Abstract
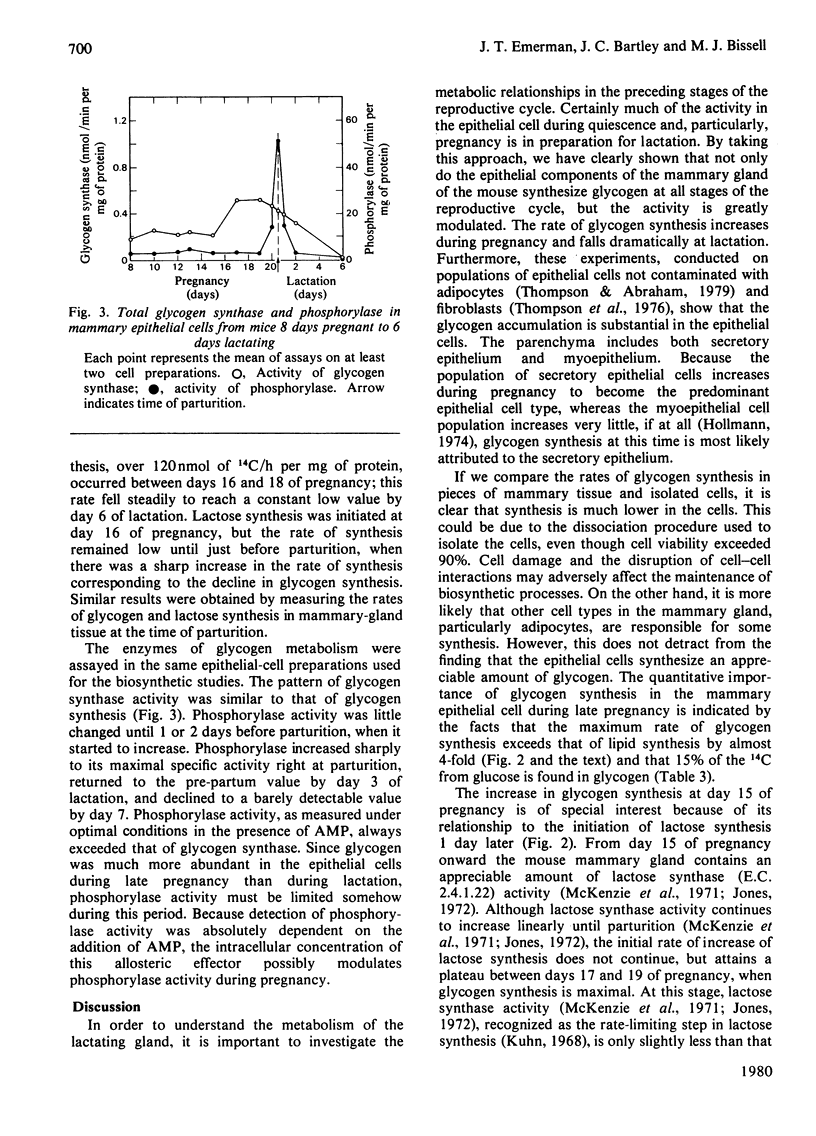
Glycogen metabolism in mammary epithelial cells was investigated (i) by studying the conversion of glucose into glycogen and other cellular products in these cells from virgin, pregnant and lactating mice and (ii) by assaying the enzymes directly involved with glycogen metabolism. We find that: (1) mammary epithelial cells synthesized glycogen at rates up to over 60% that of the whole gland; (2) the rate of this synthesis was modulated greatly during the reproductive cycle, reaching a peak in late pregnancy and decreasing rapidly at parturition, when abundant synthesis of lactose was initiated; (3) glycogen synthase and phosphorylase activities reflected this modulation in glycogen metabolism; (4) lactose synthesis reached a plateau during late pregnancy, even though lactose synthase is reported to increase in the mouse mammary gland at this time. We propose that glycogen synthesis restricts lactose synthesis during late pregnancy by competing successfully for the shared UDP-glucose pool. The physiological advantage of glycogen accumulation during late pregnancy is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
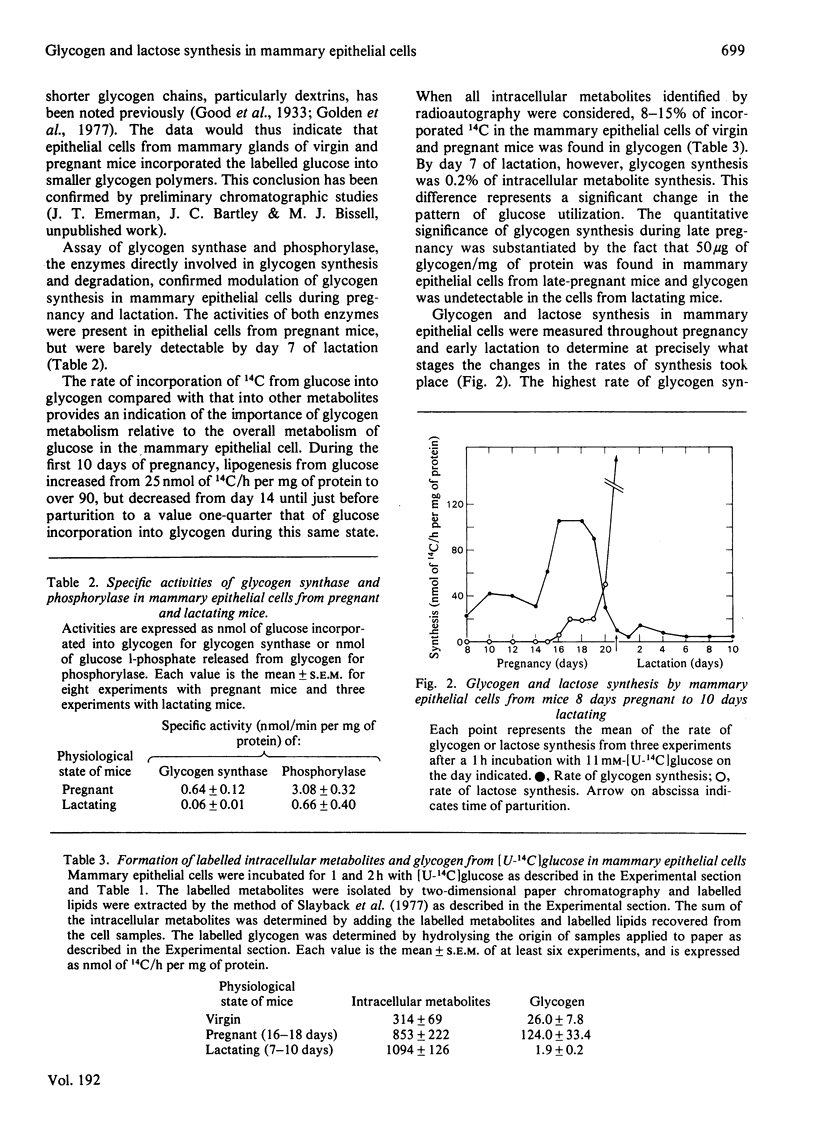
- Bartley J. C., Abraham S., Chaikoff I. L. Biosynthesis of lactose by mammary gland slices from the lactating rat. J Biol Chem. 1966 Mar 10;241(5):1132–1137. [PubMed] [Google Scholar]
- Bassham J. A., Bissell M. J., White R. C. Quantitative tracer studies of metabolic dynamics of animal cells growing in tissue culture. Anal Biochem. 1974 Oct;61(2):479–491. doi: 10.1016/0003-2697(74)90415-1. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., White R. C., Hatie C., Bassham J. A. Dynamics of metabolism of normal and virus-transformed chick cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2951–2955. doi: 10.1073/pnas.70.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani R. L., Peterson J. A., Abraham S. The removal of cell surface material by enzymes used to dissociate mammary-gland cells. In Vitro. 1978 Nov;14(11):887–894. doi: 10.1007/BF02616117. [DOI] [PubMed] [Google Scholar]
- EBNER K. E., HOOVER C. R., HAGEMAN E. C., LARSON B. L. Cultivation and properties of bovine mammary cell cultures. Exp Cell Res. 1961 Mar;23:373–385. doi: 10.1016/0014-4827(61)90046-5. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Bissell M. J. A simple technique for detection and quantitation of lactose synthesis and secretion. Anal Biochem. 1979 Apr 15;94(2):340–345. doi: 10.1016/0003-2697(79)90370-1. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Golden S., Wals P. A., Katz J. An improved procedure for the assay of glycogen synthase and phosphorylase in rat liver homogenates. Anal Biochem. 1977 Feb;77(2):436–445. doi: 10.1016/0003-2697(77)90257-3. [DOI] [PubMed] [Google Scholar]
- Jones E. A. Studies on the particulate lactose synthetase of mouse mammary gland and the role of -lactalbumin in the initiation of lactose synthesis. Biochem J. 1972 Jan;126(1):67–78. doi: 10.1042/bj1260067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITTINGER G. W., REITHEL F. J. Lactose synthesis in mammary gland preparations. J Biol Chem. 1953 Dec;205(2):527–533. [PubMed] [Google Scholar]
- Lasfargues E. Y., Moore D. H. A method for the continuous cultivation of mammary epithelium. In Vitro. 1971 Jul-Aug;7(1):21–25. doi: 10.1007/BF02619001. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- MOSES V., LONBERG-HOLM K. K. A semiautomatic device for measuring radioactivity on two-dimensional paper chromatograms. Anal Biochem. 1963 Jan;5:11–27. doi: 10.1016/0003-2697(63)90053-8. [DOI] [PubMed] [Google Scholar]
- McKenzie L., Fitzgerald D. K., Ebner K. E. Lactose synthetase activities in rat and mouse mammary glands. Biochim Biophys Acta. 1971;230(3):526–530. doi: 10.1016/0304-4165(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Mellenberger R. W., Bauman D. E. Metabolic adaptations during lactogenesis. Lactose synthesis in rabbit mammary tissue during pregnancy and lactation. Biochem J. 1974 Sep;142(3):659–665. doi: 10.1042/bj1420659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellenberger R. W., Bauman D. E., Nelson D. R. Metabolic adaptations during lactogenesis. Fatty acid and lactose synthesis in cow mammary tissue. Biochem J. 1973 Nov;136(3):741–748. doi: 10.1042/bj1360741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Ariyanayagam A. D., Kuhn N. J. Progesterone and the metabolic control of the lactose biosynthetic pathway during lactogenesis in the rat. Biochem J. 1973 Dec;136(4):1105–1116. doi: 10.1042/bj1361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. M., Chandler R. L. Ultrastructural studies on the bovine mammary gland with particular reference to glycogen distribution. Res Vet Sci. 1973 May;14(3):334–340. [PubMed] [Google Scholar]
- Slayback J. R., Cheung L. W., Geyer R. P. Quantitative extraction of microgram amounts of lipid from cultured human cells. Anal Biochem. 1977 Dec;83(2):372–384. doi: 10.1016/0003-2697(77)90046-x. [DOI] [PubMed] [Google Scholar]
- Solling H., Esmann V. A sensitive method of glycogen determination in the presence of interfering substances utilizing the filter-paper technique. Anal Biochem. 1975 Oct;68(2):664–668. doi: 10.1016/0003-2697(75)90667-3. [DOI] [PubMed] [Google Scholar]
- Speake B. K., White D. A. Lipid-linked oligosaccharides containing glucose in lactating rabbit mammary gland. Biochem J. 1978 Dec 15;176(3):993–1000. doi: 10.1042/bj1760993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J. W., Chandler J. A. The fine structure of ducts and subareolar ducts in the resting gland of the female breast. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):119–132. doi: 10.1007/BF00432157. [DOI] [PubMed] [Google Scholar]
- TWAROG J. M., LARSON B. L. INDUCED ENZYMATIC CHANGES IN LACTOSE SYNTHESIS AND ASSOCIATED PATHWAYS OF BOVINE MAMMARY CELL CULTURES. Exp Cell Res. 1964 Mar;34:88–99. doi: 10.1016/0014-4827(64)90185-5. [DOI] [PubMed] [Google Scholar]
- Thompson K., Abraham S. Identification of mouse mammary adipose cells by membrane antigens. In Vitro. 1979 Jun;15(6):441–445. doi: 10.1007/BF02618413. [DOI] [PubMed] [Google Scholar]
- Thompson K., Ceriani R. L., Wong D., Abraham S. Immunologic methods for the identification of cell types. I. Specific antibodies that distinguish between mammary gland epithelial cells and fibroblasts. J Natl Cancer Inst. 1976 Jul;57(1):167–172. doi: 10.1093/jnci/57.1.167. [DOI] [PubMed] [Google Scholar]


