Abstract
Autoimmune disorders exhibit intricate pathology. Their mechanisms are complex, which attenuates the need for novel therapeutic interventions. Frexalimab, a potent monoclonal antibody targeting the dysregulated CD40-CD40L pathway, stands out as a formidable weapon against the assault of inflammation and tissue devastation. Diverse electronic databases were searched using relevant keywords to extract data on the role of Frexalimab in combating various autoimmune diseases. This review highlights Frexalimab’s efficacy in improving various disability indicators of relapsing multiple sclerosis (RMS), alleviating fatigue in primary Sjögren’s syndrome (PSJS), and improving glycemic control in diabetic patients. Across multiple trials, its favorable safety profile has proven its superiority over first-generation drugs in minimizing side effects. Indeed, Frexalimab has become a harbinger of hope in the fight against autoimmune diseases and has pioneered a unique and unchallenging way for tackling complex autoimmune diseases in the clinical realm, however, further large-scale trials are needed to establish its therapeutic benefits across different autoimmune conditions.
Keywords: autoimmune disorders, CD40-CD40L pathway, clinical trials, efficacy, frexalimab, multiple sclerosis, pharmacodynamics, pharmacokinetics, safety, Sjögren’s syndrome, systemic lupus erythematosus, type 1 diabetes
Introduction
Highlights
Frexalimab, a monoclonal antibody targeting the CD40-CD40L pathway, shows promise in treating various autoimmune diseases by reducing inflammation and tissue damage.
The review underscores Frexalimab’s effectiveness in improving disability indicators in relapsing multiple sclerosis (RMS), alleviating fatigue in primary Sjögren’s syndrome (PSJS), and enhancing glycemic control in diabetic patients.
Multiple clinical trials have demonstrated Frexalimab’s favorable safety profile, marking it as superior to first-generation drugs by minimizing side effects.
Despite promising results, further large-scale trials are needed to establish Frexalimab’s therapeutic benefits across different autoimmune conditions.
The CD40 receptor and CD40L ligand are transmembrane proteins that are part of the tumor necrosis factor (TNF) receptor superfamily and are imperative to the induction and maintenance of inflammation1. The expression of CD40 has been recognized on APCs, such as B cells, dendritic cells (DCs), macrophages, and monocytes, as well as on non-immune cells, such as epithelial, endothelial, and mesenchymal cells. CD40L is predominantly expressed by activated CD4+ T cells as well as activated B cells and platelets, although both CD40 and CD40L can also be variably induced on other cells in the context of inflammatory conditions. Interaction of CD40 with CD40L triggers an array of downstream signaling pathways, demonstrating the significant roles they play in different cellular immune processes and, thus, in the pathogenesis of autoimmune disease processes that involve B and T cell activation2,3.
The disruption of CD40-CD40L interactions within medullary thymic epithelial cells (mTECs) is implicated in the failure of central tolerance. Furthermore, CD40 signaling prompts the generation of proinflammatory cytokines, while CD40 elevation following antigen-presenting cell (APC) activation, heightened CD40 levels—either constitutive or induced—enhancing the potency of CD40-CD154 interactions, and the anomalous expression of CD40 in tissues where it is typically absent, collectively contribute to the etiology of autoimmune disorders such as autoimmune thyroiditis, type 1 diabetes, inflammatory bowel disease, psoriasis, rheumatoid arthritis, and systemic lupus erythematosus4. In humans, both clinical and pathologic evidence also suggests the involvement of CD40L and CD40 in multiple sclerosis (MS)5.
In recent times, notable progress has been achieved in the realm of CD40/CD40L-targeted therapeutic interventions. Diverse categories of agents, encompassing agonistic/antagonistic monoclonal antibodies, cellular vaccines, adenoviral vectors, and protein antagonists, have been formulated and evaluated in preliminary clinical trials aimed at treating malignancies, autoimmune disorders, and allograft rejection6. The first generation of antibodies precipitated thromboembolic side effects, prompting the introduction of subsequent antibody generations to overcome this toxicity7.
While numerous treatments employing diverse mechanisms of action are available for relapsing forms of multiple sclerosis (RMS), therapeutic options that have been proven to be efficacious for progressive RMS remain scarce, with remyelination therapies yet to secure regulatory approval. Consequently, significant unmet needs remain in the MS landscape, particularly for interventions capable of halting the progression of disability and facilitating functional restoration following central nervous system (CNS) injury8. The blockade of CD40L has shown efficacy in ameliorating experimental autoimmune conditions, showcasing it as an appealing therapeutic strategy. Frexalimab, a second-generation anti-CD40L monoclonal antibody, is currently under evaluation for the treatment of multiple sclerosis9.
We look forward to forthcoming trials that are expected to unveil results over the next several years, hoping that they will yield substantial benefits for individuals dealing with progressive multiple sclerosis. It will be interesting to note for which disease under clinical trial this novel yet innovative immunomodulatory therapy is going to exhibit the most notable improvement in outcomes with the least incidence of adverse effects.
Review
CD40D-CD40L axis drugs and their mechanism of action
Research conducted over the past three decades has demonstrated that the CD40-CD40L system plays a crucial role in the development and progression of multiple autoimmune diseases. Therefore, targeting CD40-CD40L signaling could potentially serve as a novel therapeutic option for treating such disorders. There are several CD40L and CD40 drugs, many of which are currently in clinical trials for evaluation of therapeutic benefits. Notably, the success rate of CD40 drugs is higher owing to few thromboembolic complications as compared to CD40L, which is associated with higher risks of thromboembolic events. CD40 and CD40L drugs have been tested in various clinical trials, including phase 2 trials. They are used to treat autoimmune diseases, especially multiple sclerosis (MS). Notably, Frexalimab, an anti-CD40L (SAR44134) monoclonal antibody has even undergone a phase 3 randomized clinical trial as well. Table 1 and Table 2 attempt to summarize CD40-CD40L axis drugs and their applications.
Table 1.
Anti-CD40L drugs, their mechanism of action, and important features
| Target | Drug name | Receptor site | MOA | Notes | Diseases |
|---|---|---|---|---|---|
| CD40L | Ruplizumab | Humanized IgG1 mAB [BG9588] | Decreased levels of anti-dsDNA antibodies and increased concentrations of complement component C3, along with a reduction in spontaneously proliferating B cells, plasma cells, and IgM and IgG anti-DNA-secreting cells | Associated with thromboembolic complications | Indication research has been conducted on the potential application and therapeutic use for conditions such as thrombocytopenia, systemic lupus erythematosus, transplant rejection, unspecified blood-forming organ disorders, and multiple sclerosis |
| Toralizumab | IDEC-131 Humanized IgGmAB | NEUTRALIZES CD40L function, as it blocks the interaction between CD40L and CD40 | Safety issues, associated with thrombotic events | The treatment may be effective for managing antibody-mediated disorders such as immune thrombocytopenic purpura, lupus nephritis, and rheumatoid arthritis. Additionally, it shows promise in addressing T-cell-mediated diseases like multiple sclerosis and Crohn’s disease, as well as in transplantation procedures, including solid organ and pancreatic islet cell transplants | |
| Dapirolizumab | CDP657 [Fab fragment] | This inhibits the humoral immune response, and the improvement in activity is attributed to a decrease in mRNA expression in B-cells and PC-related changes, which results in a reduction of the dependent antigen response | No evidence of thromboembolic events | SLE | |
| Letolizumab | BMS-986004 | A homodimer antibody that produces a modified IgG1 lacking effector functions, such as Fc binding and complement fixation | No effect on coagulation function or platelet count\ | ITP and acute graft versus host disease are linked through the interaction of CD40L, a protein expressed on the surface of multiple cell types. This transmembrane protein can be found on activated T cells, eosinophils, basophils, natural killer (NK) cells, mast cells, platelets, and activated endothelial cells | |
| Frexalimab | SAR44134 | It blocks the CD40\CD40L pathway, which is required for activation and function of both innate and adaptive immune systems. [macrophages\ microglial and dendritic cells] | It treats both acute and chronic inflammation of CBC in multiple sclerosis and does not cause any lymphocyte decline | This drug is used to treat multiple sclerosis and plaque psoriasis via inhibiting CD40 ligands |
Table 2.
Anti-CD40L drugs, target, mechanism of action, and important features
| Target | Drug | MOA | Notes |
|---|---|---|---|
| CD40 | CFZ533 | The modified Fc domain of the human antagonist anti-CD40 monoclonal IgG1 antibody enables it to mediate Fc Gamma-dependent effector function | Promotes allograft survival following kidney transplant |
| Bleselumab [ASKP1240] | It is an IgG4 antibody (humanized monoclonal antibody) that causes suppression of both cellular and humoral immune systems | This drug has safety against psoriasis and it does not cause side effects like infusion reactions, cytokine release syndrome and any thromboembolic events like atherosclerosis or pulmonary embolism. It also prevents rejection of renal and hepatic transplants | |
| BI-655064 | It is a humanized antagonist IgG1 mAB. It mechanism of action is to prevent Fc-mediated effector function including cytotoxicity and platelet activation |
The mechanism of action of Frexalimab that underlies its potential to treat multiple autoimmune disorders has been illustrated in Figure 1.
Figure 1.
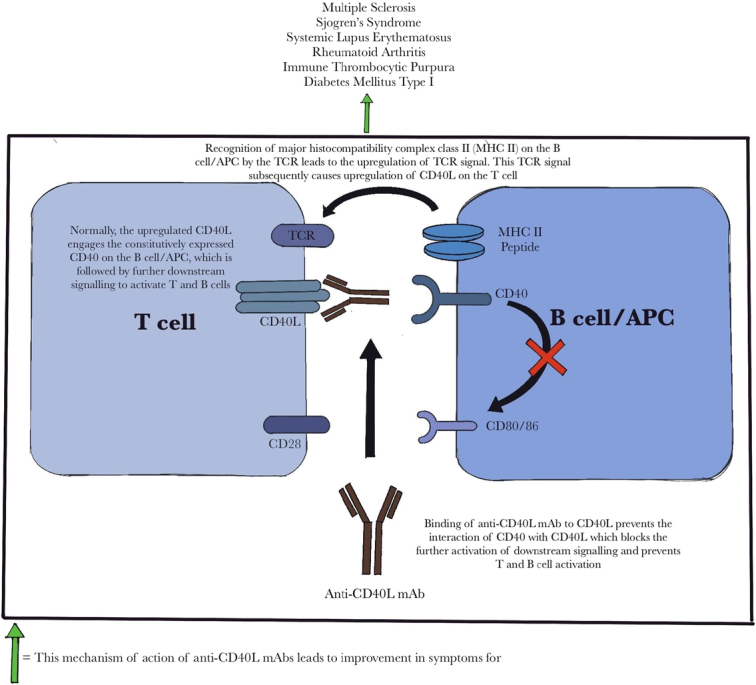
The figure demonstrates the proposed mechanism of action of anti-CD40L monoclonal antibodies and how they further contribute towards the amelioration of symptoms in various autoimmune disorders. Normally, activation of T cells by antigen-presenting cells (APCs)/B cells requires the interaction of costimulatory molecules and the subsequent activation of the T cell receptor (TCR) signal. Anti-CD40L mAbs prevent this interaction and the downstream signaling. APC, antigen presenting cell; TCR, T-cell receptor; MHC II, major histocompatibility complex class II; mAb, monoclonal antibody.
Multiple sclerosis
Multiple sclerosis (MS) is a rampant autoimmune-mediated condition of the central nervous system (CNS) that frequently causes severe physical or intellectual impairment in young people. In this condition, the body’s immune system mistakenly attacks the central nervous system (CNS), leading to inflammation in white and gray matter tissues10. This inflammation is caused by immune cells and cytokines, which disrupt normal nerve function. Studies indicate that CD4+ T cells, a type of T helper cell, and interactions between antigen-presenting cells (APCs) and T lymphocytes are crucial in the onset and progression of MS11,12. When pathogen-associated molecules bind to toll-like receptors on APCs, specific cytokines like interleukin (IL)-12, IL-23, and IL-4 are produced. These cytokines drive the differentiation of CD4+ T cells into various phenotypes such as Th1, Th2, or Th17. Th1 cells produce inflammatory cytokines like interferon and TNF-α, which can worsen inflammation by inhibiting the differentiation of Th2 cells13. The inflammation leads to focal areas in the CNS where T cells and macrophages infiltrate, causing damage to the myelin sheath that surrounds nerve fibers. This damage results in the formation of CNS plaques, characterized by inflammatory cells, demyelinated nerve fibers, and other tissue changes. These lesions disrupt the transmission of nerve signals, leading to neurological symptoms.
CD40/CD40L pathway signaling defect
B cells play a significant role in the development and progression of MS. These immune cells target autoantigen and produce antibodies that can damage tissue, leading to symptoms and disability. In fact, nearly 90% of people with MS have oligoclonal immunoglobulins (Ig) in their cerebrospinal fluid, which further supports the importance of B cells in MS. B cells can also cause direct harm to the brain and spinal cord. For example, leptomeningeal B cells have been found to contribute to neuronal degeneration and demyelination, making MS worse. Additionally, when B cells deplete anti-CD20 antibodies, it can lead to a relapse of MS symptoms and further neurological problems14.
Understanding how B cells produce oligoclonal bands (OCBs) in the cerebrospinal fluid is crucial for diagnosing and managing MS effectively. B cells require both antigen receptor stimulation and co-stimulation for robust activation15. CD40, a costimulatory molecule of the TNFR superfamily16 is a transmembrane protein that forms a trimer with its ligand (CD40L or CD154). Erroneous CD40 signaling may trigger the development or persistence of pathogenic autoimmune reactions. Studies have shown a strong correlation between the blood–brain barrier breakdown indicator, the albumin quotient, and the levels of sCD40L in MS patients’ serum. The blood of MS patients contained lower levels of sCD40L. Interestingly, a higher incidence of multiple sclerosis has been associated with a CD40 genotype with low mRNA expression17.
Quality of life (QoL)
Multiple sclerosis severely affects the quality of life by causing sensory disturbances like numbness, tingling, itching, walking issues (owing to weariness, frailness, spasticity, diminished coordination, and tremor), vision difficulties (diplopia and fuzzy vision), constipation and bladder malfunction, psychological and intellectual impairment (learning disability and despair), vertigo, and intercourse troubles. Less common symptoms include dysphagia, dysarthria, breathing abnormalities, auditory impairment, seizures, headache, etc.18.
QoL in people with MS can be assessed by using various metrics including Kurtzke’s Expanded or Extended Disability Status Scale (EDSS), the Scripps Neurological Rating Scale (SNRS), the Barthel Index (BI), and the Functional Independence Measure (FIM). They have demonstrated that MS can cause severe impairment in social, vocational, and psychological domains of life, thus utterly affecting the functional status of an individual19.
Clinical trials of frexalimab for MS and other autoimmune disorders
Frexalimab has been undergoing many clinical trials about improving various indicators of disability due to MS, such as improving composite confirmed disability progression (cCDP), along with improving recurrence of MS in the form of annualized relapse rate (ARR) and several new Gadolinium-enhancing T1 lesions. Frexalimab achieves these outcomes by inhibiting CD40-CD40L binding, which impairs the development of an aberrant immune response resulting in the progression and/or relapse of crippling autoimmune disorders. The very mechanism prevents demyelination of oligodendrocytes which can otherwise lead to exacerbation of autoimmune response seen in flares of MS in its patients. The trials are summarized in Table 3.
Table 3.
The table shows the regimen, primary outcomes, and major secondary outcomes of all the clinical trials of Frexalimab for treating multiple sclerosis
| ClinicalTrials.gov ID | Regimen of frexalimab | Primary outcome | Secondary outcomes |
|---|---|---|---|
| NCT06141486 | IV Frexalimab | Time to onset of cCDP (6 months) | Time to onset of cCDP (3 months), Time to onset of CDI, Number of new/enlarging T2 lesions, Percent change in brain volume, change in cognitive function, Change in MSIS-29v2 scores, Change in PROMIS Fatigue MS-8a, Annualized relapse rate, Adverse events, Laboratory/ECG/vital sign abnormalities, Antibody levels, change in serum Ig, Change in NfL levels, frexalimab plasma concentration |
| NCT06141473 | IV frexalimab | Annualized relapse rate (ARR) | Time to onset of cCDW (3 and 6 months), time to onset of CDI, progression independent of relapse activity, number of new/enlarging T2 lesions, number of new Gd-enhancing T1 lesions, percent change in brain volume, change in cognitive function, Change in MSIS-29v2 scores, change in PROMIS fatigue MS-8, adverse events, laboratory/ECG/vital sign abnormalities, Antidrug antibodies, Change in NfL levels, Frexalimab plasma concentration |
| NCT04879628 | 1200 mg IV frexalimab, 300 mg SC frexalimab | Number of new Gd-enhancing T1 lesions | Number of new/enlarging T2 lesions, Total number of Gd-enhancing T1 lesions, adverse events, antidrug antibodies, pharmacokinetics (Cmax, tmax, AUC0-tau, t1/2z) |
Frexalimab clinical trial summary
The descriptive statistics of a phase-2 clinical trial are given below to further elaborate its potential impact for treatment of MS.
Participants: 129 with multiple sclerosis, divided into four groups (1200 mg IV, 300 mg SC, placebo IV, and placebo SC).
Completion: 97% completed the 12-week double-blind period; four discontinued due to various reasons.
-
Primary outcomes: At week 12, frexalimab groups had fewer new gadolinium-enhancing lesions than placebo:
1200 mg IV: 0.2 lesions (85% had no new lesions).
300 mg SC: 0.3 lesions (84% had no new lesions).
Placebo: 1.4 lesions (50% had no new lesions).
Secondary outcomes: Fewer new or enlarging T2 lesions in Frexalimab groups versus placebo.
Biomarkers: Decreased levels of plasma neurofilament light chain and chemokine [C-X-C motif] ligand 13 (CXCL13) in frexalimab groups, indicating reduced disease activity.
Conclusion: Frexalimab reduced lesions and disease biomarkers, showing efficacy and a favorable safety profile9.
Other autoimmune diseases for which frexalimab is undergoing clinical trials and their salient details are summarized in Table 4.
Table 4.
The table shows different applications of Frexalimab for possibly treating various autoimmune diseases, along with its mechanism of action for each particular disease and the current status of Frexalimab with regard for being a candidate drug for treating these diseases
| Condition | ClinicalTrials.gov ID | Use | Mechanism of action | Current status |
|---|---|---|---|---|
| Sjögren’s Syndrome | NCT04572841 | Potential use in reducing inflammation and improving glandular function | Inhibits specific immune cells, reducing inflammation and tissue damage | Investigational, clinical trials ongoing |
| Diabetes | NCT06111586 | Exploring the potential to improve glycemic control and reduce immune-mediated beta cell destruction | Modulates immune response, potentially preserving pancreatic function | Preclinical/early clinical studies |
| Systemic lupus erythematosus (SLE) | NCT05039840 | Potential use in reducing disease activity and preventing organ damage | Targets immune pathways involved in lupus pathology | Clinical trials ongoing |
Frexalimab adverse events summary
During a 12-week trial, adverse events (AEs) occurred in:
1200 mg IV Group: 29%.
300 mg SC Group: 45%.
Placebo Group: 31%.
Common AEs: COVID-19 and headaches.
COVID-19: 10% in the 300 mg SC group; none in the 1200 mg IV or placebo groups.
Infections: 8% in the 1200 mg IV group, 24% in the 300 mg SC group, 4% in the placebo group.
Other events:
Liver enzyme elevations and neutropenia were reported but resolved without stopping treatment.
Overall safety: No serious or severe AEs, thromboembolic events, or deaths were reported. Frexalimab was well tolerated with mostly mild to moderate AEs9.
The aforementioned immunomodulatory effects of frexalimab have demonstrated its supremacy over previously available immunomodulatory drugs in the treatment of debilitating autoimmune diseases such as MS. This narrative review elaborates on the clinical efficacy and better safety profile of CD40L inhibitors such as frexalimab as compared to already available immunomodulatory drugs in the market. This article also clarifies the current status of frexalimab in the treatment of MS and further research and studies that must be undertaken to enable us to utilize it on a more massive scale to treat MS worldwide.
Safety and efficacy of anti-CD40L drugs
First generation anti-CD40L drugs such as toralizumab showed promise in treating autoimmune diseases and preventing transplant rejection but faced significant setbacks due to thromboembolic events, likely caused by platelet aggregation. Second-generation anti-CD40L drugs, such as dapirolizumab and frexalimab, were developed to address these safety concerns. They feature improved specificity and reduced off-target effects, focusing on minimizing treatment-emergent adverse events and disability progression. The comparison of primary outcome and key secondary outcomes of 1st generation and second generation anti-CD40L drugs has been summarized in Table 5. The table shows the major primary outcomes and secondary outcomes of all the registered clinical trials of anti-CD40L drugs, that is toralizumab, dapirolizumab, and frexalimab to date.
Table 5.
First generation vs second generation anti-CD40 ligand drugs comparative endpoint table
| Generation | Treatment | Primary outcome | Key secondary outcomes |
|---|---|---|---|
| First generation | Toralizumab | Insulin independence post-transplant | Graft failure, hypoglycemia events, glycemic control, kidney function changes, albumin excretion ratio, graft-versus-host disease (acute and chronic), malignancy relapse, nonrelapse mortality, overall survival |
| Second generation | Dapirolizumab | Treatment-emergent adverse events (TEAEs) | BICLA response, blood pressure changes, ECG findings, hematological and biochemical changes, pharmacokinetics of BIIB133, PEG concentration, anti-BIIB133 and Anti-PEG antibodies, Adverse events |
| Second generation | Frexalimab | Disability progression (cCDP), annualized relapse rate | Disability improvement (CDI), MRI lesion changes, brain volume loss, cognitive function, MSIS-29v2 scores, PROMIS fatigue, adverse events, plasma NfL levels, antidrug antibodies, insulin dose, HbA1c levels, hypoglycemia/hyperglycemia events, diabetic ketoacidosis, pediatric quality of life scores |
Table 6 shows the possible adverse effects of first generation anti-CD40L drugs such as ruplizumab and toralizumab along with the adverse effects of second generation anti-CD40L drugs such as dapirolizumab, letolizumab, and frexalimab. It is to be noted, however, that frexalimab has the highest safety profile of all of these drugs, as shown in Figure 2.
Table 6.
First generation vs second generation adverse effects
| Anti-CD40 ligand drugs | Adverse effects | |||
|---|---|---|---|---|
| 1st generation | ||||
| Ruplizumab | Thromboembolic events | |||
| Toralizumab | Upper respiratory tract infections | Thromboembolic events | ||
| Second generation | ||||
| Dapirolizumab | Upper respiratory tract infections | Thromboembolic events | ||
| Letolizumab | Infusion-related reactions | Infections | Thrombocytopenia | Hepatotoxicity |
| Frexalimab | Nasopharyngitis | Infusion reactions | Allergic reactions | |
Figure 2.
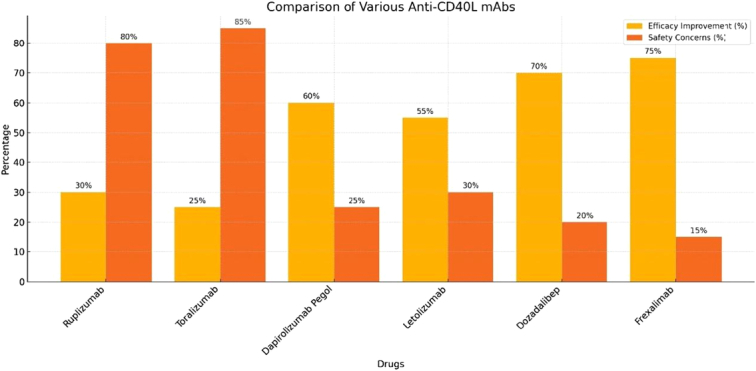
Efficacy and safety profile of first generation anti-CD40L drugs (ruplizumab and toralizumab) compared with that of second generation anti-CD40L drugs (dapirolizumab pegol, letolizumab, dozadalibep, and frexalimab).
The histogram in Figure 2 shows the efficacy improvement in percentage and safety concerns in the percentage of first generation anti-CD40L drugs (ruplizumab and toralizumab) and second generation anti-CD40L drugs (dapirolizumab pegol, letolizumab, dozadalibep, and frexalimab). It is evident from the figure that frexalimab has the highest efficacy improvement and has the least safety concerns among all anti-CD40L drugs discovered till now.
Ongoing analysis of biomarkers such as neurofilament light chain (NfL) and CXCL13 could provide early indications of neural and immune response changes, potentially predicting adverse outcomes before they become clinically apparent. These biomarkers could help in developing personalized treatment plans that mitigate risks for individual patients.
Comparison of frexalimab with other gold standard treatments
While other traditional treatments focus on broad immunosuppression or specific cytokine inhibition, frexalimab helps to modulate the immune system by addressing the dysregulated CD40-CD40L pathway. Nowadays, the mainstay of treatment for autoimmune diseases includes corticosteroids and disease-modifying antirheumatic drugs (DMARDs). Although these drugs are beneficial in controlling inflammation and slowing disease progression, they can still cause significant side effects and have limited capacity for long-term disease modification. However, the management of autoimmune diseases has now been advanced by gold standard treatments like tumor necrosis factor (TNF) inhibitors. They target specific inflammatory pathways, offering improved efficacy and reduced side effects compared to older treatments. Through frexalimab, we can now have a targeted and refined approach that can enhance efficacy and minimize adverse effects compared to both traditional and biologic therapies. But we still need extensive clinical trials to prove its efficacy and safety profile, especially in comparison to established treatments that already have evidence of managing autoimmune diseases effectively. Table 7 compares the efficacy and safety of Frexalimab with other current, investigational, and gold standard treatments.
Table 7.
Comparison of Frexalimab with other current, investigational, and gold-standard treatments for various autoimmune diseases
| Condition | Therapy | Comparison with frexalimab |
|---|---|---|
| Multiple sclerosis (MS) | DMTs 1. Natalizumab (Tysabri) 2. Ocrelizumab (Ocrevus) |
1. Natalizumab (Tysabri) MOA: Monoclonal antibody targeting α4-integrin to prevent leukocyte migration into the CNS Efficacy: Highly effective in reducing relapse rates and slowing progression in relapsing MS. Demonstrated significant reduction in relapse rates and MRI lesion activity. Sixty-eight percent reduction compared to placebo. Limited data is available on frexalimab specifically for MS. Safety: Risk of PML (Progressive Multifocal Leukoencephalopathy), infections, and liver enzyme abnormalities. Frexalimab has immune-related adverse events (e.g. pneumonitis, colitis). The safety profile is still under investigation. Overall: Well-established gold standard for relapsing MS with proven efficacy and a known risk profile; requires monitoring for PML. While on frexalimab ongoing trials will be crucial to determine its role in MS treatment. Potential for novel dosing and monitoring protocols based on immune modulation effects. 2. Ocrelizumab (Ocrevus) MOA: Monoclonal antibody targeting CD20+ B cells to deplete autoreactive B cells Efficacy: Highly effective in reducing relapse rates and slowing progression in both relapsing and primary progressive MS while Frexalimab showed 71–84% reduction vs placebo in ARR Safety: Infusion-related reactions, increased risk of infections, potential malignancies; generally well-tolerated Frexalimab: Available from pivotal trials; ongoing long-term studies Overall: Established gold standard for both relapsing and primary progressive MS; effective with a generally manageable safety profile |
| 2. Sjögren’s syndrome | 1, Hydroxychloroquine (Plaquenil) 2, Secukinumab (Cosentyx) |
1, Hydroxychloroquine (Plaquenil) MOA: Antimalarial drug with immunomodulatory effects; reduces inflammation and autoimmunity Efficacy: -Widely used as part of standard treatment. Effective in reducing disease activity and improving symptoms in some patients. Safety: -Generally well-tolerated. - Side effects include gastrointestinal disturbances, retinal toxicity (rare), and skin rashes. Overall - Hydroxychloroquine is a gold standard for managing symptoms in Sjögren’s Syndrome with a well-established efficacy profile. - Generally well-tolerated and effective for many patients. Frexalimab could potentially offer a novel treatment for Sjögren’s Syndrome through its unique mechanism of targeting PD-1/PD-L1.However, Clinical trials and data are needed to establish its efficacy and safety specifically for Sjögren’s Syndrome. Promising as an investigational therapy Secukinumab (Cosentyx) MOA: Monoclonal antibody targeting IL-17A to reduce inflammation and immune response Efficacy: -Investigational for Sjögren’s Syndrome. Early studies showed potential in reducing disease activity and symptoms but not yet standard treatment. Safety: -Investigational safety profile. Possible side effects include infections, injection site reactions, and effects on the immune system. overall Secukinumab is an investigational therapy with promising early data |
| Type 1 diabetes SLE |
1.Insulin therapy (Gold Standard) 2. Teplizumab (Tzield) 1.Hydroxychloroquine (Plaquenil) 2. Belimumab (Benlysta) |
1. Insulin therapy (Gold Standard) Not directly disease-modifying; provides external insulin to regulate blood glucose levels while for Frexalimab early-phase data is needed for Type 1 Diabetes. Efficacy: Does not modify the underlying disease but is critical for managing symptoms and preventing complication While Frexalimab has the ability to modulate immune response and restore β-cell function. Safety: -Well-established safety profile. - Risks include hypoglycemia, insulin allergic reactions, and weight gain. - Requires careful management to avoid complications. While in case of Frexalimab safety profile is under investigation. Also, there is also potential for immune-related adverse events such as pneumonitis, colitis, and endocrinopathies. However, for specific safety data for Type 1 Diabetes is not yet established Overall - Insulin Therapy is the gold standard for managing Type 1 Diabetes. Frexalimab has potential to modulate immune response and preserve β-cell function, which could modify disease progression unlike insulin therapy. 2. Teplizumab (Tzield) Moa: Monoclonal antibody targeting CD3 to modulate immune response and preserve pancreatic β-cell function Efficacy: -Demonstrated efficacy in delaying the onset of insulin dependence in recent studies. - Shows potential in preserving β-cell function in newly diagnosed patients. Safety: Safety profile includes risks of infections, infusion reactions, and potential effects on the immune system. – Generally well-tolerated in clinical trials but needs further monitoring. Overall Teplizumab is an investigational therapy with promising results in delaying the progression of Type 1 Diabetes. It is a potential disease-modifying treatment. Not yet widely available but shows potential for preserving β-cell function in newly diagnosed patients. 1.Hydroxychloroquine (Plaquenil) Moa: Modulates immune response and reduces inflammation; interferes with antigen processing and immune cell activity Efficacy: -Well-established for managing SLE symptoms. It has been shown to reduce the risk of disease flares, decrease steroid dosage, prevent organ damage, and reduce thrombotic effects. In the case of Frexalimab, early data suggest it may improve disease outcomes by targeting specific immune pathways. However, the results are not yet fully established. Safety: Generally well-tolerated. - Side effects include gastrointestinal disturbances, retinal toxicity (rare), and skin rashes. For Frexalimab, the safety profile is under investigation. Specific safety data for SLE is not yet established. Overall: Hydroxychloroquine is a gold standard for managing SLE symptoms. 2. Belimumab (Benlysta) moa: Monoclonal antibody targeting B-lymphocyte stimulator (BLyS) to reduce B-cell activity and autoantibody production Efficacy: helps in reducing disease activity and flares in SLE. Proven to improve outcomes in clinical trials for moderate to severe SLE. Safety: Potential side effects include infusion reactions, infections, and potential impact on immune function. Generally well-tolerated in clinical trials but requires monitoring Overall Belimumab is an effective treatment for moderate to severe SLE, targeting a specific component of the immune system. Frexalimab could provide a new method for modulating immune response, complementing or offering an alternative to targeting BLyS |
Future of frexalimab
Research on its pharmacokinetics and ideal dosage pharmacodynamics is ongoing. Frexalimab, which targets the CD40-CD40L pathway, is a crucial medication in treating autoimmune disorders and can reduce disease activity and symptoms. Larger studies and extended follow-ups are necessary, though, to fully comprehend its therapeutic effect. All things considered, frexalimab has a bright future as a major contributor to the treatment of autoimmune diseases, with the potential to enhance patient outcomes and quality of life around the globe.
Potential long-term safety issues:
Infections: Higher rates of COVID-19 and other infections suggest potential immune suppression.
Neutropenia: Cases of neutropenia indicate possible effects on blood cells.
Liver enzyme elevations: Elevated ALT levels suggest potential liver toxicity.
Ongoing research:
Extended trials: Longer studies are needed to monitor long-term adverse effects.
Postmarketing surveillance: Tracking real-world safety in broader patient populations.
Comparative studies: Comparing frexalimab with other treatments to assess relative safety.
Biomarker monitoring: Analyzing biomarkers to predict and manage risks.
Conclusion
Complex mechanisms underlying autoimmune illnesses highlight the need for novel therapeutic approaches that go beyond existing ones. The immune system begins to attack the body’s tissues during an autoimmune illness. Overactivity of the CD40-CD40L pathway has been linked to inflammation and tissue destruction in several autoimmune disorders. By blocking this pathway anti-CD40L drugs have been found successful in alleviating experimental illnesses. Therefore, targeting signaling could serve as a novel therapeutic option for various autoimmune diseases. Recently, primary and secondary outcomes of frexalimab, a second-generation anti-CD40 drug, have been massively studied for clinical research. Due to its ability to reduce inflammation and the autoimmune response, frexalimab has completely revolutionized clinical research. With relapsing multiple sclerosis, frexalimab shows strong effectiveness in reducing the inflammatory cascade, which in turn reduces disease activity and may even help with very crippling relapses. Moreover, it shows promise in treating primary Sjögren’s syndrome, diabetes mellitus, and systemic lupus erythematosus (SLE). Trial results have shown that frexalimab is a better treatment choice for newly diagnosed type 1 diabetes mellitus (DM) patients. Amongst first-generation and second-generation anti-CD40 ligand drugs, frexalimab has the highest safety profile. It successfully lowers disease activity and symptoms while maintaining insulin secretion. It exhibits encouraging outcomes in lowering active brain lesions in relapse multiple sclerosis (MS). It shows promise in mitigating fatigue and decreasing the course of primary Sjögren’s syndrome (PSJS). Clinical research on frexalimab has turned out to be groundbreaking, predicting its golden future in treating autoimmune illnesses and patients’ health quality. Healthcare professionals are eagerly looking forward to forthcoming trials, yielding results that can provide substantial help to patients suffering from illnesses and bring more improvement to their quality of life.
Ethical approval
Not applicable.
Consent
Not applicable.
Source of funding
The authors did not receive any financial support for this work. No funding has been received for the conduct of this study.
Author contribution
All authors are contributed equally.
Conflicts of interest disclosure
The authors declare no conflict of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Aymar Akilimali.
Data availability statement
Not applicable.
Provenance and peer review
Not applicable.
Acknowledgement
The authors acknowledge all the authors for their equal contribution to this paper.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Tehreem Fatima, Email: tehreemfatima@kemu.edu.pk.
Aeliya Mirza, Email: aeliyaamirza@gmail.com.
Faiza Fatima, Email: faiza.fatima.039@gmail.com.
Riyan I. Karamat, Email: karamat.riyan@gmail.com.
Bilal Ahmad, Email: bilalahmadjajja@gmail.com.
Silla Naeem, Email: sillanaeem22@gmail.com.
Iqra Shahid, Email: Iqrashahid1412@gmail.com.
Aymar Akilimali, Email: aymarakilimali@gmail.com.
References
- 1. Ots HD, Tracz JA, Vinokuroff KE, et al. CD40–CD40L in neurological disease. Int J Mol Sci 2022;23:4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawabe T, Matsushima M, Hashimoto N, et al. CD40/CD40 Ligand interactions in immune responses and pulmonary immunity. Nagoya J Med Sci 2011;73:69–78. [PMC free article] [PubMed] [Google Scholar]
- 3. Elgueta R, Benson MJ, De Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol 2009;21:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadul CE, Mao-Draayer Y, Ryan KA, et al. Safety and immune effects of blocking CD40 ligand in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation 2021;8:e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang T, Cheng X, Truong B, et al. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther 2021;219:107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vial G, Gensous N, Duffau P. L’axe CD40-CD40L : implications actuelles et futures en immunologie clinique. Rev Médecine Interne 2021;42:722–728. [DOI] [PubMed] [Google Scholar]
- 8. Cree BAC, Hartung HP, Barnett M. New drugs for multiple sclerosis: new treatment algorithms. Curr Opin Neurol 2022;35:262–270. [DOI] [PubMed] [Google Scholar]
- 9. Vermersch P, Granziera C, Mao-Draayer Y, et al. Inhibition of CD40L with frexalimab in multiple sclerosis. N Engl J Med 2024;390:589–600. [DOI] [PubMed] [Google Scholar]
- 10. Delva I. Fatigue in multiple sclerosis. East Ukr Med J 2022;10:309–317. [Google Scholar]
- 11. Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 2010;221:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kasper LH, Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology 2010;74(suppl_1):S2–S8. [DOI] [PubMed] [Google Scholar]
- 13. Wynn TA. IL-13 effector functions. Annu Rev Immunol 2003;21:425–456. [DOI] [PubMed] [Google Scholar]
- 14. Arneth BM. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation 2019;16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharpe AH. Mechanisms of costimulation. Immunol Rev 2009;229:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. June CH, Ledbetter JA, Linsley PS, et al. Role of the CD28 receptor in T-cell activation. Immunol Today 1990;11:211–216. [DOI] [PubMed] [Google Scholar]
- 17. Karnell JL, Rieder SA, Ettinger R, et al. Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv Drug Deliv Rev 2019;141:92–103. [DOI] [PubMed] [Google Scholar]
- 18. Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J Yakhteh 2017;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neagos D, Cretu R, Tutulan-Cunita A, et al. Methylenetetrahydrofolate dehydrogenase (MTHFD) enzyme polymorphism as a maternal risk factor for trisomy 21: a clinical study. J Med Life 2010;3:454–457. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


