Abstract
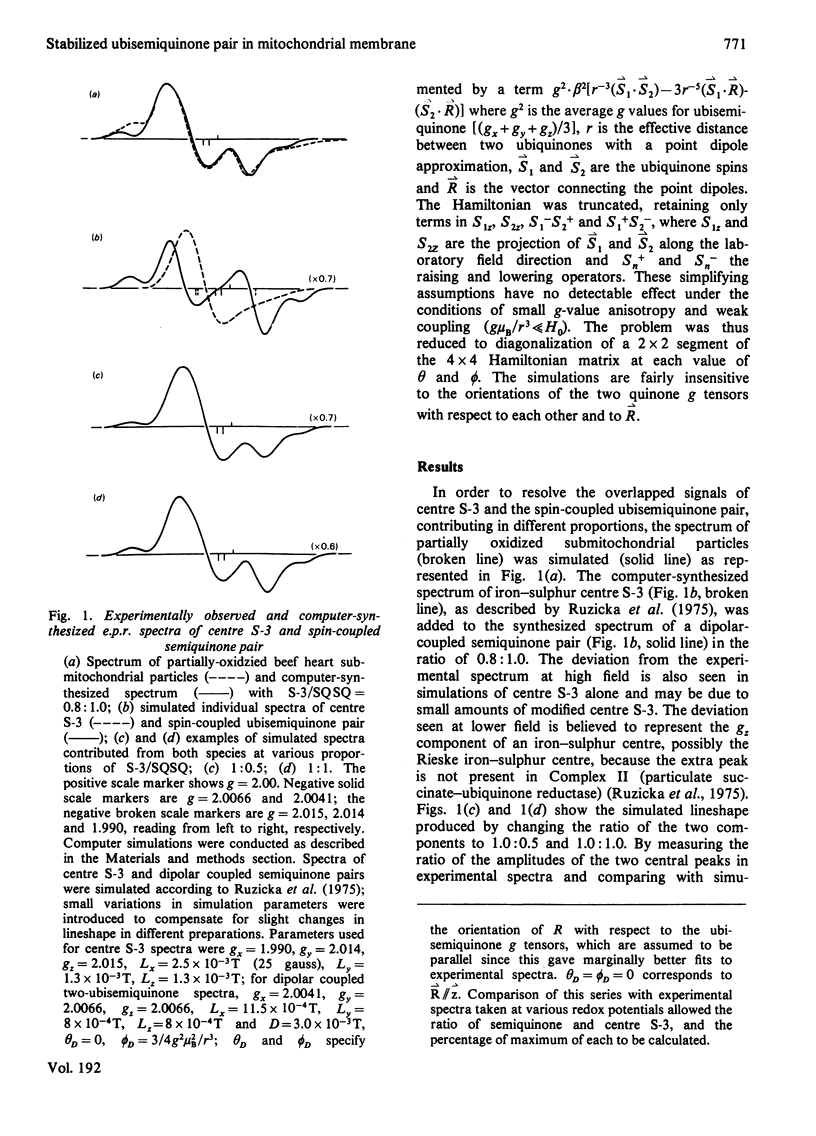
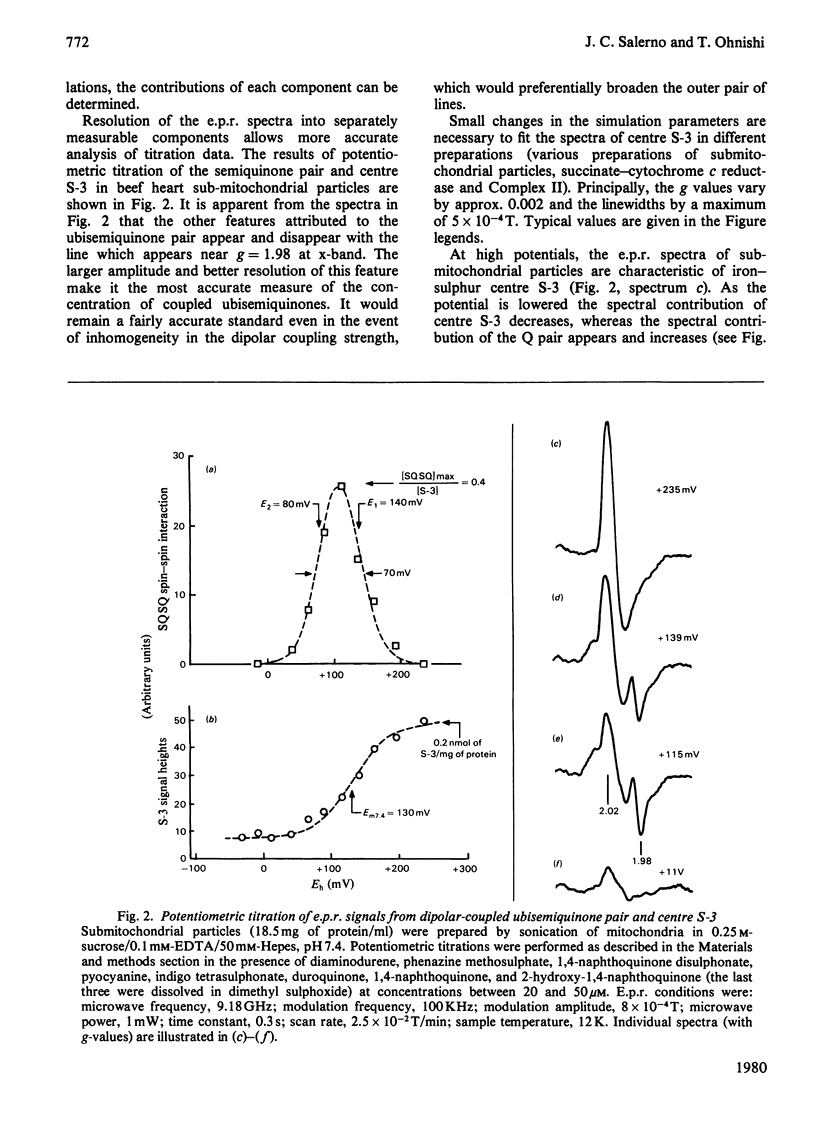
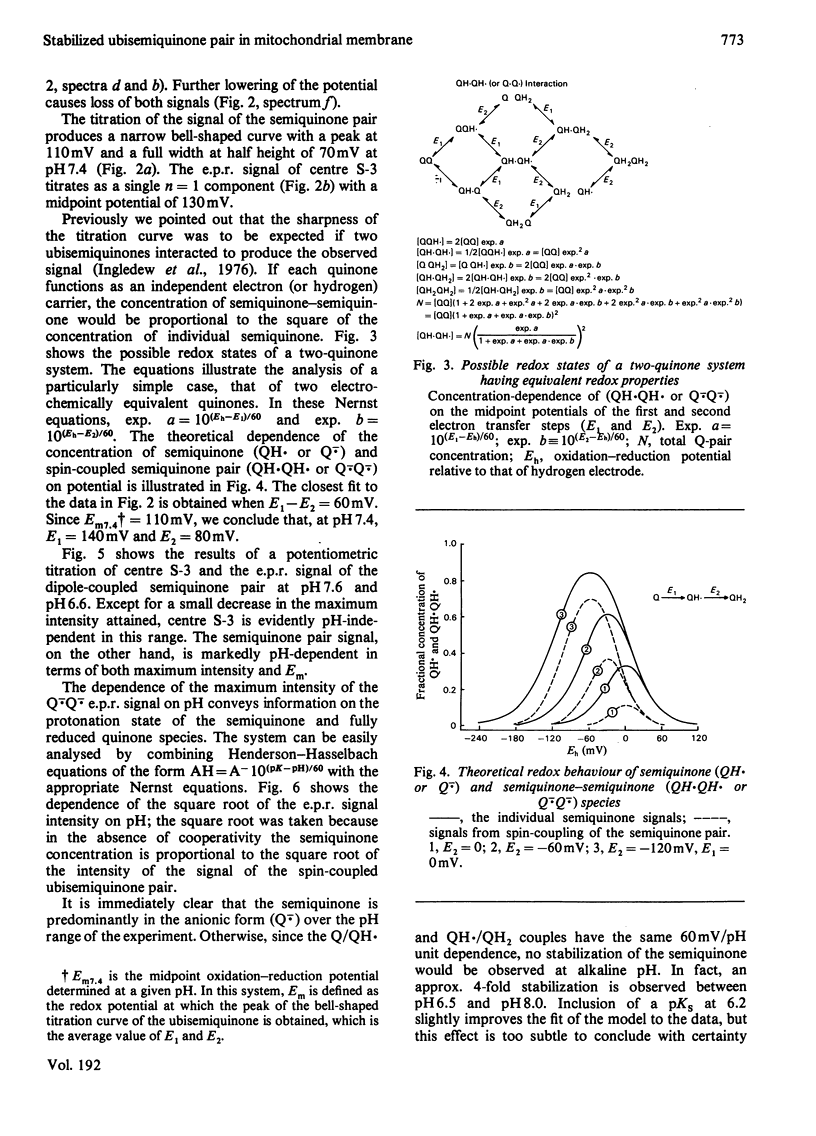
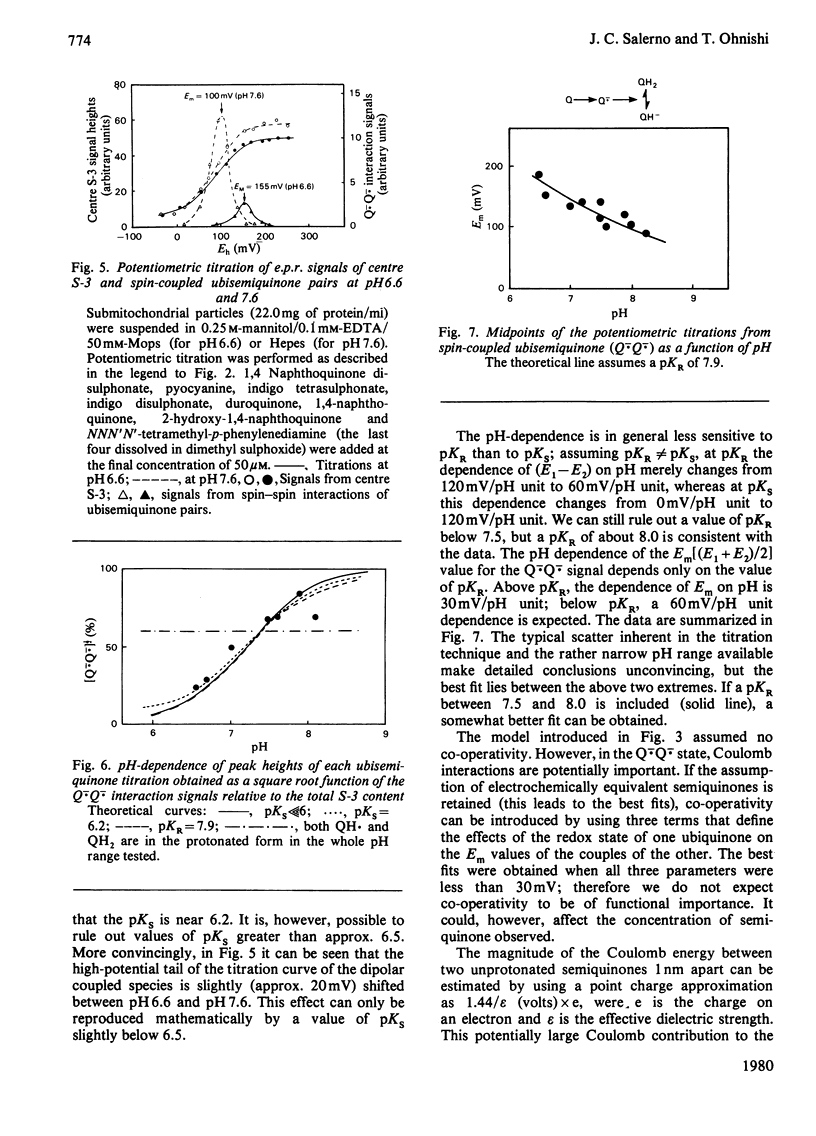
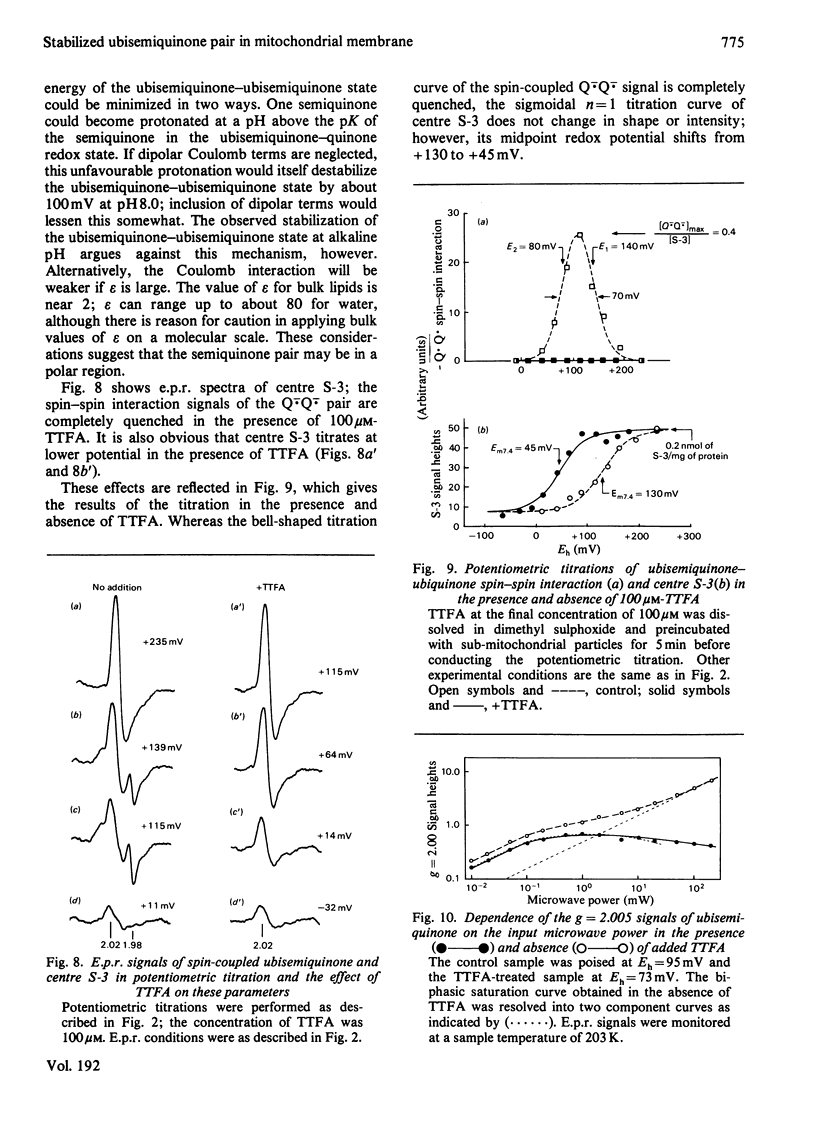
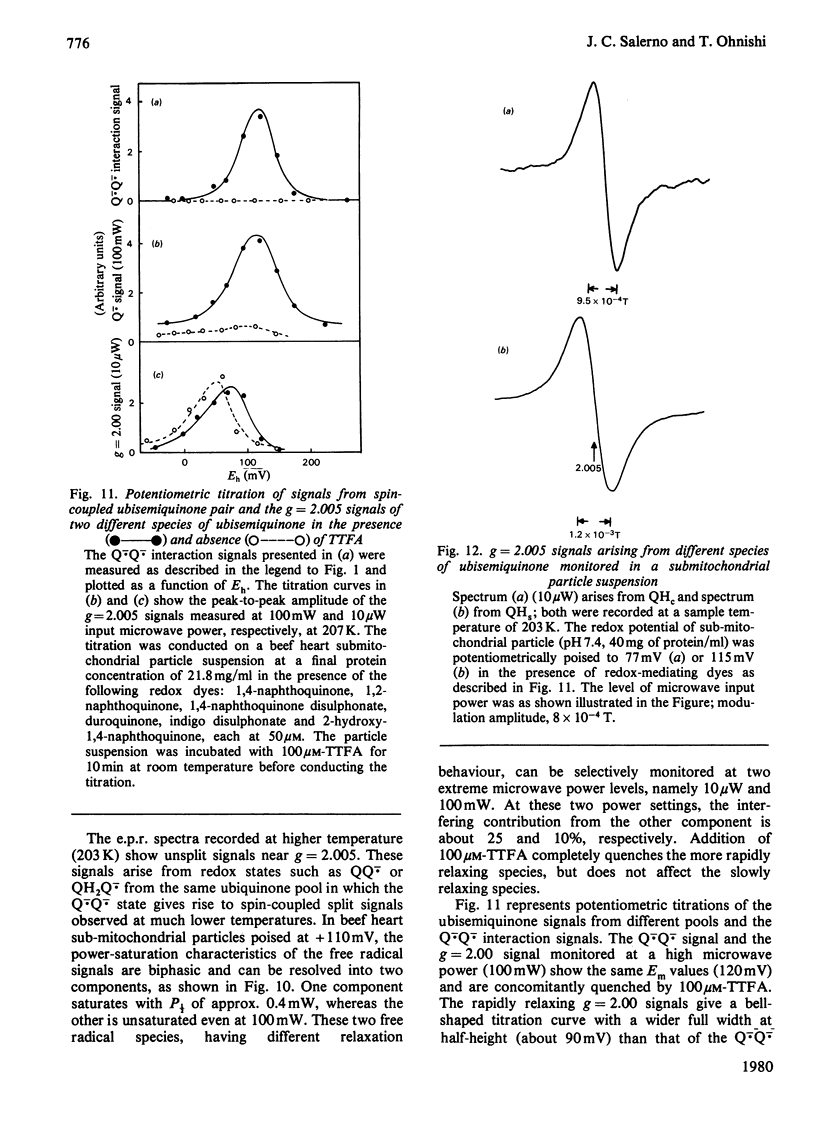
1. Evidence is presented for the presence of a stable ubisemiquinone pair in the vicinity of iron-sulphur centre S-3, based on its thermodynamic and spin relaxation properties. 2. These semiquinones are coupled by dipolar interaction; quantitative analysis of the signals of the spin-coupled semiquinones (at pH 7.4) gives midpoint redox potentials E1 (oxidized to semiquinone state) and E2 (semiquinone to fully reduced state) of 140 and 80mV, respectively, for individual ubiquinones. 3. Values of pKS (pK of the semiquinone form) below 6.5 and pKR (pK of the fully reduced ubiquinone) of about 8.0 or above were estimated from the pH-dependence of the midpoint potentials of the spin coupled signals. Thus the ubisemiquinone associated with succinate dehydrogenase (designated as SQS) functions mostly in the anionic form of the physiological pH range. 4. Theonyltrifluoroacetone, a specific inhibitor of the succinate-ubiquinone reductase segment of the respiratory chain, destabilized the intermediate redox state; thus it quenches both the g = 2.00 signal and ubisemiquinone (SQS) and split signals from the spin coupled pair. This inhibitor has no significant effect on another bound ubisemiquinone species present in the cytochrome bc1 region (designated as SQC). 5. The possible function and location of these stabilized ubisemiquinone species were discussed in connection with Site-II energy transduction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Coles C. J., Singer T. P., Beinert H., Wan Y. P., Folkers K. Kinetics of the reoxidation of succinate dehydrogenase. Arch Biochem Biophys. 1977 Jul;182(1):107–117. doi: 10.1016/0003-9861(77)90288-0. [DOI] [PubMed] [Google Scholar]
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Beinert H., Ackrell B. A., Kearney E. B., Singer T. P. Iron-sulfur components of succinate dehydrogenase: stoichiometry and kinetic behavior in activated preparations. Eur J Biochem. 1975 May;54(1):185–194. doi: 10.1111/j.1432-1033.1975.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Heron C., Ragan C. I., Trumpower B. L. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J. 1978 Sep 15;174(3):791–800. doi: 10.1042/bj1740791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Ohnishi T. Properties of the S-3 iron-sulphur centre of succinate dehydrogenase in the intact respiratory chain of beef heart mitochondria. FEBS Lett. 1975 Jun 15;54(2):167–171. doi: 10.1016/0014-5793(75)80067-6. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Ohnishi T. The probable site of action of thenolytrifluoracetone on the respiratory chain. Biochem J. 1977 Jun 15;164(3):617–620. doi: 10.1042/bj1640617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Salerno J. C., Ohnishi T. Studies on electron paramagnetic resonance spectra manifested by a respiratory chain hydrogen carrier. Arch Biochem Biophys. 1976 Nov;177(1):176–184. doi: 10.1016/0003-9861(76)90427-6. [DOI] [PubMed] [Google Scholar]
- King T. E. Reconstitution of the respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1966;28:155–236. doi: 10.1002/9780470122730.ch3. [DOI] [PubMed] [Google Scholar]
- Konstantinov A. A., Ruuge E. K. Semiquinone Q in the respiratory chain of electron transport particles: electron spin resonance studies. FEBS Lett. 1977 Sep 1;81(1):137–141. doi: 10.1016/0014-5793(77)80946-0. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973 Nov 15;39(2):313–323. doi: 10.1111/j.1432-1033.1973.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Massey V., Palmer G. On the existence of spectrally distinct classes of flavoprotein semiquinones. A new method for the quantitative production of flavoprotein semiquinones. Biochemistry. 1966 Oct;5(10):3181–3189. doi: 10.1021/bi00874a016. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976 Oct 21;62(2):327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Lim J., Winter D. B., King T. E. Thermodynamic and EPR characteristics of a HiPIP-type iron-sulfur center in the succinate dehydrogenase of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2105–2109. [PubMed] [Google Scholar]
- Ohnishi T., Trumpower B. L. Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate . cytochrome c reductase complex. J Biol Chem. 1980 Apr 25;255(8):3278–3284. [PubMed] [Google Scholar]
- PUMPHREY A. M. Studies on the electron transfer system. XLV. Some effects of antimycin on cytochrome b. J Biol Chem. 1962 Jul;237:2384–2390. [PubMed] [Google Scholar]
- Petty K., Jackson J. B., Dutton P. L. Factors controlling the binding of two protons per electron transferred through the ubiquinone and cytochrome b/c2 segment of Rhodopseudomonas sphaeroides chromatophores. Biochim Biophys Acta. 1979 Apr 11;546(1):17–42. doi: 10.1016/0005-2728(79)90167-1. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bonner W. D., Jr An EPR analysis of cyanide-resistant mitochondria isolated from the mutant poky strain of Neurospora crassa. Biochim Biophys Acta. 1978 Dec 7;504(3):345–363. doi: 10.1016/0005-2728(78)90059-2. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L., Ingledew W. J., Bonner W. D., Jr EPR studies of higher plant mitochondria. I Ubisemiquinone and its relation to alternative respiratory oxidations. Biochim Biophys Acta. 1977 Dec 23;462(3):501–514. doi: 10.1016/0005-2728(77)90097-4. [DOI] [PubMed] [Google Scholar]
- Ruuge E. K., Konstantinov A. A. Signal EPR semikhinona Q10 s neobychnymi relaksatsionnymi parametrami. Biofizika. 1976 May-Jun;21(3):586–588. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H., Schepler K. L., Dunham W. R., Sands R. H. Interaction of ubisemiquinone with a paramagnetic component in heart tissue. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2886–2890. doi: 10.1073/pnas.72.8.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Blum H., Ohnishi T. The orientation of iron-sulfur clusters and a spin-coupled ubiquinone pair in the mitochondrial membrane. Biochim Biophys Acta. 1979 Aug 14;547(2):270–281. doi: 10.1016/0005-2728(79)90010-0. [DOI] [PubMed] [Google Scholar]
- Takamiya K. I., Dutton P. L. Ubiquinone in Rhodopseudomonas sphaeroides. Some thermodynamic properties. Biochim Biophys Acta. 1979 Apr 11;546(1):1–16. doi: 10.1016/0005-2728(79)90166-x. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L., Simmons Z. Diminished inhibition of mitochondrial electron transfer from succinate to cytochrome c by thenoyltrifluoroacetone induced by antimycin. J Biol Chem. 1979 Jun 10;254(11):4608–4616. [PubMed] [Google Scholar]
- Wikström M. K., Berden J. A. Oxidoreduction of cytochrome b in the presence of antimycin. Biochim Biophys Acta. 1972 Dec 14;283(3):403–420. doi: 10.1016/0005-2728(72)90258-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecinska M., Dutton P. L., Tsudzuki T. The oxidation-reduction potentials of the iron-sulfur proteins in mitochondria. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1273–1278. doi: 10.1016/0006-291x(70)90225-1. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Reconstitution of succinate-Q reductase. Biochem Biophys Res Commun. 1977 Dec 7;79(3):939–946. doi: 10.1016/0006-291x(77)91201-3. [DOI] [PubMed] [Google Scholar]


