Abstract
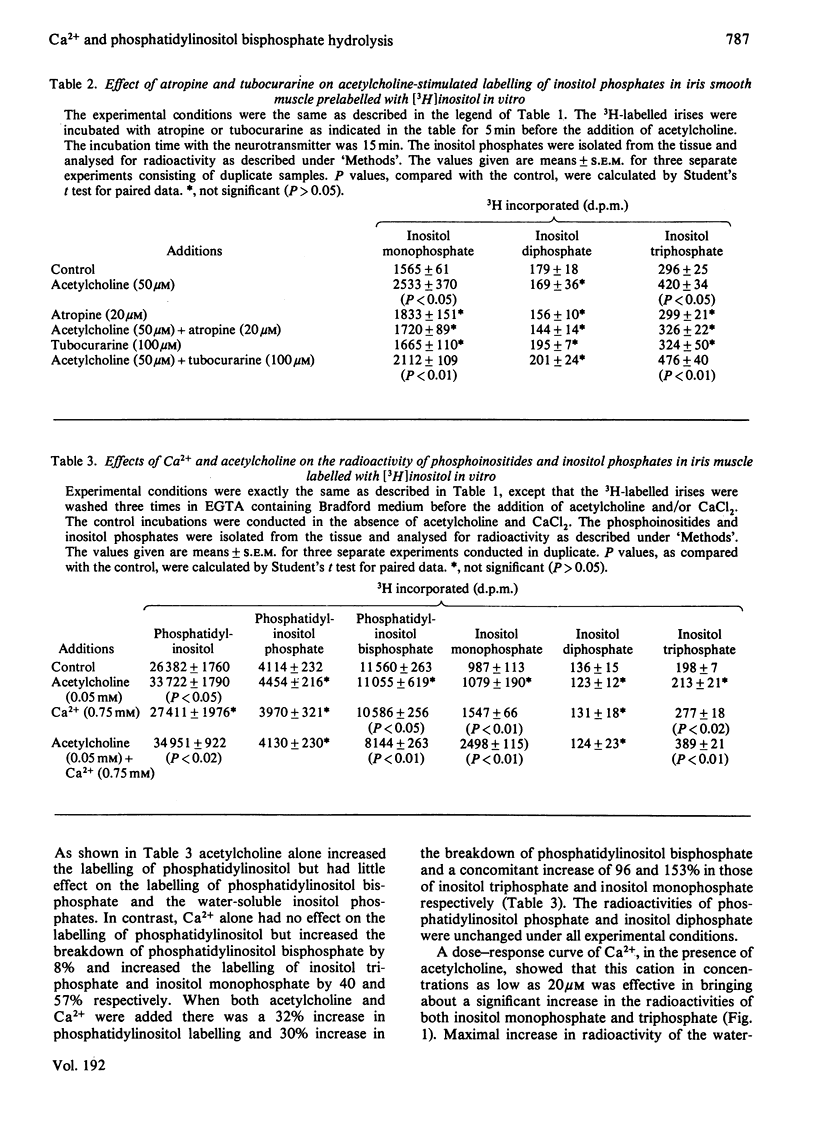
1. The mechanism of acetylcholine-stimulated breakdown of phosphatidyl-myo-inositol 4,5-bisphosphate and its dependence on extracellular Ca2+ was investigated in the rabbit iris smooth muscle. 2. Acetylcholine (50μm) increased the breakdown of phosphatidylinositol bisphosphate in [3H]inositol-labelled muscle by 28% and the labelling of phosphatidylinositol by 24% of that of the control. Under the same experimental conditions there was a 33 and 48% increase in the production of 3H-labelled inositol trisphosphate and inositol monophosphate respectively. Similarly carbamoylcholine and ionophore A23187 increased the production of these water-soluble inositol phosphates. Little change was observed in the 3H radioactivity of inositol bisphosphate. 3. Both inositol trisphosphatase and inositol monophosphatase were demonstrated in subcellular fractions of this tissue and the specific activity of the former was severalfold higher than that of the latter. 4. The acetylcholine-stimulated production of inositol trisphosphate and inositol monophosphate was inhibited by atropine (20μm), but not tubocurarine (100μm); and it was abolished by depletion of extracellular Ca2+ with EGTA, but restored on addition of low concentrations of Ca2+ (20μm). 5. Calcium-antagonistic agents, such as verapamil (20μm), dibenamine (20μm) or La3+ (2mm), also abolished the production of the water-soluble inositol phosphates in response to acetylcholine. 6. Release of inositol trisphosphate from exogenous phosphatidylinositol bisphosphate by iris muscle microsomal fraction (`microsomes') was stimulated by 43% in the presence of 50μm-Ca2+. 7. The results indicate that increased Ca2+ influx into the iris smooth muscle by acetylcholine and ionophore A23187 markedly activates phosphatidylinositol bisphosphate phosphodiesterase and subsequently increases the production of inositol trisphosphate and its hydrolytic product inositol monophosphate. The marked increase observed in the production of inositol monophosphate could also result from Ca2+ activation of phosphatidylinositol phosphodiesterase. However, there was no concomitant decrease in the 3H radioactivity of this phospholipid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Green K., Smith J. P., McPherson J. C., Jr, Matheny J. L. Norepinephrine-stimulated breakdown of triphosphoinositide of rabbit iris smooth muscle: effects of surgical sympathetic denervation and in vivo electrical stimulation of the sympathetic nerve of the eye. J Neurochem. 1978 Mar;30(3):517–522. doi: 10.1111/j.1471-4159.1978.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Calcium ion requirement for acetylcholine-stimulated breakdown of triphosphoinositide in rabbit iris smooth muscle. J Pharmacol Exp Ther. 1978 Mar;204(3):655–668. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Effects of acetylcholine and norepinephrine on 45Ca uptake and efflux in rabbit iris smooth muscle. Gen Pharmacol. 1979;10(6):445–450. doi: 10.1016/0306-3623(79)90006-5. [DOI] [PubMed] [Google Scholar]
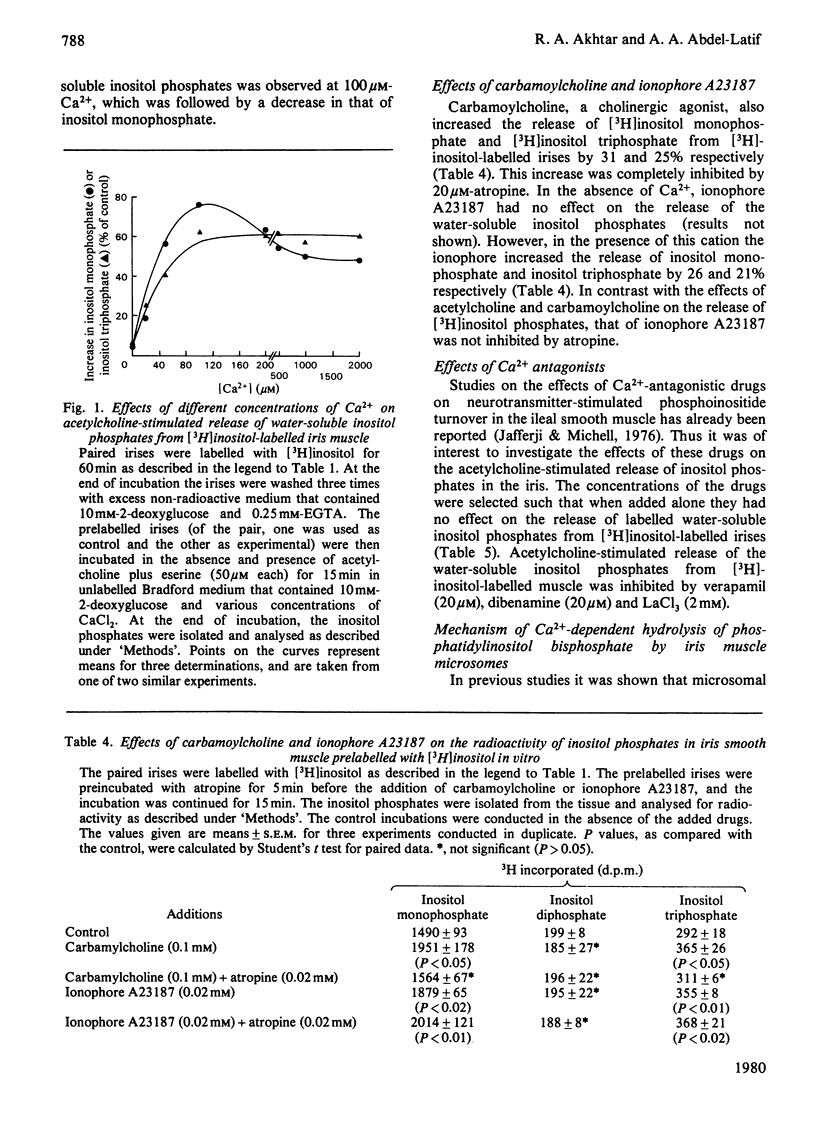
- Akhtar R. A., Abdel-Latif A. A. Studies on the properties of triphosphoinositide phosphomonoesterase and phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1978 Nov 10;527(1):159–170. doi: 10.1016/0005-2744(78)90265-6. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978 Apr 4;508(2):277–286. doi: 10.1016/0005-2736(78)90330-9. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
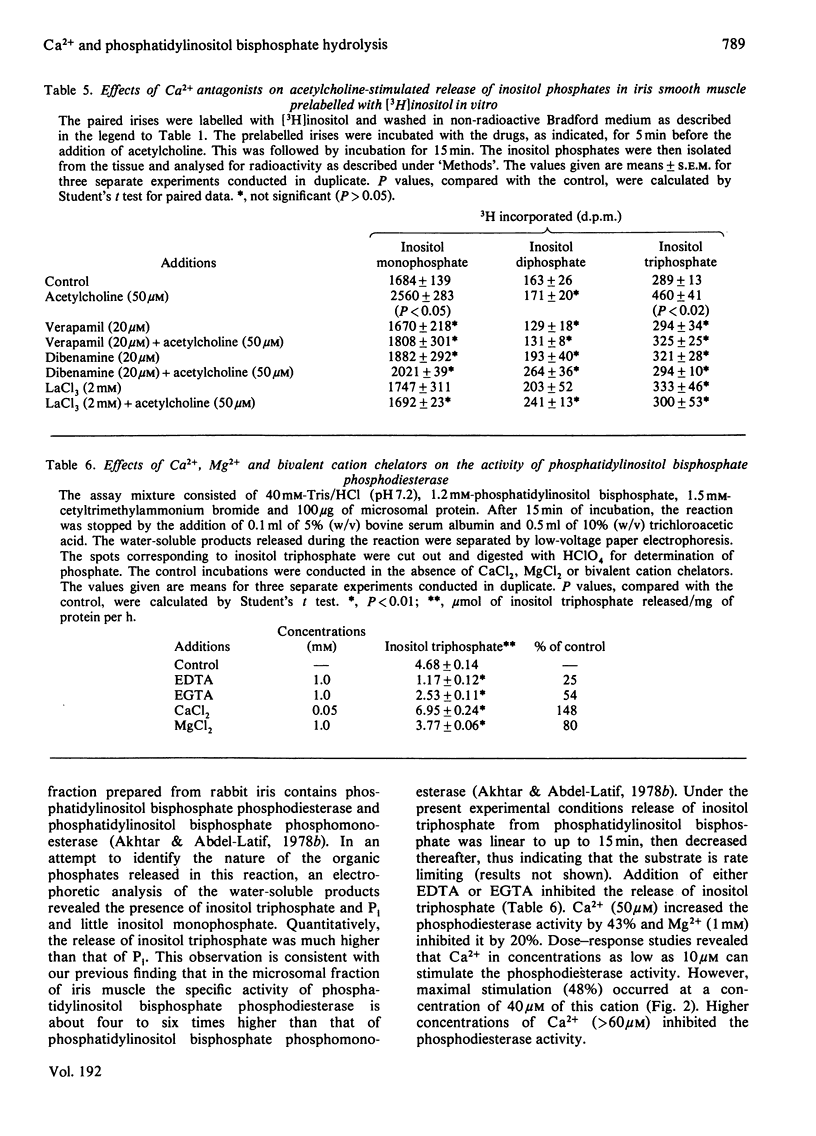
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
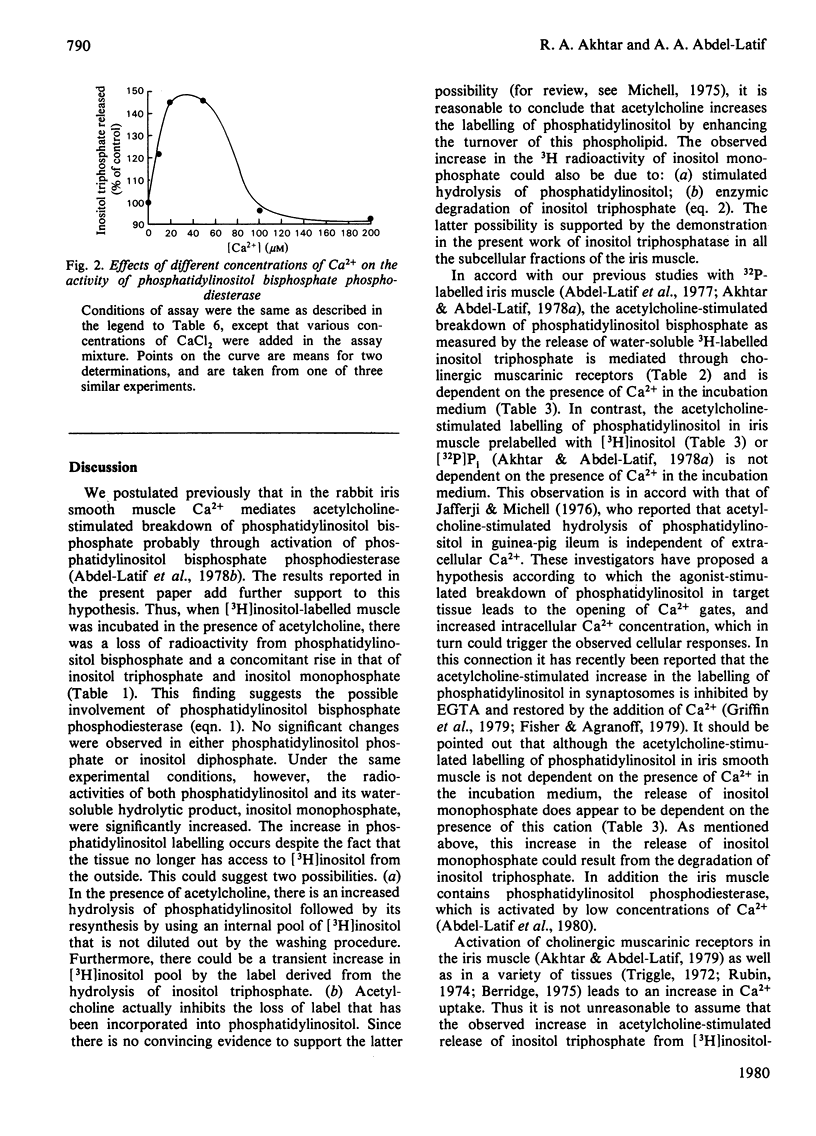
- Bradford H. F. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 May;16(5):675–684. doi: 10.1111/j.1471-4159.1969.tb06444.x. [DOI] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N. Calcium-activated hydrolysis of phosphatidyl-myo-inositol 4-phosphate and phosphatidyl-myo-inositol 4,5-bisphosphate in guinea-pig synaptosomes. Biochem J. 1978 Nov 15;176(2):541–552. doi: 10.1042/bj1760541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N., Sykes M., Orlacchio A. A calcium requirement for the phosphatidylinositol response following activation of presynaptic muscarinic receptors. Biochem Pharmacol. 1979 Apr 1;28(7):1143–1147. doi: 10.1016/0006-2952(79)90320-4. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HENDRICKSON H. S., BALLOU C. E. ION EXCHANGE CHROMATOGRAPHY OF INTACT BRAIN PHOSPHOINOSITIDES ON DIETHYLAMINOETHYL CELLULOSE BY GRADIENT SALT ELUTION IN A MIXED SOLVENT SYSTEM. J Biol Chem. 1964 May;239:1369–1373. [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Jafferji S. S., Michell R. H. Effects of calcium-antagonistic drugs on the stimulation by carbamoylcholine and histamine of phosphatidylinositol turnover in longitudinal smooth muscle of guinea-pig ileum. Biochem J. 1976 Nov 15;160(2):163–169. doi: 10.1042/bj1600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]


