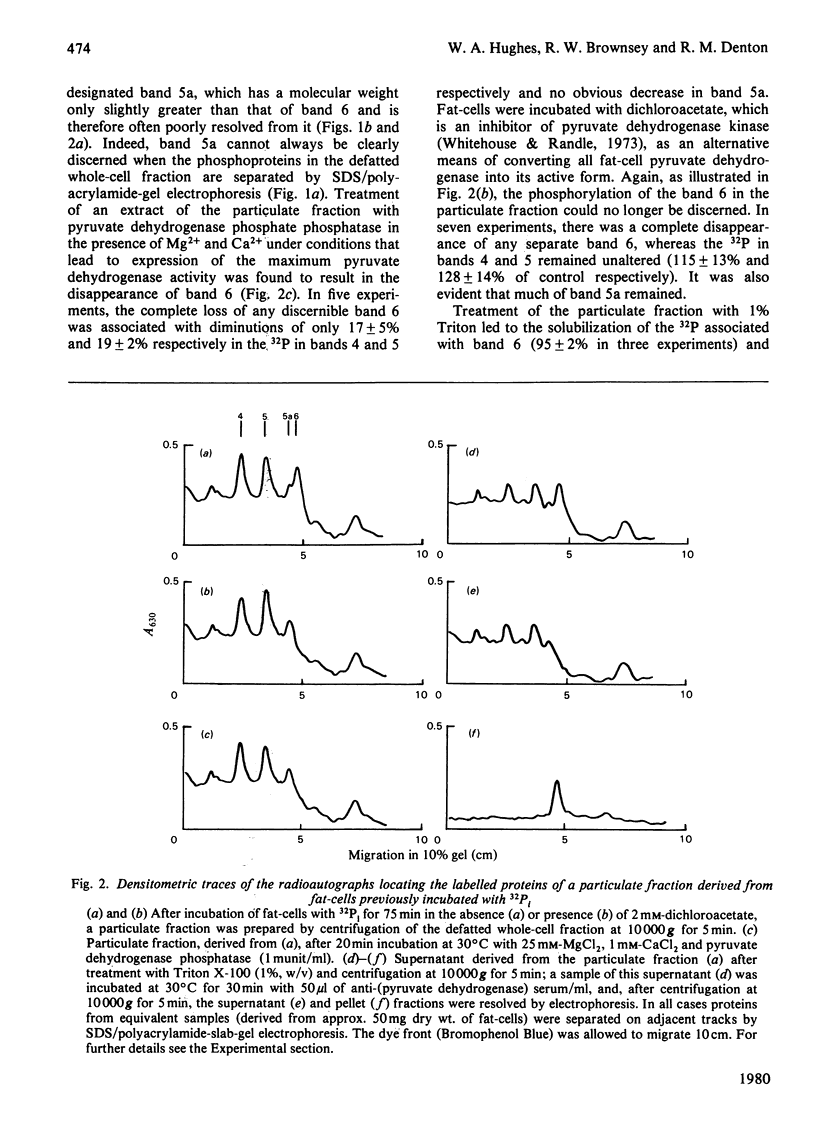
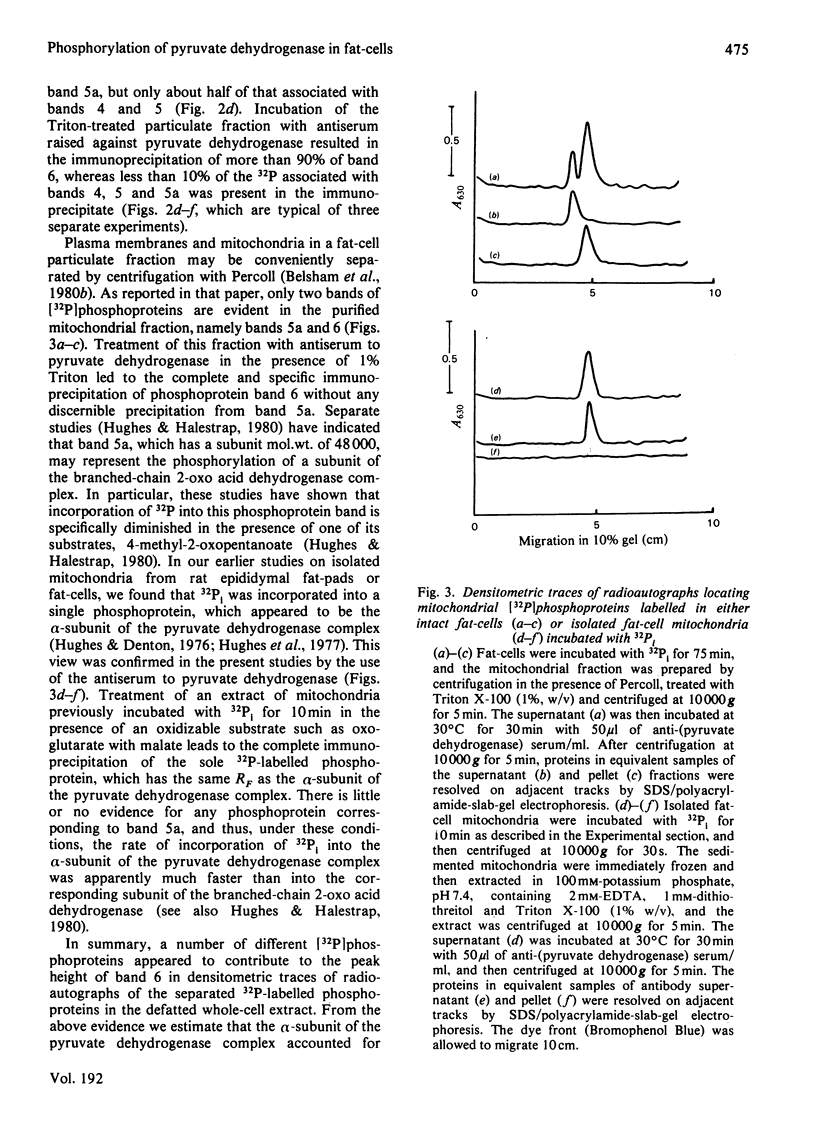
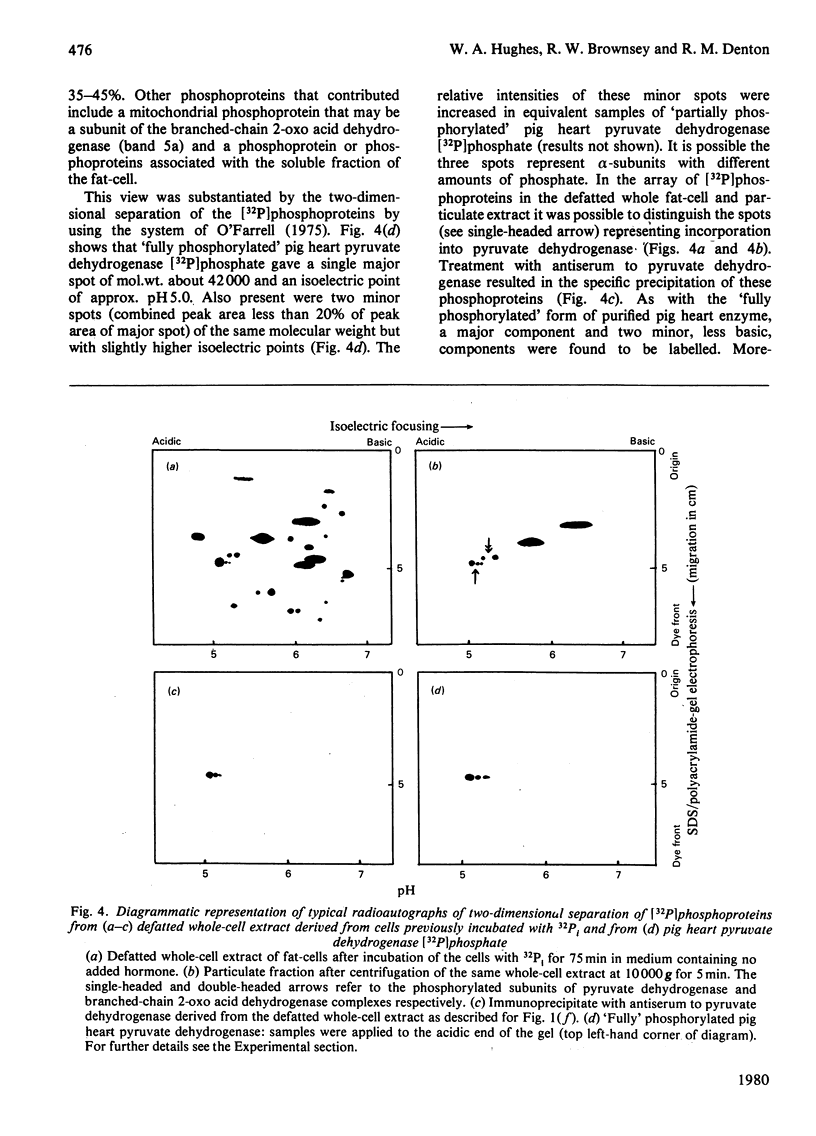
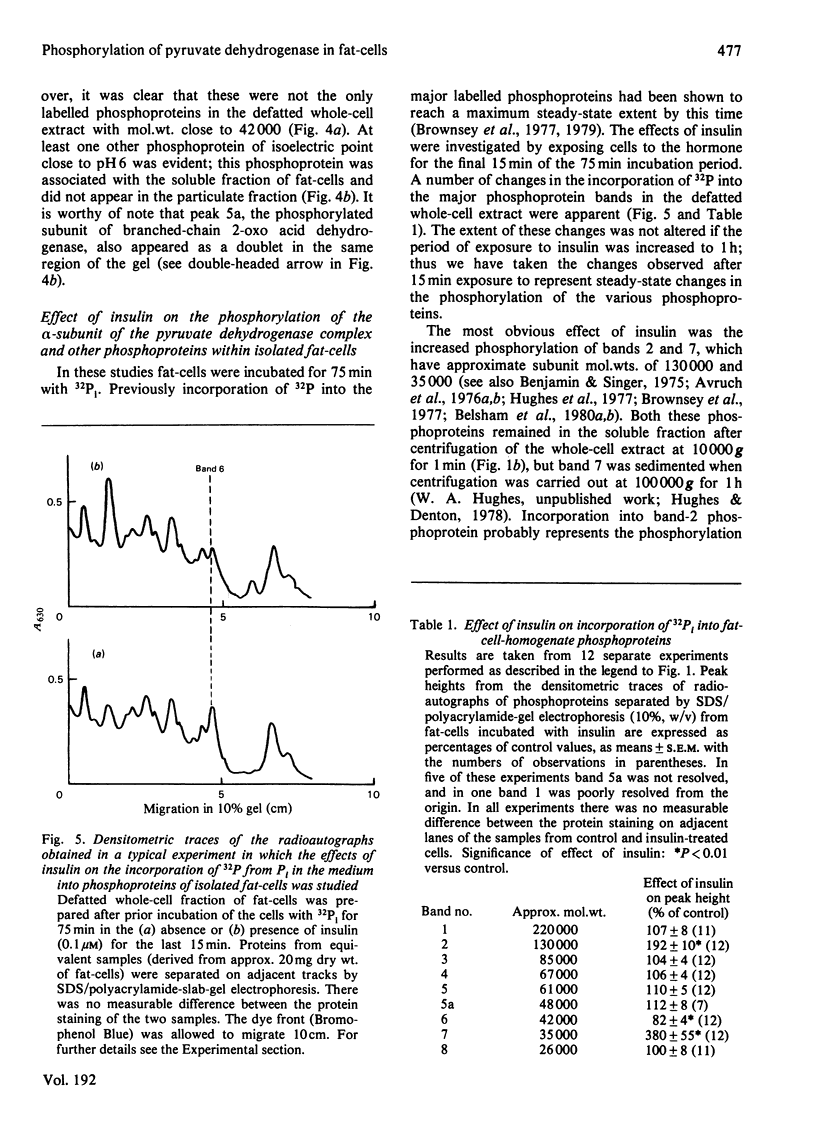
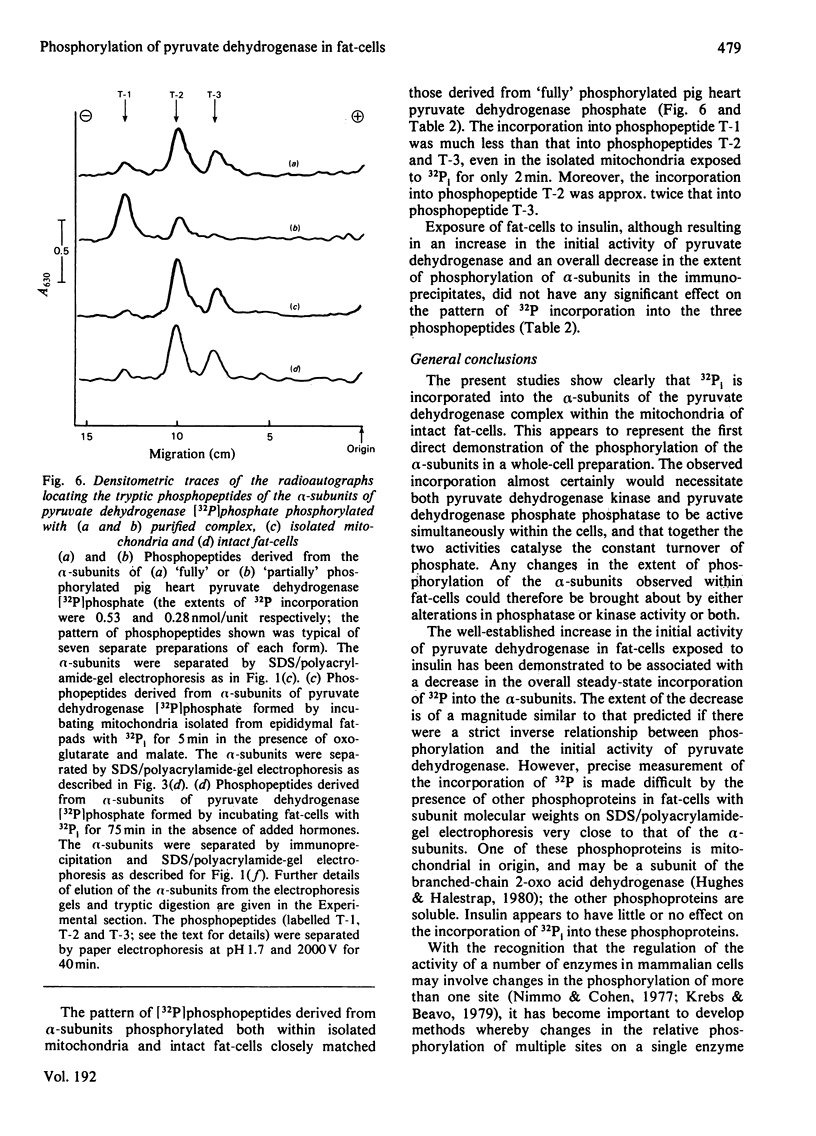
1. Intact rat epididymal fat-cells were incubated with 32Pi, and the intracellular proteins were separated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. One of the separated bands of phosphorylated proteins had an apparent subunit mol.wt. of 42 000, which is the same as that of the alpha-subunit of the pyruvate dehydrogenase complex. By using a combination of subcellular fractionation, immunoprecipitation with antiserum raised against pyruvate dehydrogenase complex and two-dimensional electrophoresis it was apparent that the incorporation into alpha-subunits accounted for 35--45% of the total incorporation into this band of phosphoproteins. 2. The increase in the initial activity of pyruvate dehydrogenase that follows brief exposure of fat-cells to insulin was shown to be associated with a decrease in the steady-state incorporation of 32P into the alpha-subunits of pyruvate dehydrogenase. 3. Tryptic peptide analysis of pyruvate dehydrogenase [32P]phosphate, labelled in intact fat-cells, indicated that three serine residues on the alpha-subunit were phosphorylated, corresponding to the three sites phosphorylated when purified pig heart pyruvate dehydrogenase was incubated with [gamma-32P]ATP. The relative phosphorylation of all three serine residues appeared to be similar in 32P-labelled alpha-subunits in both control and insulin-treated fat-cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. C., Kowaloff E. M., Witters L. A., Dennihy D. T., Avruch J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J Biol Chem. 1979 Aug 25;254(16):8052–8056. [PubMed] [Google Scholar]
- Avruch J., Leone G. R., Martin D. B. Effects of epinephrine and insulin on phosphopeptide metabolism in adipocytes. J Biol Chem. 1976 Mar 10;251(5):1511–1515. [PubMed] [Google Scholar]
- Avruch J., Leone G. R., Martin D. B. Identification and subcellular distribution of adipocyte peptides and phosphopeptides. J Biol Chem. 1976 Mar 10;251(5):1505–1510. [PubMed] [Google Scholar]
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Belsham G. J., Brownsey R. W., Hughes W. A., Denton R. M. Anti-insulin receptor antibodies mimic the effects of insulin on the activities of pyruvate dehydrogenase and acetylCoA carboxylase and on specific protein phosphorylation in rat epididymal fat cells. Diabetologia. 1980 Apr;18(4):307–312. doi: 10.1007/BF00251011. [DOI] [PubMed] [Google Scholar]
- Belsham G. J., Denton R. M., Tanner M. J. Use of a novel rapid preparation of fat-cell plasma membranes employing Percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat-cells. Biochem J. 1980 Nov 15;192(2):457–467. doi: 10.1042/bj1920457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Actions of insulin, epinephrine, and dibutyryl cyclic adenosine 5'-monophosphate on fat cell protein phosphorylations. Cyclic adenosine 5'-monophosphate dependent and independent mechanisms. Biochemistry. 1975 Jul 29;14(15):3301–3309. doi: 10.1021/bi00686a003. [DOI] [PubMed] [Google Scholar]
- Brownsey R. W., Hughes W. A., Denton R. M. Adrenaline and the regulation of acetyl-coenzyme A carboxylase in rat epididymal adipose tissue. Inactivation of the enzyme is associated with phosphorylation and can be reversed on dephosphorylation. Biochem J. 1979 Oct 15;184(1):23–32. doi: 10.1042/bj1840023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R. W., Hughes W. A., Denton R. M. Demonstration of the phosphorylation of acetyl-coenzyme A carboxylase within intact rat epididymal fat-cells. Biochem J. 1977 Dec 15;168(3):441–445. doi: 10.1042/bj1680441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Davis P. F., Pettit F. H., Reed L. J. Peptides derived from pyruvate dehydrogenase as substrates for pyruvate dehydrogenase kinase and phosphatase. Biochem Biophys Res Commun. 1977 Apr 11;75(3):541–549. doi: 10.1016/0006-291x(77)91506-6. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Hughes W. A. Pyruvate dehydrogenase and the hormonal regulation of fat synthesis in mammalian tissues. Int J Biochem. 1978;9(8):545–552. doi: 10.1016/0020-711x(78)90113-1. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. A simple modification to an enzymic method for the preparation of [gamma-32P]ATP free of salt and buffer. Anal Biochem. 1979 Mar;93(2):272–274. doi: 10.1016/s0003-2697(79)80151-7. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. A., Denton R. M. Evidence for multi-site phosphorylation of pyruvate dehydrogenase within intact mitochondria [proceedings]. Biochem Soc Trans. 1978;6(6):1228–1230. doi: 10.1042/bst0061228. [DOI] [PubMed] [Google Scholar]
- Hughes W. A., Denton R. M. Incorporation of 32Pi into pyruvate dehydrogenase phosphate in mitochondria from control and insulin-treated adipose tissue. Nature. 1976 Dec 2;264(5585):471–473. doi: 10.1038/264471a0. [DOI] [PubMed] [Google Scholar]
- Hughes W. A., Halestrap A. P. Phosphorylation of branched-chain 2-oxo acid dehydrogenase within intact mitochondria [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):374–374. doi: 10.1042/bst0080374. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Hormonal regulation of pyruvate dehydrogenase. Metabolism. 1971 Jan;20(1):43–53. doi: 10.1016/0026-0495(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J. Role of multi-site phosphorylation in regulation of pig heart pyruvate dehydrogenase phosphatase. FEBS Lett. 1979 Dec 15;108(2):485–488. doi: 10.1016/0014-5793(79)80594-3. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Srere P. A. Identification of ATP citrate lyase as a phosphoprotein. J Biol Chem. 1979 Mar 10;254(5):1691–1698. [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980 Jul 15;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Fat cell protein phosphorylation. Identification of phosphoprotein-2 as ATP-citrate lyase. J Biol Chem. 1979 Sep 25;254(18):9232–9236. [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Jarett L. Insulin effect on protein phosphorylation of plasma membranes and mitochondria in a subcellular system from rat adipocytes. II. Characterization of insulin-sensitive phosphoproteins and conditions for observation of the insulin effect. J Biol Chem. 1979 Aug 10;254(15):6997–7001. [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Bridges B. J., Randle P. J. Exchangeable and total calcium pools in mitochondria of rat epididymal fat-pads and isolated fat-cells. Role in the regulation of pyruvate dehydrogenase activity. Biochem J. 1976 Jan 15;154(1):209–223. doi: 10.1042/bj1540209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith P. A., Vernon R. G., Mayer R. J. Lipogenic enzymes in rat maternal adipose tissue in the perinatal period. Biochem J. 1980 Mar 15;186(3):937–944. doi: 10.1042/bj1860937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Wejksnora P. J., Warner J. R., Rubin C. S., Rosen O. M. Insulin-stimulated protein phosphorylation in 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2725–2729. doi: 10.1073/pnas.76.6.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Kerbey A. L., Randle P. J., Waller C. A., Reid K. B. Amino acid sequences around the sites of phosphorylation in the pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):419–426. doi: 10.1042/bj1810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Randle P. J. Regulation of pig heart pyruvate dehydrogenase by phosphorylation. Studies on the subunit and phosphorylation stoicheiometries. Biochem J. 1978 Aug 1;173(2):659–668. doi: 10.1042/bj1730659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague W. M., Pettit F. H., Yeaman S. J., Reed L. J. Function of phosphorylation sites on pyruvate dehydrogenase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):244–252. doi: 10.1016/0006-291x(79)91672-3. [DOI] [PubMed] [Google Scholar]
- Weiss L., Löffler G., Schirmann A., Wieland O. Control of pyruvate dehydrogenase interconversion in adipose tissue by insulin. FEBS Lett. 1971 Jun 24;15(3):229–231. doi: 10.1016/0014-5793(71)80318-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Randle P. J. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication). Biochem J. 1973 Jun;134(2):651–653. doi: 10.1042/bj1340651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


