Abstract
Introduction and importance:
Bartter syndrome is a rare autosomal recessive disorder affecting renal tubular function leading to disturbances in electrolyte and volume homeostasis. It can also manifest as Bartter-like syndrome (BLS), a rare side effect of certain medications. Polymyxin-B, an antibiotic used to treat multidrug-resistant infections is infrequently associated with BLS. Hence, early diagnosis of this adverse effect is crucial to prevent severe electrolyte imbalances.
Case presentation:
A 73-year-old female with coronary artery disease, chronic obstructive pulmonary disease, and hyperlipidemia, presented with fever, respiratory distress, and hypoxia on mechanical ventilation. Initial labs showed leukocytosis, anemia, and normal potassium. Despite receiving broad-spectrum antibiotics there was no improvement in her clinical condition. A sputum culture revealed pandrug-resistant Acinetobacter baumannii, sensitive only to Polymyxin-B. After six days of receiving polymyxin-B, the patient developed fever, hypotension, hypokalemia, hypomagnesemia, and polyuria. Urine studies indicated increased potassium excretion. A diagnosis of BLS was made. Polymyxin-B was discontinued, and the patient’s electrolytes normalized. She was discharged with daily potassium and magnesium supplements.
Clinical discussion:
BLS can result from polymyxin-B-induced tubular dysfunction characterized by hypokalemia and hypomagnesemia. Early recognition allowed for the timely discontinuation of polymyxin-B, which rapidly reversed her electrolyte disturbances.
Conclusion:
This case underscores the importance of recognizing polymyxin-B-induced BLS. Clinicians should be vigilant for electrolyte disturbances in patients receiving treatment with polymyxin-B, ensuring timely interventions to mitigate adverse outcomes.
Keywords: Bartter-like syndrome, hereditary disorder, pneumonia, polymyxin-B
Introduction
Highlights
Bartter syndrome is a rare autosomal recessive disorder affecting 1 in 1,000,000 individuals.
Bartter syndrome can also present as a rare side effect called Bartter-like syndrome after receiving polymyxin-B.
A 73-year-old female being treated for pneumonia caused by Acinetobacter baumanii developed Bartter-like syndrome after receiving 6 days of polymyxin-B.
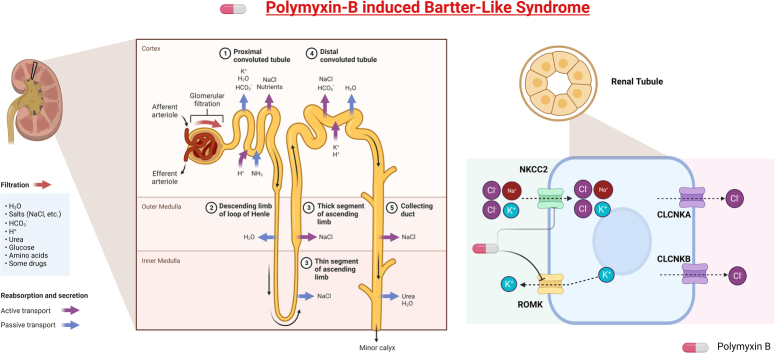
Bartter syndrome is a rare hereditary disorder with an autosomal recessive inheritance affecting 1 in 1,000,000 individuals. The disorder primarily affects the thick ascending limb of loop of Henle in the kidneys, hindering the ability to reabsorb sodium and chloride leading to unwanted losses of salt and water1. The condition is characterized by hypokalemia, hypomagnesemia, and metabolic alkalosis. Clinically, patients may present with low/normal blood pressure alongside metabolic derangements2. Polymyxins is a class of antibiotic with bactericidal properties comprising of polymyxin-B and polymyxin-E (Colistin). It is indicated for use in patients with multidrug-resistant gram-negative bacteria such as Enterobacteriaceae, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter Baumannii 3,4. The polymyxin class of drugs have shown to be useful for infections of the blood-stream, urinary tract, respiratory tract, and central nervous system3. In this particular case, the patient was treated with Polymyxin-B for a pandrug-resistant Acinetobacter Baumani respiratory infection. The patient was treated for a total of 6 days following which she developed Bartter-Like syndrome (BLS). An adverse effect of Polymyxin-B was suspected and discontinuation of the offending drug resulted in full clinical and metabolic recovery (Fig. 1).
Figure 1.
Polymyxin-B induced Bartter-Like Syndrome. CLCNKA and CLCNKB, Chloride voltage-gated Channels; NKCC2, Sodium Potassium 2 Chloride transporter; ROMK, Renal Outer Medullary Potassium Channel. Created with BioRender.com.
Case presentation (ST)
A 73-year-old female with a past medical history of coronary artery disease, chronic obstructive pulmonary disease, hyperlipidemia, hypothyroidism, schizoaffective disease, and seizure disorder presented to the hospital from a nursing home with complaints of fever, respiratory distress, and hypoxia with an oxygen saturation of 74% on mechanical ventilation. On initial presentation, the patient had a temperature of 103oF, a heart rate (HR) of 134 bpm, blood pressure (BP) of 132/68 mmHg, and was saturating 100% with mechanical ventilation (MV) settings of 20/400/100/5. On physical examination, pertinent findings included bilateral rhonchi, tachycardia, and the abdominal examination was remarkable for percutaneous endoscopic gastrostomy (PEG) site with black/brown colored discharge, presumably gastric contents. Initial laboratory values showed: hemoglobin: 9 gm/dl, white blood cell: 19.4 ku/l, Platelets: 171 ku/l, blood urea nitrogen: 22 mg/dl, creatinine: 0.70 mg/dl, albumin: 2.7 gm/l, sodium: 136 mmol/l, potassium: 4.6 mmol/l, phosphate: 3.9 mg/dl, Calcium: 8.7 mg/dl, magnesium: 1.7 mg/dl, and procalcitonin: 0.80 ng/ml, lactate: 2.9 mmol/l. A baseline Chest X-Ray (CXR) was obtained, which showed right perihilar lung base atelectasis/infiltrates (Fig. 2). Broad spectrum antibiotic treatment with intravenous (IV) Meropenem 1 g Q8HR and IV Vancomycin 1 g Q12HR were initiated for suspected pneumonia, while sputum culture, blood culture, and urine culture were pending.
Figure 2.
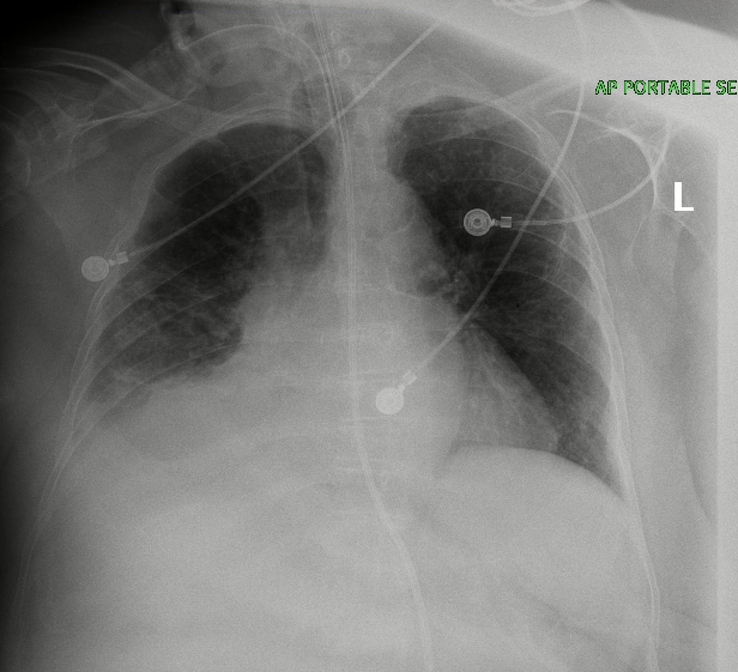
Initial CXR on admission: Right perihilar lung base atelectasis/infiltrates.
During her course of stay in the hospital, a nasogastric tube (NGT) was placed and PEG tube leakage site was clipped, a repeated CXR showed worsening right basilar pneumonia (Fig. 3). On day four, the sputum culture returned positive for pandrug-resistant Acinetobacter Baumannii with sensitivity to only polymyxin-B. IV polymyxin-B 800 000 units Q12HR was initiated and vancomycin was discontinued. Six days following the administration of polymyxin-B, the patient developed a fever with a temperature of 104.3°F and her BP had decreased to 87/53 mmHg with a stable respiratory status on MV with settings 20/400/40/5. Laboratory values showed: potassium: 2.9 mmol/l, magnesium: 1.03 mg/dl, and calcium: 7.4 mg/dl. Serum sodium and chloride were within the reference range. Due to persistent hypotension, norepinephrine drip, and stress dose hydrocortisone were administered to stabilize the blood pressure. For acute features of sepsis, vancomycin 1G IV Q12H was restarted on Day 10 before being stopped due to supratherapeutic treatment for 3 days. Vancomycin was subsequently switched to Linezolid 600 mg IV Q12H on Day 13 and at this time, the patient was also receiving Micafungin 100 mg IV daily with polymyxin-B.
Figure 3.
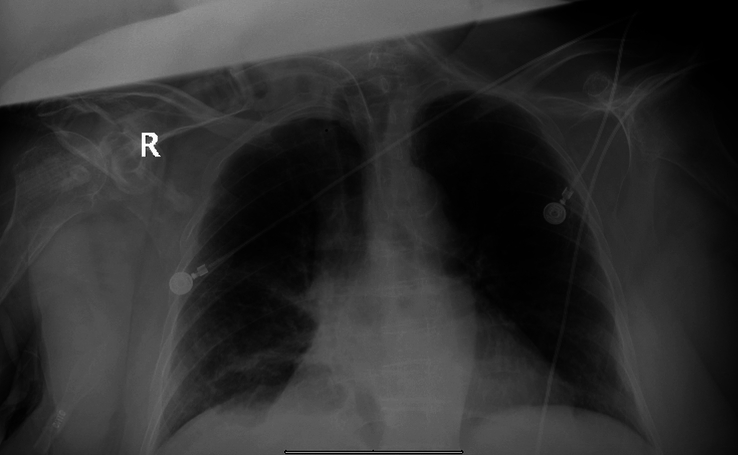
CXR depicting right basilar pneumonia.
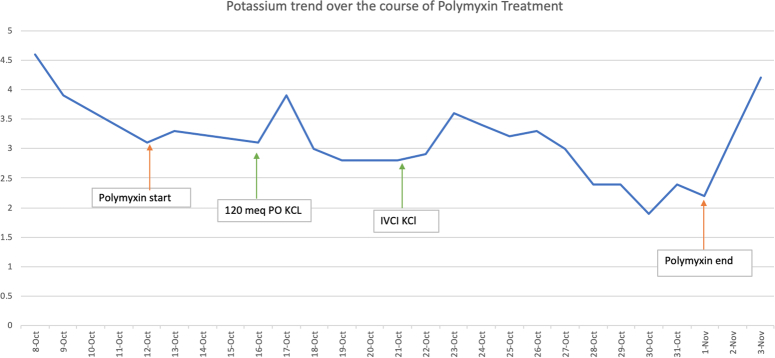
On Day 6 of treatment with polymyxin-B, the patient started developing hypomagnesemia from a level of 1.82 mg/dl to 1.03 mg/dl and subsequently developed marked hypokalemia on the same day from a potassium level of 3.9 mmol/l to 3.0 mmol/l. This hypokalemia and hypomagnesemia markedly decreased in the following days of treatment with polymyxin-B. During this time, the patient also had polyuria (6 l, 2.17–3.27 ml/kg/h) with normal blood pressure. The patient did not have vomiting or diarrhea, but there was gastric content leaking from her PEG site. Except for hypokalemia and hypomagnesemia, other basic metabolic profile levels were within the normal range. Urine studies showed a low urine potassium level and urine creatinine was 4.1 mg/dl. All of the above antibiotics were discontinued due to persistent hypokalemia/hypomagnesemia and resolving sepsis. The urinary potassium significantly increased after the polymyxin-B was discontinued. The patient’s baseline urinary K+ was around 19 mmol/l, and it subsequently increased to 33.2 mmol/l following the discontinuation of polymyxin-B.
2 days after discontinuation of polymyxin-B, the serum potassium level improved significantly, and polyuria resolved. After all the electrolytes were replenished to the normal range, the PEG tube was replaced, and the patient was discharged to a short-term rehabilitation facility with daily potassium and magnesium supplements (Figs 2–4).
Figure 4.
Timeline and trend of potassium following initiation of polymyxin-B.
Discussion
This case demonstrates the importance of recognizing the side effects and rare syndromes associated with administering antibiotics. This patient’s cause of hypokalemia was multifactorial, however, the renal loss of potassium due to BLS was the most significant contributor to hypokalemia as Tubular Transmembrane Potassium Gradient (TTKG) 7 and Fractional excretion of potassium (FeK) 42% supported the renal potassium loss. As demonstrated in the flow chart by Anju et al.5;(Fig. 1)], the patient had persistent hypokalemia despite adequate replacement of potassium which eventually improved after discontinuation of polymyxin-B. This patient had a spot urinary potassium of 35 meq/l (15–20 mEq/l), a metabolic alkalosis with a serum bicarbonate of 28 mmol/l and an elevated urine chloride of 140 mmol/l, TTKG 7 and FeK 42%, supporting the diagnosis of BLS5. In addition, the urine potassium/creatinine ratio was 350 mmol/l indicating active renal potassium losses. This patient’s spot elevated urine potassium, elevated fractional excretion of potassium, elevated urine potassium/creatinine ratio points towards active renal losses even though the patient did have extra renal losses through the PEG tube, which overlapped with the duration of polymyxin-B.
The median time of onset after exposure to the inciting agent was 10 days (range 7–18 days)2,5,6. In this case, the patient developed electrolyte abnormalities after only 6 days of polymyxin-B 800 000U Q12HR. As a result of BLS, this patient’s hypokalemia became very difficult to correct, which required her to be transferred to the coronary care unit for continuous telemetry monitoring. Numerous attempts to correct this patient’s potassium with both enteral and parenteral potassium chloride were made, with a goal potassium of 4.0 mmol/l.
BLS occurs more commonly in patients who are prescribed aminoglycosides (65.1%) and gentamicin (41.8%), however, polymyxins are responsible in 32.5% of cases5. Nine previous cases of polymyxin-B induced BLS have been shown in Table 1. Table 2 depicts the five different types of Bartter syndromes and their characteristics. The search criteria for these cases included not only the disease process and antibiotics of interest but also similar age groups and severity of illness.
Table 1.
Previous cases reported in the literature.
| Case | Age | Sex | Diagnosis | # of days of BLS following Polymyxin | Electrolyte abnormalities | Management/ Outcome | PMID/Reference |
|---|---|---|---|---|---|---|---|
| 1 | 32 | F | Respiratory infection with Klebsiella and Pseudomonas | 6 | hypokalaemia, hypocalcemia | Not reported | 382297822 |
| 2 | 28 weeks | F | Bloodstream infection with Acinetobacter baumannii | 13 | hypokalemic metabolic alkalosis, hypomagnesemia, hypocalcemia | Electrolyte recovery following cessation | 233429926 |
| 3 | 46 | M | Respiratory infection with Acinetobacter baumannii | 3 | Hypokalaemia, hypocalcaemia, hypomagnesemia, and metabolic alkalosis | Electrolyte recovery following cessation | 320295157 |
| 4 | 58 | F | Lower Limb Ulcer infection by extensively drug-resistant (XDR) Pseudomonas aeruginosa | 2 weeks | Hypokalaemia, hypocalcaemia, hypophosphatemia, and hypomagnesaemia | Electrolyte recovery following cessation | 86991148 |
| 5 | 32 | M | Sacral osteomyelitis by XDR Klebsiella pneumoniae | 30 days | hypomagnesaemia, hypokalaemia, and metabolic alkalosis | Electrolyte recovery following cessation | https://www.emjreviews.com/wp-content/uploads/2018/07/Prolonged-Intravenous-Colistin-Use-Associated-with-Acquired-Bartter-Like-Syndrome-in-An-Adult-Patient.pdf |
| 6 | 43 | F | Cholecystitis | 12 days | hypomagnesemia and hypocalcemia | Electrolyte recovery following cessation | 357127405 |
| 7 | 23 | M | Respiratory infection with Acinetobacter baumannii | 7 days | hypokalemia, hypomagnesemia, and metabolic alkalosis | Electrolyte recovery following cessation | 357127405 |
| 8 | 57 | M | Peritonitis | 7 days | hypokalemia, hypomagnesemia, and hypocalcemia, along with metabolic alkalosis | Electrolyte recovery following cessation | 357127405 |
| 9 | 50 | M | Bloodstream infection with Acinetobacter baumannii | 8 days | hypokalemia, hypomagnesemia, hypocalcemia, and metabolic alkalosis | Electrolyte recovery following cessation | 357127405 |
| 10 | 20s | M | multidrug-resistant Klebsiella pneumoniae | 7 days | metabolic alkalosis with hypokalaemia and hypomagnesaemia | 6 days | 387020709 |
Table 2.
Bartter syndrome types.
| Types | Affected gene | Laboratory findings | Clinical characteristics | References/ PMID |
|---|---|---|---|---|
| Type I | NKCC2 | Hypokalemia, hypercalciuria, hypercalcemia, increased urinary potassium and chloride | Fetal polyuria, polyhydramnios, nephrocalcinosis, osteopenia | 2872304810,11 |
| Type II | ROMK | Hyperkalemia at birth, then hypokalemia; hypercalciuria, increased urinary potassium and chloride, hyposthenuria | Nephrocalcinosis, failure to thrive, osteopenia, vomiting, diarrhea | |
| Type III | CIC-Kb | Hypokalemia, metabolic alkalosis, increased urinary potassium and chloride, hypocalciuria or normocalciuria | Short stature, mental retardation, hypotensive | |
| Type IVa | BSND | Hypokalemia, hyponatremia, hypochloremia, increased urinary sodium, potassium and chloride | Renal failure, sensorineural deafness, motor retardation | |
| Type IVb | CIC-Ka, CIC-Kb | |||
| Type V | CaSr | Hypokalemia, metabolic alkalosis, hypocalcemia, hypercalciuria | Nephrocalcinosis, hypotension, seizures |
11
3359715912 |
Given the resistance of the Acinetobacter Baumannii, polymyxin-B was the drug of choice by the infectious disease specialist for a recommended total duration of 21–28 days. After a multidisciplinary approach with the nephrologist and infectious disease specialist, the decision to discontinue polymyxin-B after 21 days of therapy was made given the clinical improvement of this patient’s ventilator-associated pneumonia.
After discontinuing polymyxin-B, the patient’s electrolytes were corrected within 2 days of discontinuing therapy (Fig. 4). The patient concomitantly had PEG tube leakage of gastric contents further complicating the management of electrolyte derangements. The urine electrolytes supported extra renal losses which was evident by GI losses. However, following polymyxin-B initiation, urine electrolyte excretion doubled despite ongoing PEG tube leakage indicating polymyxin-B’s effect on the renal tubules as well. In other reported cases of BLS, the median time for resolution after stopping the drug was 14 days (range 7–31 days)5. This case is unique, given how quickly the BLS presented and resolved after discontinuation of the medication. The onset of the BLS was on Day 6 of treatment for this patient, and the resolution of symptoms after polymyxin-B discontinuation was at 2 days following a prolonged treatment course. When compared to other reported cases of BLS secondary to polymyxin use, this patient is of a markedly older age-group and the electrolyte derangements were complicated by GI losses through the PEG-tube. Most of the cases have been reported in age groups ranging from infants to middle-aged adults and a similar case was reported in a 23-year-old male with a respiratory infection due to Acinetobacter Baumannii5 just as this patient. All forms of Bartter syndrome present before birth, soon after birth or in very early childhood except for Type 3 Bartter Syndrome. Laboratory findings of hypokalemia, metabolic alkalosis, increased urinary potassium and chloride in Type 3 Bartter syndrome closely mimic the laboratory findings of this reported patient, except for the classic clinical findings. Given the rise in multi-drug resistant bacteria and the necessary use of polymyxin-B antibiotics for the treatment of these organisms, BLS should be a part of our differential diagnosis when encountering persistent electrolyte disturbances in clinical practice despite appropriate medical therapy.
Conclusion
The authors of this case report shed light on the occurrence of BLS secondary to polymyxin-B use, highlighting a rare adverse effect. While the disorder’s onset due to pharmacological agents has been reported, instances linked to polymyxin-B are exceptionally rare. This case presentation emphasizes the importance of vigilant monitoring for adverse effects, aiding clinicians in early identification and timely management. In this case, the patient made a full recovery.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
S.T., R.H., G.P., M.A., M.F., D.F., P.K., M.T., and Y.S.: writing – original draft and writing – review and editing; A.M.: overview of manuscript, final review and editing, and submission process; I.P.: conceptualization, writing – original draft, writing – review and editing, supervision, review, and final approval; T.B.E.: writing – reviewing and editing, visualization, and supervision.
Conflicts of interest disclosure
The authors have no conflict of interest. Also, the authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research registration unique identifying number (UIN)
Name of the registry: Not applicable.
Unique Identifying number or registration ID: Not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Talha Bin Emran.
Data availability statement
All data are available in manuscript.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Acknowledgement
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Sophia Taik, Email: dhawanmanish5@gmail.com.
Razi Hashmi, Email: manish.e13@cumail.in.
Arun Mahtani, Email: arun.mahtani@nyu.edu.
Gianpaolo Piccione, Email: alkaabin1@gmail.com.
Mohamed Albakri, Email: maha7@gmail.com.
Meena Farid, Email: MSA@HOTMAIL.COM.
Daniel Fabian, Email: Youse@machs.edu.sa.
Merschelle Tindoy, Email: Tindoy34@gmail.com.
Yashendra Sethi, Email: yashendrasethi@gmail.com.
Inderbir Padda, Email: ipadda@rumcsi.org.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd;talhabmb@gmail.com.
References
- 1. Bokhari SRA, Zulfiqar H, Mansur A. Bartter Syndrome [Updated 2023 Sep 4] In: StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK442019/ [PubMed] [Google Scholar]
- 2. Dessai S, Deshpande H. IV colistin: a rare cause of bartter-like syndrome in adults. Cureus 2023;15:e50672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shatri G, Polymyxin TP. StatPearls. StatPearls Publishing; 2023. Accessed 4 July 2023.https://www.ncbi.nlm.nih.gov/books/NBK557540/. [PubMed] [Google Scholar]
- 4. Allison DG, Lambert PA. Chapter 32 - modes of action of antibacterial agents. Molecular Medical Microbiology 2nd edn. Academic Press; 2015:583–598. [Google Scholar]
- 5. Kumari A, Gupta P, Verma H, et al. Colistin-induced Bartter-like syndrome: ponder before treatment!. Indian J Crit Care Med 2022;26:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cakir U, Alan S, Zeybek C, et al. Acquired bartter-like syndrome associated with colistin use in a preterm infant. Ren Fail 2013;35:411–413. [DOI] [PubMed] [Google Scholar]
- 7. Tabish M, Mahendran M, Ray A, et al. Colistin-induced acquired Bartter-like syndrome: an unusual cause of meltdown. BMJ Case Rep 2020;13:e232630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamal Eldin T, Tosone G, Capuano A, et al. Reversible Hypokalemia and Bartter-Like syndrome during prolonged systemic therapy with colistimethate sodium in an adult patient. Drug Saf Case Rep 2017;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohan Lal B, Musthafa Hafeesa N, Vikram NK, et al. Polymyxin B-induced Bartter syndrome. BMJ Case Rep 2024;17:e255242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bokhari SRA, Zulfiqar H, Mansur A. Bartter Syndrome. 2023 Sep 4. StatPearls. StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK442019/ [PubMed] [Google Scholar]
- 11. Yu ASL, Chertow GM, Luyckx V, et al. Brenner & Rector’s the Kidney, 11th edn. Elsevier; 2020. [Google Scholar]
- 12. Hussain A, Atlani M, Goyal A, et al. Type-5 Bartter syndrome presenting with metabolic seizure in adulthood. BMJ Case Rep 2021;14:e235349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in manuscript.