Abstract
The opioid system plays crucial roles in modulating social behaviors in both humans and animals. However, the pharmacological profiles of opioids regarding social behavior and their therapeutic potential remain unclear. Multiple pharmacological, behavioral, and immunohistological c-Fos mapping approaches were used to characterize the effects of μ-opioid receptor agonists on social behavior and investigate the mechanisms in naive mice and autism spectrum disorder–like (ASD-like) mouse models, such as prenatally valproic acid–treated mice and Fmr1-KO mice. Here, we report that low-dose morphine, a μ-opioid receptor agonist, promoted social behavior by selectively activating neurons in prosocial brain regions, including the nucleus accumbens, but not those in the dorsomedial periaqueductal gray (dmPAG), which are only activated by analgesic high-dose morphine. Critically, intra-dmPAG morphine injection counteracted the prosocial effect of low-dose morphine, suggesting that dmPAG neural activation suppresses social behavior. Moreover, buprenorphine, a μ-opioid receptor partial agonist with less abuse liability and a well-established safety profile, ameliorated social behavior deficits in two mouse models recapitulating ASD symptoms by selectively activating prosocial brain regions without dmPAG neural activation. Our findings highlight the therapeutic potential of brain region–specific neural activation induced by low-dose opioids for social behavior deficits in ASD.
Keywords: Neuroscience, Therapeutics
Keywords: Behavior, Neurodevelopment, Pharmacology
Non-analgesic low-dose opioid activates pro-social brain regions to ameliorate sociability deficits in ASD models.
Introduction
Animals including humans and rodents live in a world that is largely socially constructed, where they conduct a wide miscellany of complex social interactions. Social behaviors are essential for the health, survival, and reproduction of animals. Conversely, social behavioral deficits are key features of several neuropsychiatric disorders, especially autism spectrum disorder (ASD). ASD is a neurodevelopmental disorder defined by social communication impairments and restricted, repetitive behaviors (1, 2). Despite the global increase in the ASD burden and the growing demand for effective medications (3), there is currently no approved drug for the core symptoms of ASD ASD; rather, there are only drugs for its comorbidities, such as irritability.
Neuropeptides, such as oxytocin and arginine vasopressin, play a central role in social function. Several molecules targeting oxytocin and vasopressin signaling have been developed in clinical trials for ASD therapeutics. However, they all failed to demonstrate clinically meaningful improvement of social function in patients with ASD (4, 5). The reasons for the failures include (a) a substantial disparity in these pathways between humans and rodents and (b) the optimal levels of oxytocin and vasopressin signaling are unknown.
The opioid system plays a critical role in social behavior as well as pain sensation. In particular, the crucial role of μ-opioid receptors (MORs) in social function has been revealed by genetic and pharmacological studies. For instance, deletions and duplications of genomic regions covering the OPRM1 gene were identified in patients with ASD (6), and Oprm1-mutant mice showed ASD-like symptoms, including sociability deficits and repetitive behaviors (7). However, the optimal level and even directionality (activation/inhibition) of MOR signaling regulation to promote social behavior are controversial (8–11) and have yet to be elucidated, particularly under the pathological condition of ASD.
Here, we aim to elucidate the effects of a wide range of doses of morphine and buprenorphine on social behavior and pain sensation in naive and ASD-like model mice. Moreover, we performed c-Fos expression mapping across the brain regions involved in social behavior to obtain mechanistic insights into the roles of each region in the pharmacological effects of MOR agonists. Thus, we report that only low doses of MOR agonists without an analgesic effect promoted social behaviors in mice by specifically activating prosocial brain regions, such as the nucleus accumbens (NAc). These doses did not activate the dorsomedial periaqueductal gray (dmPAG), which was only activated by high doses of MOR agonists, resulting in inhibition of social behavior. Nonanalgesic low doses of MOR agonists could be potential therapeutic options for sociability deficits in ASD.
Results
Low-dose morphine promotes social behavior in naive and valproic acid model mice.
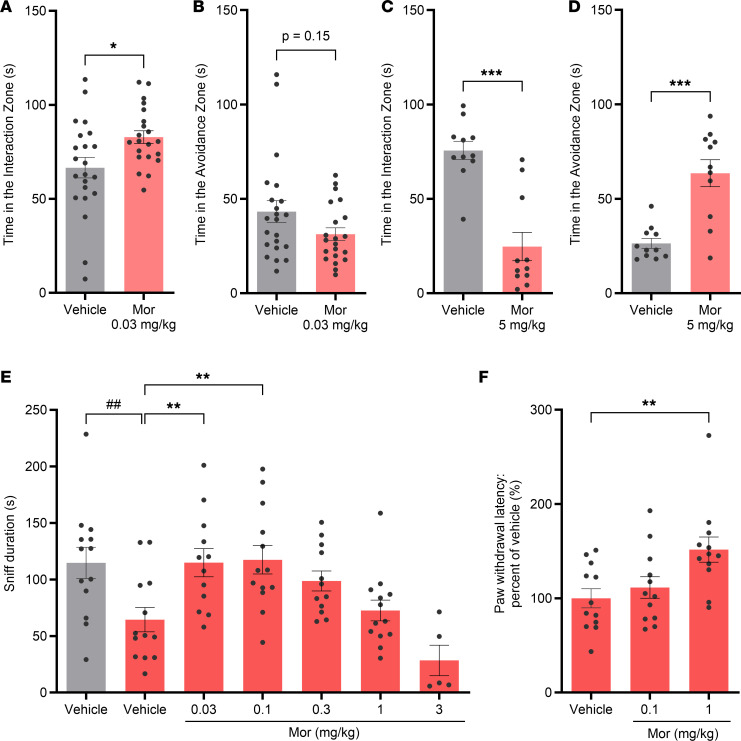
First, we assessed the effects of systemic morphine administration on social behaviors of naive C57BL/6J (B6) mice using the single-chamber social interaction test (SIT). Morphine (0.03 mg/kg, s.c. administration) significantly increased the time spent in the interaction zone when an unfamiliar B6 mouse was present (Figure 1A), whereas it did not change the time spent in the empty session without an unfamiliar mouse (Supplemental Figure 2, A and B; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.182060DS1). The time spent in the avoidance zone tended to be decreased by the administration of 0.03 mg/kg morphine (P = 0.15 compared with vehicle-treated animals: Figure 1B), whereas such a trend was not observed in the empty session. In contrast, 5 mg/kg morphine significantly decreased the time spent in the interaction zone and increased that in the avoidance zone (Figure 1, C and D). In the empty session, 5 mg/kg morphine decreased the time spent in the interaction zone but did not change the time spent in the avoidance zone (Supplemental Figure 2, D and E). High doses of morphine have been reported to induce hyperlocomotion (12), which could affect the readouts in SIT. Therefore, we examined the effect of morphine on locomotor activity (Supplemental Figure 3A) and found that only 5 mg/kg morphine significantly increased locomotor activity. While hyperlocomotion could contribute to the decrease in time spent in the interaction zone in SIT, it would be unlikely that hyperlocomotion would lead to an increase in time spent in the avoidance zone. Taken together with a greater decrease in time spent in the interaction zone in the target session compared with that spent in the empty session, 5 mg/kg morphine would, at least to some extent, suppress the sociability of the mice. Thus, systemic morphine administration enhanced social interaction behaviors only when a low dose was administered, and that effect was inverted with a higher dose.
Figure 1. Morphine increased social behaviors in mice only at low doses.
(A–D) Effects of systemic administration of morphine on social behaviors in the single-chamber social interaction test (SIT). Single-housed C57BL/6J mice were s.c. injected with morphine (0.03 or 5 mg/kg) or vehicle at 12–13 weeks of age. The time spent in the interaction zone (A and C) and the avoidance zone (B and D) during a 3-minute test period with an unfamiliar mouse kept in a cage (target session) is shown. n = 21–22 (A and B) and 11 animals (C and D). (E) Effects of systemic administration of morphine on social interaction deficits in mice prenatally exposed to valproic acid (VPA) in the reciprocal SIT. VPA (500 mg/kg) or saline was intraperitoneally injected into pregnant CD-1 mice at embryonic day 12.5. Male offspring at 8 weeks of age were s.c. injected with morphine (0.03, 0.1, 0.3, 1, 3 mg/kg) or vehicle, and the duration of sniffing was measured during a 20-minute test period. n = 5–13 animals. (F) Effects of morphine on thermal nociception in prenatally VPA-treated mice. The withdrawal latency at 49°C was measured with the hot-plate test at 1 hour after administration of morphine (0.1, 1 mg/kg, s.c.). n = 12 animals. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, by the parametric tests (A, D, and E) or nonparametric ones (B, C, and F) compared with vehicle-treated mice. ##P < 0.01 compared with mice prenatally exposed to saline (control). Mor, morphine.
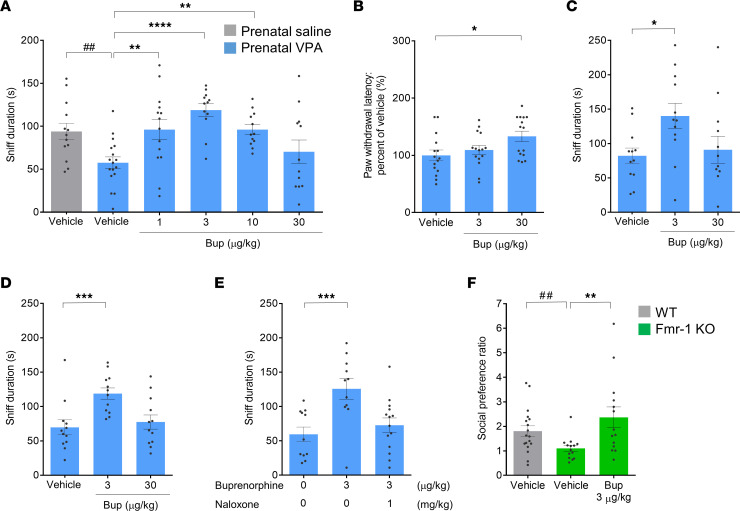
Next, we examined the effect of morphine on social interaction deficits in mice prenatally exposed to valproic acid (VPA), which are commonly used as an animal model of ASD (13–16), based on the evidence that fetal valproate exposure is associated with an increased risk of autism and ASDs in humans (17, 18). VPA model was reported to show social behavior deficit in reciprocal SIT but not in the chamber-based SIT without direct physical interactions (19). Therefore, we evaluated the effect of morphine on social behavior of the VPA model using reciprocal SIT. Consistent with results of previous studies, we observed social interaction deficits in VPA-treated mice compared with the behavior of prenatal saline-treated control mice (Figure 1E). These deficits were significantly improved by s.c. administration of lower doses of morphine (0.03 and 0.1 mg/kg), but not with higher doses (0.3, 1, or 3 mg/kg), resulting in an inverted U-shaped dose response (Figure 1E). These findings suggest that systemic administration of low doses of morphine can improve social behaviors in VPA-treated ASD-like animals as well as naive animals (Figure 1, A–D).
To further clarify the dose-response relationship of the effects of morphine on sociability and pain sensitivity, we evaluated pain-related behaviors in mice. Dose-dependent antinociceptive efficacy of morphine in intact mice was detected at doses greater than 1 mg/kg (s.c. administration) (20–24). In VPA-treated mice, we found that 1 mg/kg, but not 0.1 mg/kg, morphine significantly increased the paw-withdrawal latency in the hot-plate test (Figure 1F). These data indicate a difference between the effective doses for suppressing pain behavior and promoting social behavior.
The effects of morphine on neural activation across several brain regions in naive mice.
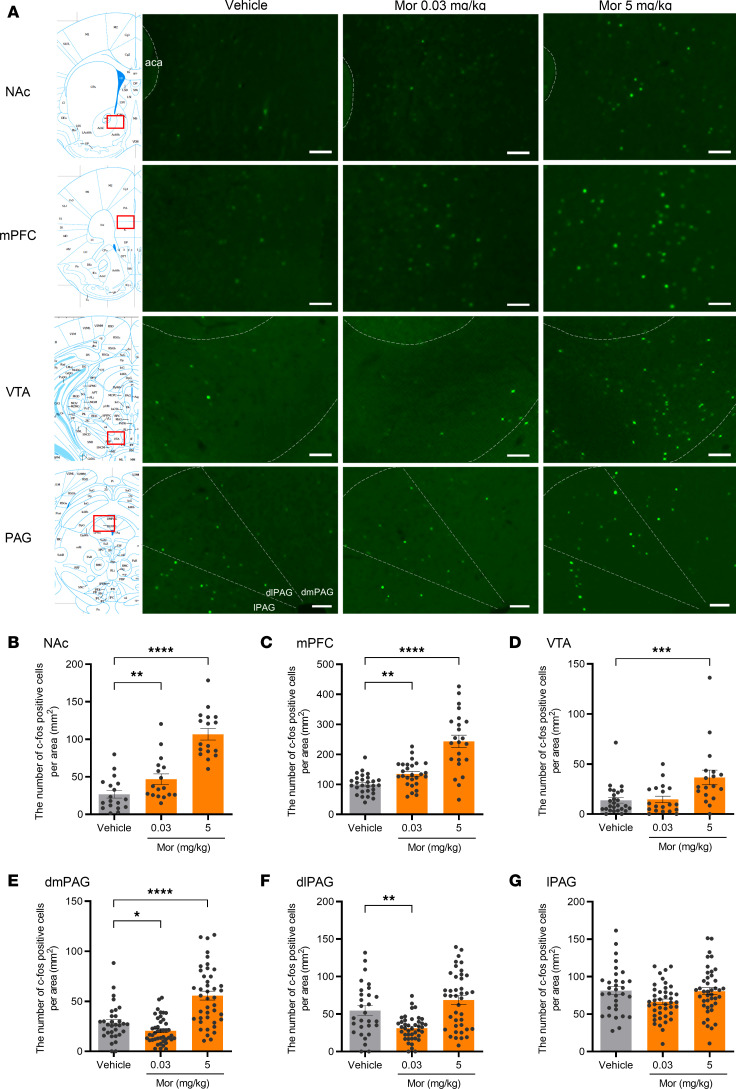
To identify the brain regions involved in the effects of morphine in promoting social behavior, we quantified neuronal activation in several brain regions using c-Fos immunohistochemistry, which is a marker of neuronal activity. Mice were euthanized 1.5 hours after s.c. administration of morphine (0.03 and 5 mg/kg), and the number of c-Fos–positive cells was quantified using our established automated counting system (Supplemental Figure 1). Initially, we focused on the NAc and medial prefrontal cortex (mPFC) because these regions are known to have a high density of MORs (25–28) and play critical roles in modulating social behavior (10, 29, 30). At the low dose (0.03 mg/kg), we observed a significant increase in the number of c-Fos–positive cells in both the NAc and mPFC (Figure 2, A–C) (31), indicating that, even at such a low dose, morphine could affect neuronal activity in the central nervous system. Consistent with findings in previous reports (32–35), the higher dose of morphine (5 mg/kg) further increased the number of c-Fos–positive cells in both regions (Figure 2, A–C).
Figure 2. Brain area–specific activation by systemic morphine administration in mice.
Morphine (0.03 or 5 mg/kg) or vehicle was s.c. administered to naive C57BL/6J mice 1.5 hours before sampling the brain. (A) Representative images of c-Fos–immunostained brain sections of the NAc, mPFC, VTA, and dorsal PAG with the brain atlas map (31). Scale bar: 100 μm. (B–G) Quantification of c-Fos–positive cells in each brain region. Results from the NAc (B, 17–18 sections/group from 4 mice/group), mPFC (C, 23–28 sections/group from 4 mice/group), VTA (D, 18–26 sections/group from 4 mice/group), dmPAG (E, 32–43 sections/group from 4 mice/group), dlPAG (F, 29–42 sections/group from 4 mice/group), and lPAG (G, 32–42 sections/group from 4 mice/group) are shown. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.01, ****P < 0.0001, by the parametric tests (C, F, and G) or nonparametric ones (B, D, and E) compared with vehicle-treated mice. Mor, morphine; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; VTA, ventral tegmental area; dmPAG, dorsomedial periaqueductal gray; dlPAG, dorsolateral periaqueductal gray; lPAG, lateral periaqueductal gray.
Next, we evaluated neuronal activity in the ventral tegmental area (VTA), a brain region associated with addiction and analgesia induced by opioids (36). Although 5 mg/kg morphine, which is analgesic and produces tolerance and dependence with repeated administration (37, 38), significantly increased the number of c-Fos–positive cells in the VTA, 0.03 mg/kg morphine, a prosocial dose, did not have this effect (Figure 2, A and D).
We further investigated the dorsal PAG, which plays critical roles in opioid-induced analgesia and social behaviors (39, 40). Because Franklin et al. reported that activation of the PAG, especially the dorsomedial part (dmPAG), suppressed social interaction behavior (41), we postulated that a high dose of morphine with an analgesic effect would activate the dmPAG and induce an inhibitory effect on social behavior. Expectedly, the dmPAG was activated by administration of 5 mg/kg morphine (Figure 2, A and E), which did not promote social behavior (Figure 1, C and D). In contrast, 0.03 mg/kg morphine did not increase the number of c-Fos–positive cells in any of the 3 subregions (dorsomedial, dorsolateral, or lateral) of the PAG (Figure 2, A and E–G).
The prosocial effect of low-dose morphine is counteracted by activation of the dmPAG.
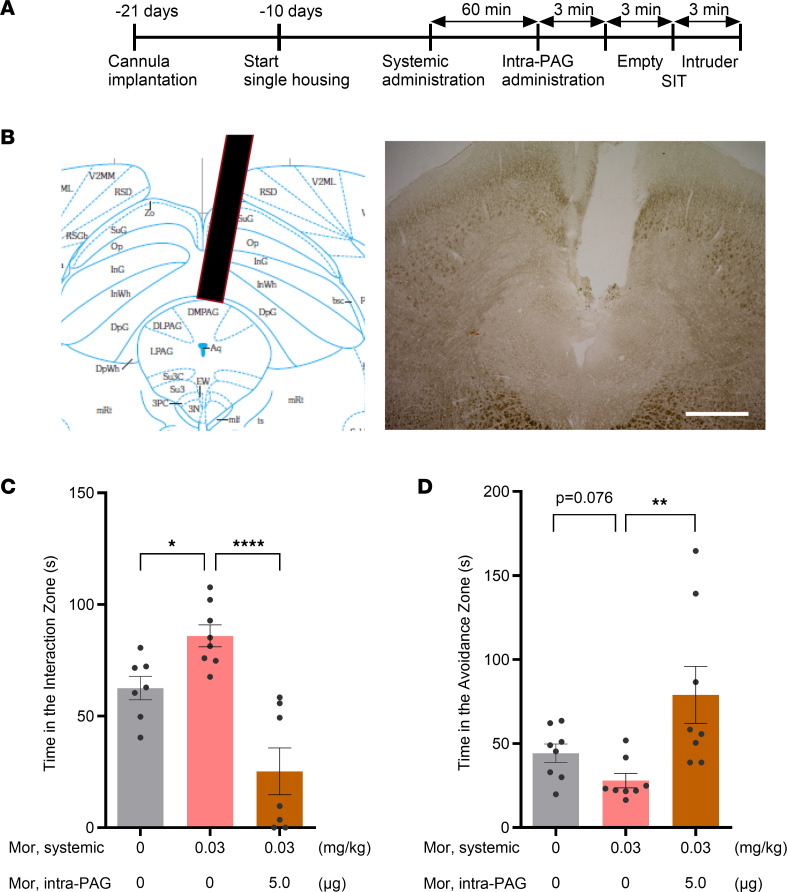
Our c-Fos mapping results led us to hypothesize that, while low-dose morphine selectively activates prosocial brain regions such as the NAc and mPFC, high-dose morphine additionally activates the dmPAG, which negatively regulates social behavior, thereby counteracting the prosocial signals from the activated NAc and mPFC. To examine our hypothesis, we determined whether intra-dmPAG injection of morphine would inhibit the prosocial effect induced by systemic treatment with low-dose morphine. For intra-dmPAG injection, a single cannula was implanted into the dorsal PAG of B6 mice. Following a 3-week recovery period, the SIT was performed after systemic administration accompanied by intra-PAG infusion of drugs (Figure 3, A and B). Systemic administration of low-dose morphine (0.03 mg/kg, s.c.) significantly increased the time spent in the interaction zone when an intruder mouse was presented (Figure 3C; cf. Figure 1A). However, the interaction behavior was significantly decreased by concomitant intra-PAG infusion of 5.0 μg of morphine, which has been reported to induce analgesic effects (42, 43) (Figure 3C). On the other hand, the time spent in the avoidance zone was increased by concomitant intra-dmPAG infusion of morphine (Figure 3D). These results support the idea that stimulation of MORs in the dmPAG, which is induced only by high-dose morphine, counteracts the positive effects of NAc and mPFC activation on social interaction behavior, which underlies the inverted U-shaped dose response of the prosocial effect of morphine.
Figure 3. Social behavior induced by morphine was antagonized by topical administration of morphine into the dorsal PAG.
(A) Timeline of the experiment. (B) Schematic of cannula implantation into the dorsomedial part of the PAG, and a representative image of mouse brain sections obtained after the experiment. Scale bar: 500 μm. (C and D) Effects of intra-PAG administration of morphine on social behaviors in the single-chamber social interaction test (SIT). Single-housed C57BL/6J mice were s.c. injected with morphine (0.03 mg/kg) or vehicle 1 hour before the test. Immediately before the test, morphine (5 μg) or vehicle was infused through the cannula (see Supplemental Methods). The time spent in the interaction zone (C) and the avoidance zone (D) during a 3-minute test period with an unfamiliar mouse kept in a cage (target session) is shown. n = 7–8 animals. *P<0.05, **P < 0.01, ****P < 0.0001, by parametric (C) and nonparametric (D) tests compared with the group treated with systemic morphine 0.03 mg/kg plus vehicle intra-PAG infusion. PAG, periaqueductal gray; Mor, morphine.
The effects of buprenorphine on social behavior in VPA model and Fmr1-KO mice.
Low doses of morphine without analgesic or addictive effects induced prosocial effects, suggesting that maximal stimulation of MORs is unnecessary to promote social behavior, and only partial stimulation of MORs would be sufficient. This finding prompted us to examine the therapeutic potential of buprenorphine, a MOR partial agonist with κ-opioid receptor antagonist potency, in ASD-like model animals. Buprenorphine is a schedule III controlled drug according to Drug Enforcement Administration regulations, indicating its better safety profile and lower abuse liability compared with those of other opioids, such as morphine. Indeed, buprenorphine is broadly used to treat pain and opioid use disorder. Here, we examined the effect of a broad range of buprenorphine doses on social behavior in VPA model mice. We found that at 1 hour after s.c. administration of buprenorphine, reciprocal social interaction deficits of VPA model mice were significantly improved with low doses (1, 3, 10 μg/kg), but not for a higher dose (30 μg/kg), of buprenorphine indicating an inverted U-shaped dose response (Figure 4A). Conversely, in the evaluation of its analgesic effect in the hot-plate test, 30 μg/kg buprenorphine, but not 3 μg/kg, significantly increased the paw-withdrawal latency (Figure 4B), indicating distinct effective doses for promoting social behavior and suppressing pain sensation.
Figure 4. Low-dose buprenorphine increased social behaviors in VPA-treated and Fmr1-KO mice.
(A) Effects of systemic buprenorphine administration on social behavior deficits in mice prenatally exposed to valproic acid (VPA) in the reciprocal social interaction test. Buprenorphine (1, 3, 10, or 30 μg/kg) or vehicle was s.c. injected into male offspring at 8 weeks of age. One hour after administration, the sniffing duration was measured during a 20-minute test period. (B) Effects of buprenorphine on thermal nociception in VPA-treated mice. The withdrawal latency at 49°C was measured with a hot-plate test 1 hour after buprenorphine administration (3 or 30 μg/kg, s.c.). (C and D) Effects of buprenorphine (3 or 30 μg/kg, s.c.) on social interaction deficits in VPA-treated mice were examined at 3 (C) or 12 hours (D) after administration. (E) The effects of buprenorphine (3 μg/kg, s.c.) on social interaction deficits were antagonized by naloxone (1 mg/kg, s.c.), an MOR antagonist, in VPA-treated mice. (F) Effects of systemic buprenorphine administration (3 μg/kg, s.c.) on disrupted social preference in Fmr1-KO mice. The time spent in the zone with or without an unfamiliar mouse was measured during a 10-minute test period. n = 11–18 animals. Data are shown as the mean ± SEM. ##P < 0.01, by parametric test (A) or nonparametric one (F) compared with vehicle-treated control mice prenatally exposed to saline (A) or vehicle-treated wild-type (C57BL/6J) mice (F). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, by parametric tests (A–C) or nonparametric ones (D–F) compared with vehicle-treated mice prenatally exposed to VPA (A–E) or vehicle-treated Fmr1-KO mice (F). Bup, buprenorphine; MOR, μ-opioid receptor; Fmr1, fragile X mental retardation 1.
Next, considering the long-lasting efficacy of buprenorphine previously reported (44–46), we assessed the duration of the effects of buprenorphine on social interaction in VPA model mice. Buprenorphine (3 μg/kg, s.c., but not 30 μg/kg) showed sustained effects of improving social behavior at 3 and 12 hours (Figure 4, C and D) as well as at 1 hour after administration (Figure 4A).
To clarify the receptor-mediating prosocial effect of buprenorphine, we examined the effect of naloxone (1 mg/kg, s.c.), a MOR antagonist, in a reciprocal SIT when it was concomitantly injected with buprenorphine (3 μg/kg, s.c.). Cotreatment of naloxone and buprenorphine almost completely blocked the effect of buprenorphine to improve social interaction deficits (Figure 4E). This result suggests that the effect of buprenorphine on social behavior could be mediated mainly by MORs.
Furthermore, to corroborate the therapeutic potential of buprenorphine on social behavior deficits, we performed an efficacy study using Fmr1-KO mice, a well-established animal model of fragile X syndrome. Many individuals with fragile X syndrome show ASD-like social behavior impairments, some of which are recapitulated in Fmr1-KO mice (47). Since Fmr1-KO mice showed the impairments only in a social novelty session but not in a sociability session in the 3-chamber test in the previous study (47), we evaluated the effect of buprenorphine on social novelty behaviors of Fmr1-KO mice. After s.c. administration of 3 μg/kg buprenorphine, social behavior impairment in Fmr1-KO mice was significantly improved (Figure 4F), whereas exploratory behavior around the cages was not altered (Supplemental Figure 4). This result strengthens the notion that buprenorphine has the therapeutic potential to ameliorate social behavioral deficits in ASD.
The effects of buprenorphine on neural activation across several brain regions in VPA model mice.
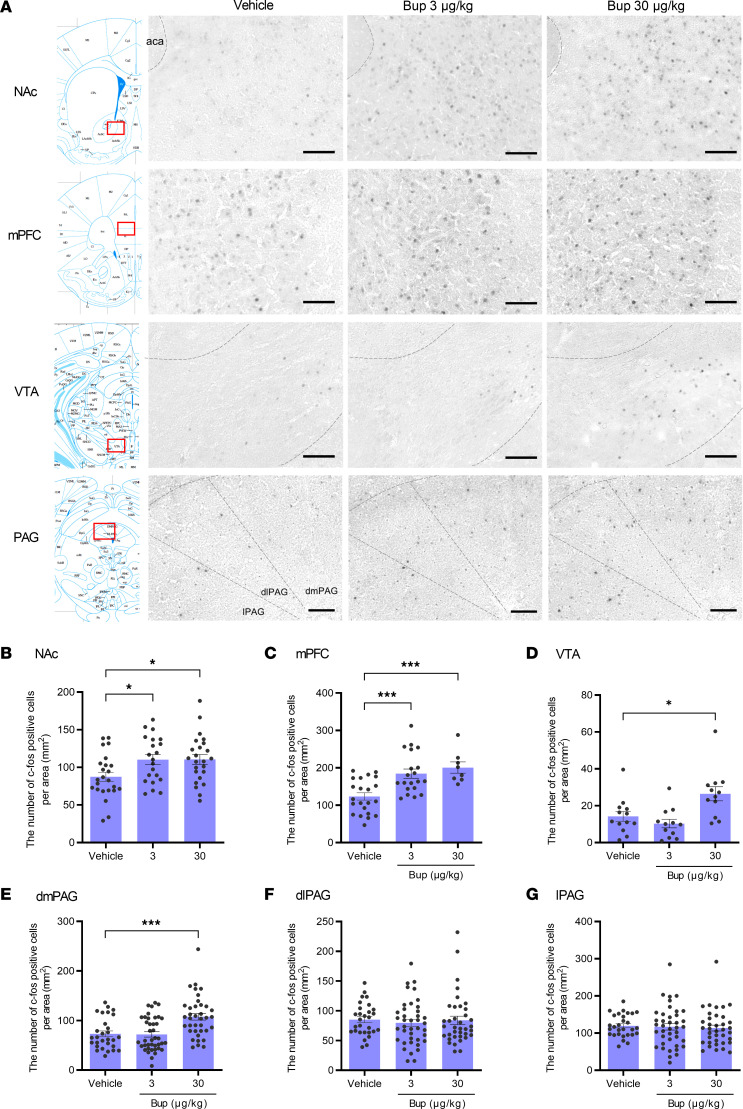
To identify the brain regions involved in the effect of buprenorphine on social behavioral deficits, we performed c-Fos immunohistochemistry, as described above. Mice were euthanized 1.5 hours after s.c. administration of buprenorphine (3 and 30 μg/kg), and the number of c-Fos–positive cells was quantified. Buprenorphine at a dose of 3 μg/kg, which improved social interaction deficits, significantly increased the number of c-Fos–positive cells in both the NAc (Figure 5, A and B) and mPFC (Figure 5, A and C) but not in the VTA (Figure 5, A and D) or in 3 subregions (dorsomedial, dorsolateral, and lateral) of the PAG (Figure 5, A and E–G). Buprenorphine, at a dose of 30 μg/kg, which showed an analgesic effect (Figure 4B) (44) but not a prosocial effect (Figure 4, A, C, and D), significantly increased the number of c-Fos–positive cells in the NAc, mPFC, VTA, and dmPAG. The relationship between the effect on social behavior and the pattern of brain activation was consistent between morphine and buprenorphine. These results further suggest that the dmPAG determines the effects of MOR agonists on social behavior, in addition to activation of the NAc and mPFC.
Figure 5. Brain area–specific activation by systemic buprenorphine administration in VPA-treated mice.
Male offspring born to mothers injected with valproic acid (500 mg/kg, intraperitoneal) were subjected to immunohistochemical analysis at 8 weeks of age. Buprenorphine (3 or 30 μg/kg) or vehicle was s.c. administered 1.5 hours before sampling the brain. (A) Representative images of c-Fos–immunostained brain sections containing the NAc, mPFC, VTA, and dorsal PAG. Scale bar: 100 μm. (B–G) Quantification of c-Fos–positive cells in each brain region. The results in the NAc (B, 21–24 sections/group from 4 mice/group), mPFC (C, 8–22 sections/group from 3–7 mice/group), VTA (D, 12–13 sections/group from 2 mice/group), dmPAG (E, 29–40 sections/group from 6 mice/group), dlPAG (F, 29–40 sections/group from 6 mice/group), and lPAG (G, 29–40 sections/group from 6 mice/group) are shown. Data are shown as the mean ± SEM. *P < 0.05, ***P < 0.001, by parametric (B and D) or nonparametric (C and E–G) tests compared with vehicle-treated mice. Bup, buprenorphine; NAc, nucleus accumbens, mPFC, medial prefrontal cortex; VTA, ventral tegmental area; dmPAG, dorsomedial periaqueductal gray; dlPAG, dorsolateral periaqueductal gray; lPAG, lateral periaqueductal gray.
Discussion
In the present study, we investigated the effects of MOR agonists on sociability impairments as observed in ASD from a nonclinical perspective. Low doses of both morphine and buprenorphine enhanced or improved sociability without exhibiting analgesic effects. At those doses, activation of the NAc and mPFC was observed with both drugs, implying the involvement of these brain regions in facilitating social behavior. Conversely, at analgesic doses, neither of the drugs enhanced or improved social behaviors. Activation of the dmPAG, in addition to the NAc and mPFC, was induced by both drugs at those doses, indicating a potential role of the dmPAG in inhibiting social behaviors, as reported in a previous study (41). Indeed, when morphine was injected into the dmPAG, the enhancement in sociability induced by systemic administration of low-dose morphine was antagonized. These results suggest the important role of brain region–specific neural activation by low-dose opioids in promoting social behaviors.
Previous studies reported that activation of MORs by administration of their agonists at analgesic doses reduced social investigatory behavior (11, 48, 49) and responses to social play (11). Conversely, attenuation of MOR signaling also has a negative effect on sociability, considering that social interaction behavior is decreased in Oprm1-KO mice (7) and animals treated with MOR antagonists (10, 49–51). Based on these findings, it has been hypothesized that maintaining an optimal activation state of the MORs is important in driving social behavior (6). In the present study, we demonstrated that morphine and buprenorphine improved sociability with doses lower than the analgesic doses in naive and ASD-like mice, indicating that an optimally activated state of MOR signaling for sociability could be achieved by administering nonanalgesic low-dose opioids in both naive and ASD-like conditions.
As previously mentioned, it has been suggested that, while excessive activation of MORs suppresses social behavior, appropriate activation of MORs promotes this behavior. The mechanism underlying such a relationship between MOR activation and social behavior, however, remains to be elucidated. In this study, we found that prosocial doses of morphine and buprenorphine induced neural activation in the NAc and mPFC but not in the dmPAG. In a previous study, local injection of a MOR agonist into the NAc enhanced social behavior, suggesting that MOR activation in the NAc may be sufficient to promote social behavior (10). The NAc of both humans and rodents has a high density of MORs (25, 28), indicating that the NAc is an important site of action of MOR agonists. In addition, [35S]GTPγS binding stimulated by a MOR agonist was 2–3 times higher in the NAc than that in the PAG and VTA, suggesting a higher expression level of functional MORs in the NAc (27). Likewise, the mPFC is also suggested to play a crucial role in the control of social behavior (29, 30, 52), and the MOR expression level in the PFC is higher than that in other cortical regions (25, 26). Thus, the higher density of MORs in the NAc and mPFC would facilitate formation of an agonist and receptor complex and transduce signaling, even at low agonist concentrations, leading to higher sensitivity of the NAc and mPFC to MOR agonists than that in the PAG and VTA.
The role of the PAG in social behavior has been investigated and its activation has been reported to induce anxiety, avoidance, and defensive behaviors (39, 40). In particular, Franklin et. al. directly demonstrated, through chemogenetic manipulation followed by c-Fos analysis, that neural activation in the dmPAG negatively controls social behaviors (41). Taken together with our results, high-dose, but not low-dose, opioids can induce neural activation in the dmPAG and thereby counteract the prosocial signals caused by activation of the NAc and mPFC, which would underlie the inverted U-shaped dose-dependent effects of morphine and buprenorphine on social behavior. We, however, cannot exclude the involvement of other brain regions reported to be activated by analgesic opioid doses (33–35), and further investigation is necessary to comprehensively elucidate the neural circuit underlying the control of social behavior by opioids.
When opioids are used for therapeutic purposes, caution should be exercised regarding their dependence and abuse liabilities, particularly when considering the opioid crisis in the US and its societal problems (53). The reward circuitry, primarily involving dopaminergic neurons in the VTA, plays a central role in the development of opioid dependence (36). In this study, we showed that opioids could improve social behavior at doses that did not increase neural activation in the VTA (Figures 2 and 5). Consistently, prosocial doses of morphine did not induce conditioned place preference in a previous study (54). Thus, the risk of dependence when prosocial doses of opioids are used is expected to be low, which needs to be cautiously examined in clinical trial. Furthermore, buprenorphine is categorized as a schedule III controlled drug, similar to methylphenidate, which is widely used to treat attention deficit hyperactivity disorder, a developmental disorder. On the basis of these findings, low-dose buprenorphine could be a viable option to treat the pediatric population with ASD.
Regarding the therapeutic potential for ASD, low-dose opioids, especially buprenorphine, have several advantages compared with drug candidates that were highly expected but failed to meet the endpoint in clinical trials, such as oxytocin, balovaptan, and bumetanide. First, the opioid system is highly conserved among species, as exemplified by the analgesic effect commonly observed in humans and rodents, implying that our preclinical findings regarding the role of opioids in social behavior could be translated into clinical results. In fact, MOR activation in the NAc was correlated with an increased desire for social interaction (55), and specifically, low-dose buprenorphine was reported to enhance positive responses to social stimuli (55), which are consistent with our findings in mice. In addition, buprenorphine was reported to improve depressive symptoms and suicidal ideation, supporting the potential of buprenorphine to treat psychiatric disorders involving motivational deficits (56, 57). Second, opioids showed positive effects on social behavior in multiple types of mice, including naive, VPA-treated, and Fmr1-KO mice. Combined with the clinical results mentioned above, buprenorphine is expected to be efficacious for a wide range of heterogeneous individuals with ASD. Finally, buprenorphine showed a sustained effect in promoting social behavior (Figure 4, C and D), which is in contrast to the short duration of efficacy of oxytocin in the VPA model (58). This finding could be attributed to the slow dissociation of buprenorphine from MORs (59, 60). In addition, prolonged exposure of humans to buprenorphine further extended the duration of the therapeutic effect to longer than 28 hours (46). Thus, the receptor binding and pharmacokinetic characteristics of buprenorphine could be suitable to facilitate sociability throughout the day, which would be critical to improve social behavior deficits in individuals with ASD during a reasonable time frame. Taken together, the findings indicate that buprenorphine has great potential to treat sociability deficits and achieve an acceptable safety profile as an ASD therapy. On the basis of these results and a case report of 1 patient with ASD taking buprenorphine, implying its efficacy for ASD symptoms (61), clinical testing of the efficacy and safety of buprenorphine in ASD is warranted.
In conclusion, low doses of MOR agonists promoted social behaviors without inducing analgesic effects in naive and ASD-like model animals by activating the NAc and mPFC. These effects on social behaviors were diminished at higher analgesic doses for which the dmPAG was activated. Thus, we clearly show dose-dependent differences in the pharmacological effects of opioids on social behaviors and provide evidence that brain region–specific neural activation is a key factor for opioids to appropriately promote these behaviors. This study sheds light on drug development for sociability impairments in psychiatric disorders and specifically suggests that low-dose MOR agonists could be promising treatments for sociability symptoms in ASD.
Methods
Sex as a biologic variable.
Our study examined male mice, because ASD is more common in male than female individuals and male animals exhibited less variability in behavioral assays.
Animals.
Experimental animals were purchased from Nihon CLEA (male B6 mice) and from Japan SLC Inc. (ICR [CD-1] mice). Animals were housed on a standard 12-hour light/dark cycle and given food and water ad libitum. Mice were housed in groups of 3–4 animals unless otherwise noted.
Drug treatment.
Morphine was purchased from Takeda Pharmaceuticals. Buprenorphine was purchased from Otsuka Pharmaceutical Co. Ltd. Unless otherwise stated, both drugs were dissolved in 0.9% NaCl solution (saline, Otsuka Pharmaceutical Co. Ltd.) and s.c. administrated at a dose of 10 mL/kg.
Preparation of a VPA-induced ASD-like mouse model.
Mouse model development was conducted as previously reported (13, 58, 62). In brief, pregnant CD-1 mice were randomly divided into 2 groups and intraperitoneally administered either 500 mg/kg VPA (Sigma-Aldrich) or saline on embryonic day 12.5. VPA was dissolved in saline (Otsuka Pharmaceutical Co. Ltd.), and the volume of injection was 10 mL/kg. Offspring born to VPA- and saline-treated mothers were housed in groups of 5–6 mice of the same sex on postnatal day 21. Only male offspring were subjected to experiments at 8 weeks of age.
Single-chamber SIT.
Animals were housed individually for 10 days before the test and were habituated to the interaction arena (42 cm width × 42 cm depth × 42 cm height) for 15 minutes on the day before the test. The test was performed as previously reported with minor modifications (63, 64). On the test day, test mice were placed in an arena with an empty wire cage (10 cm width × 6.5 cm depth × 42 cm height). The animals were given 3 minutes to explore the arena (empty session) and then removed. A novel B6 mouse (male, 1 week younger than the test mouse) enclosed in a wire cage was placed in the arena, and the same procedure as that used in the empty session was repeated (target session). The time spent in the area surrounding the wire cage (interaction zone, 24 cm × 14 cm) and in the corner zones (9 cm × 9 cm) opposite to the interaction zone were measured as the social interaction time and avoidance time, respectively, using an ANY-maze Video Tracking System (Stoelting Co.).
Reciprocal SIT.
The test was conducted as previously reported (13, 65). In brief, test mice (resident mice) were individually habituated to the experimental cage for 60 minutes. Then, an intruder mouse (male CD-1, 1 week younger than the test mouse) was introduced into the cage. The sniffing behaviors (face and anogenital sniffs) of the resident mouse were measured over the total experimental period of 20 minutes.
Three-chamber social preference test.
The test was conducted as previously reported with minor modifications (66). In brief, under an illumination of 5 lx, test mice [10- to 12-week-old male B6 or Fmr1 KO (B6.129P2-Fmr1tm1Cgr/J, JAX, stock no. 003025)] were placed in the central chamber of the social interaction apparatus (67), which was composed of a clear Plexiglas box (41 cm × 60 cm × 23.5 cm) divided into 3 interconnected chambers. The test mouse was given the choice to interact with either a wire cup with a familiar cage mate (located in 1 side chamber) or a similar wire cup containing an unfamiliar male B6 mouse (male, 3 weeks younger than the test mouse, located in the opposite chamber). The amount of time the test mouse spent in each of the 3 chambers and in the area surrounding each cup was measured during a 10-minute trial using EthoVision XT (Noldus Information Technology).
Statistics.
All statistical analyses were carried out using GraphPad Prism 6 and python scikit-posthocs package. Normality of the data distribution was tested using Shapiro-Wilk’s test. The data with Gaussian distribution was tested using parametric methods such as 2-tailed unpaired t test, Welch’s test when comparing 2 groups with the different standard deviations, or 1-way analysis of variance followed by Holm-Šidák’s multiple comparisons test. The data not following normal distribution was tested using nonparametric Mann-Whitney’s test or Kruskal-Wallis test followed by Conover’s multiple comparison test. In all cases, the comparisons were considered statistically significant at P < 0.05. All data are presented as the mean ± SEM.
Study approval.
All animal procedures were approved by the Institutional Animal Care and Use Committees of Osaka University, Hiroshima University, and Shionogi & Co. Ltd. and were performed in accordance with the Guidelines for Animal Care and Use of Osaka University, Hiroshima University, and Shionogi & Co. Ltd.
Data availability.
All data needed to evaluate the conclusions of the manuscript are present in the paper and/or supplemental materials. Values for graphs in the figures and supplemental figures are provided in the Supporting Data Values file.
Author contributions
AN, HY, and Y Ago conceptualized and designed the study. SO, MN, HK, MH, SH, K Takasu, RK, Y Azuma, RY, TK, EI, TT, and Y Ago conducted the experiments. NO established the automated counting system of c-Fos–positive cells. SO, MN, HY, and Y Ago constructed the graphs. K Takuma and Y Ago provided the reagents. YK, SA, AN, KO, K Takuma, HH, HY, and Y Ago supervised the study. SO and HY wrote the manuscript. SO, HY, and Y Ago reviewed and edited the manuscript. SO was assigned as the first co–first author because of his contribution to writing.
Supplementary Material
Acknowledgments
We thank N. Yasuda and K. Kuroda at SK project for technical assistance. We thank Y. Furue, K. Shibata, and M. Fujikawa at Shionogi TechnoAdvance Research Co. Ltd. for supporting the behavioral tests of Fmr1-KO mice. We thank L. Kreiner from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This project was supported by Japan Society for the Promotion of Science KAKENHI grants JP20H03392 (to Y Ago), JP21K19714 (to Y Ago), JP24K02185 (to Y Ago), JP24K23296 (to RY), JP23K19455 (to EI), and JP23H00450 (to YK); The Nakatomi Foundation (to Y Ago); Astellas Foundation for Research on Metabolic Disorders (to Y Ago); Mochida Memorial Foundation for Medical and Pharmaceutical Research (to Y Ago), and collaborative research between Osaka University and Shionogi & Co. Ltd. (to Y Ago). This research was partially supported by the Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research) from Japan Agency for Medical Research and Development under grants JP23ama121052 and JP23ama121054.
Version 1. 12/06/2024
Electronic publication
Footnotes
Conflict of interest: SO, K Takasu, Y Azuma, AN, KO, and HY are full-time employees of Shionogi & Co. Ltd. The authors declare that this study received funding from Shionogi & Co. Ltd. The funder had the following involvement in the study: study design, interpretation of data, writing of the article, and the decision to submit it for publication. Y Ago, AN, and HY are the inventors of the patent, PCT/JP2022/047004 (“Use of opioid for treatment of autism spectrum disorders”).
Copyright: © 2024, Ohnami et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2024;9(23):e182060.https://doi.org/10.1172/jci.insight.182060.
Contributor Information
Soichiro Ohnami, Email: souichirou.oonami@shionogi.co.jp.
Megumi Naito, Email: naiba7110@gmail.com.
Haruki Kawase, Email: haruki.kawase@takano-net.co.jp.
Momoko Higuchi, Email: momohik1@yahoo.co.jp.
Shigeru Hasebe, Email: shigeru.hasebe@setsunan.ac.jp.
Keiko Takasu, Email: keiko.takasu@shionogi.co.jp.
Ryo Kanemaru, Email: ryo.kanemaru@shionogi.co.jp.
Yuki Azuma, Email: yuki.azuma@shionogi.co.jp.
Rei Yokoyama, Email: reiyokoyama@hiroshima-u.ac.jp.
Takahiro Kochi, Email: t.kochi0330@gmail.com.
Eiji Imado, Email: imadoeiji@hiroshima-u.ac.jp.
Takeru Tahara, Email: m230602@hiroshima-u.ac.jp.
Yaichiro Kotake, Email: yaichiro@hiroshima-u.ac.jp.
Satoshi Asano, Email: sasano@hiroshima-u.ac.jp.
Naoya Oishi, Email: noishi@kuhp.kyoto-u.ac.jp.
Kazuhiro Takuma, Email: takuma.kazuhiro.dent@osaka-u.ac.jp.
Hitoshi Hashimoto, Email: hasimoto@phs.osaka-u.ac.jp.
Koichi Ogawa, Email: kouichi.ogawa@shionogi.co.jp.
Atsushi Nakamura, Email: atsushi_nakamura@shionogi.co.jp.
Hidekuni Yamakawa, Email: hidekuni.yamakawa@pingan-shionogi.com.
Yukio Ago, Email: yukioago@hiroshima-u.ac.jp.
References
- 1.Hirota T, King BH. Autism spectrum disorder: a review. JAMA. 2023;329(2):157–168. doi: 10.1001/jama.2022.23661. [DOI] [PubMed] [Google Scholar]
- 2. American Psychiatric Association, eds. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Li Z, et al. Global, regional and national burden of autism spectrum disorder from 1990 to 2019: results from the Global Burden of Disease Study 2019. Epidemiol Psychiatr Sci. 2022;31:e33. doi: 10.1017/S2045796022000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollander E, et al. Balovaptan vs placebo for social communication in childhood autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79(8):760–769. doi: 10.1001/jamapsychiatry.2022.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sikich L, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385(16):1462–1473. doi: 10.1056/NEJMoa2103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellissier LP, et al. μ opioid receptor, social behaviour and autism spectrum disorder: reward matters. Br J Pharmacol. 2018;175(14):2750–2769. doi: 10.1111/bph.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker JA, et al. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology. 2014;39(9):2049–2060. doi: 10.1038/npp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes EM, et al. Mu-opioid receptor agonism differentially alters social behaviour and immediate early gene expression in male adolescent rats prenatally exposed to valproic acid versus controls. Brain Res Bull. 2021;174:260–267. doi: 10.1016/j.brainresbull.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Piccin A, et al. Morphine reduces the interest for natural rewards. Psychopharmacology (Berl) 2022;239(8):2407–2419. doi: 10.1007/s00213-022-06131-7. [DOI] [PubMed] [Google Scholar]
- 10.Trezza V, et al. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31(17):6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achterberg EJM, et al. Opioid modulation of social play reward in juvenile rats. Neuropharmacology. 2019;159:107332. doi: 10.1016/j.neuropharm.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Narita M, et al. Suppression of dopamine-related side effects of morphine by aripiprazole, a dopamine system stabilizer. Eur J Pharmacol. 2008;600(1–3):105–109. doi: 10.1016/j.ejphar.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Kataoka S, et al. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int J Neuropsychopharmacol. 2013;16(1):91–103. doi: 10.1017/S1461145711001714. [DOI] [PubMed] [Google Scholar]
- 14.Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 15.Wagner GC, et al. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36(6):779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 16.Roullet FI, et al. Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience. 2010;170(2):514–522. doi: 10.1016/j.neuroscience.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 17.Bromley RL, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643. doi: 10.1136/jnnp-2012-304270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen J, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fereshetyan K, et al. Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder. Sci Rep. 2021;11(1):23471. doi: 10.1038/s41598-021-02994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross FB, et al. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 2000;84(2–3):421–428. doi: 10.1016/S0304-3959(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 21.Vermeirsch H, Meert TF. Morphine-induced analgesia in the hot-plate test: comparison between NMRI(nu/nu) and NMRI mice. Basic Clin Pharmacol Toxicol. 2004;94(2):59–64. doi: 10.1111/j.1742-7843.2004.pto940202.x. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, et al. Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology. 2008;33(5):1097–1112. doi: 10.1038/sj.npp.1301471. [DOI] [PubMed] [Google Scholar]
- 23.Devilliers M, et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat Commun. 2013;4(1):2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 24.Minami K, et al. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci. 2009;111(1):60–72. doi: 10.1254/jphs.09139FP. [DOI] [PubMed] [Google Scholar]
- 25.Smith CJW, et al. Robust age, but limited sex, differences in mu-opioid receptors in the rat brain: relevance for reward and drug-seeking behaviors in juveniles. Brain Struct Funct. 2018;223(1):475–488. doi: 10.1007/s00429-017-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis ME, et al. Opiate receptor localization in rat cerebral cortex. J Comp Neurol. 1983;216(3):339–358. doi: 10.1002/cne.902160310. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, et al. Brain region- and sex-specific alterations in DAMGO-stimulated [(35) S]GTPγS binding in mice with Oprm1 A112G. Addict Biol. 2014;19(3):354–361. doi: 10.1111/j.1369-1600.2012.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nummenmaa L, et al. μ-opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9(1):1500. doi: 10.1038/s41467-018-03848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kietzman HW, Gourley SL. How social information impacts action in rodents and humans: the role of the prefrontal cortex and its connections. Neurosci Biobehav Rev. 2023;147:105075. doi: 10.1016/j.neubiorev.2023.105075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohapatra AN, Wagner S. The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front Psychiatry. 2023;14:1205199. doi: 10.3389/fpsyt.2023.1205199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franklin KBJ, Paxinos G, eds. The Mouse Brain in Stereotaxic Coordinates. Elsevier; 2007. [Google Scholar]
- 32.Spielewoy C, et al. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur J Neurosci. 2000;12(5):1827–1837. doi: 10.1046/j.1460-9568.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan GB, et al. Intra-VTA adenosine A1 receptor activation blocks morphine stimulation of motor behavior and cortical and limbic Fos immunoreactivity. Eur J Pharmacol. 2009;602(2–3):268–276. doi: 10.1016/j.ejphar.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Moulédous L, et al. Modulation of basal and morphine-induced neuronal activity by a NPFF(2) selective agonist measured by c-Fos mapping of the mouse brain. Synapse. 2010;64(9):672–681. doi: 10.1002/syn.20774. [DOI] [PubMed] [Google Scholar]
- 35.Nakamoto K, et al. Changes in opioid receptors, opioid peptides and morphine antinociception in mice subjected to early life stress. Eur J Pharmacol. 2020;881:173173. doi: 10.1016/j.ejphar.2020.173173. [DOI] [PubMed] [Google Scholar]
- 36.Doyle MA, Mazei-Robison MS. Opioid-induced molecular and cellular plasticity of ventral tegmental area dopamine neurons. Cold Spring Harb Perspect Med. 2021;11(2):a039362. doi: 10.1101/cshperspect.a039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabaeizadeh M, et al. The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naive versus morphine-dependent mice. Behav Brain Res. 2013;237:41–48. doi: 10.1016/j.bbr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Lutfy K, et al. Prohormone convertase 2 (PC2) null mice have increased mu opioid receptor levels accompanied by altered morphine-induced antinociception, tolerance and dependence. Neuroscience. 2016;329:318–325. doi: 10.1016/j.neuroscience.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George DT, et al. Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci. 2019;42(5):349–360. doi: 10.1016/j.tins.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Vázquez-León P, et al. Defensive and emotional behavior modulation by serotonin in the periaqueductal gray. Cell Mol Neurobiol. 2023;43(4):1453–1468. doi: 10.1007/s10571-022-01262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin TB, et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat Neurosci. 2017;20(2):260–270. doi: 10.1038/nn.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes-de-Souza RL, et al. Strain-dependent effects of morphine injected into the periaqueductal gray area of mice. Braz J Med Biol Res. 1991;24(3):291–299. [PubMed] [Google Scholar]
- 43.Miczek KA, et al. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology (Berl) 1985;87(1):39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- 44.Cowan A, et al. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol. 1977;60(4):547–554. doi: 10.1111/j.1476-5381.1977.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuh KJ, et al. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl) 1999;145(2):162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- 46.Greenwald M, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61(1):101–110. doi: 10.1016/j.biopsych.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Sørensen EM, et al. Hyperactivity and lack of social discrimination in the adolescent Fmr1 knockout mouse. Behav Pharmacol. 2015;26(8 spec no):733–740. doi: 10.1097/FBP.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 48.Landauer MR, Balster RL. Opiate effects on social investigatory behavior of male mice. Pharmacol Biochem Behav. 1982;17(6):1181–1186. doi: 10.1016/0091-3057(82)90117-4. [DOI] [PubMed] [Google Scholar]
- 49.Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23(1):75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- 50.Panksepp J, et al. Morphine reduces social cohesion in rats. Pharmacol Biochem Behav. 1979;11(2):131–134. doi: 10.1016/0091-3057(79)90002-9. [DOI] [PubMed] [Google Scholar]
- 51.Ragen BJ, et al. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38(11):2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang W-C, et al. Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Rep. 2020;32(2):107899. doi: 10.1016/j.celrep.2020.107899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hornberger J, Chhatwal J. Opioid misuse: a global crisis. Value Health. 2021;24(2):145–146. doi: 10.1016/j.jval.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Koek W. Morphine-induced conditioned place preference and effects of morphine pre-exposure in adolescent and adult male C57BL/6J mice. Psychopharmacology (Berl) 2016;233(11):2015–2024. doi: 10.1007/s00213-014-3695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bershad AK, et al. Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology. 2016;63:43–49. doi: 10.1016/j.psyneuen.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yovell Y, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173(5):491–498. doi: 10.1176/appi.ajp.2015.15040535. [DOI] [PubMed] [Google Scholar]
- 57.Karp JF, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75(8):e785–e793. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara Y, et al. Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav. 2017;96:130–136. doi: 10.1016/j.yhbeh.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Bidlack JM, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367(2):267–281. doi: 10.1124/jpet.118.249839. [DOI] [PubMed] [Google Scholar]
- 60.Boas RA, Villiger JW. Clinical actions of fentanyl and buprenorphine. The significance of receptor binding. Br J Anaesth. 1985;57(2):192–196. doi: 10.1093/bja/57.2.192. [DOI] [PubMed] [Google Scholar]
- 61.Skoglund C, et al. The partial μ-opioid agonist buprenorphine in autism spectrum disorder: a case report. J Med Case Rep. 2022;16(1):152. doi: 10.1186/s13256-022-03384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hara Y, et al. Reduced prefrontal dopaminergic activity in valproic acid-treated mouse autism model. Behav Brain Res. 2015;289:39–47. doi: 10.1016/j.bbr.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 63.Inaba H, et al. GPCR-mediated calcium and cAMP signaling determines psychosocial stress susceptibility and resiliency. Sci Adv. 2023;9(14):eade5397. doi: 10.1126/sciadv.ade5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakai Y, et al. Gene-environment interactions mediate stress susceptibility and resilience through the CaMKIIβ/TARPγ-8/AMPAR pathway. iScience. 2021;24(5):102504. doi: 10.1016/j.isci.2021.102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hara Y, et al. Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid-induced autism. Autism Res. 2016;9(9):926–939. doi: 10.1002/aur.1596. [DOI] [PubMed] [Google Scholar]
- 66.Ago Y, et al. The female encounter test: a novel method for evaluating reward-seeking behavior or motivation in mice. Int J Neuropsychopharmacol. 2015;18(11):pyv062. doi: 10.1093/ijnp/pyv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions of the manuscript are present in the paper and/or supplemental materials. Values for graphs in the figures and supplemental figures are provided in the Supporting Data Values file.