Abstract
Objectives:
Describe clinical and demographic associations with inpatient medication for opioid use disorder (MOUD) initiation on general medicine services and to examine associations between inpatient MOUD initiation by generalists and subsequent patient healthcare utilization.
Methods:
This is a retrospective study using medical record data from general medicine services at an urban safety-net hospital before an inpatient addiction consultation service. The patients were adults hospitalized for acute medical illness who had an opioid-related ICD-10 code associated with the visit. Associations with MOUD initiation were assessed using multivariable logistic regression. Hospital readmission, emergency department use, linkage to opioid treatment programs (OTP), and mortality at 30- and 90-days postdischarge were compared between those with and without hospital MOUD initiation using χ2 tests.
Results:
Of 1,284 hospitalized patients with an opioid-related code, 59.81% received MOUD and 31.38% of these were newly initiated in-hospital. In multivariable logistic regression, Black race, mood disorder, psychotic disorder, and alcohol use disorder were negatively associated with MOUD initiation, while being aged 25–34, having a moderate hospital severity of illness score, and experiencing homelessness were positively associated. There were no bivariate associations between MOUD initiation and postdischarge emergency department use, hospital readmission, or mortality at 30- and 90-days, but those initiated on MOUD were more likely to present to an OTP within 90 days (30.57% vs 12.80%, P < 0.001).
Conclusions:
MOUD prescribing by inpatient generalists may help to increase the number of patients on treatment for opioid use disorder after hospital discharge. More research is needed to understand the impact of inpatient MOUD treatment without addiction specialty consultation.
Keywords: health disparities, inpatient medicine, methadone, opioid use disorder, treatment initiation
Every year hundreds of thousands of patients with opioid use disorder (OUD) are hospitalized, yet few receive evidence-based treatment for OUD.1–4 Hospitalized patients with OUD frequently have high readmission rates and high healthcare costs.5–7 These hospitalizations may represent a missed opportunity to address OUD and better support vulnerable patients.
Specialty programs that provide integrated medical and behavioral OUD treatment for hospitalized patients have been shown to increase engagement in outpatient treatment, indicating that hospitalizations may be a good opportunity to address substance use and connect patients to ongoing care.8,9 Furthermore, specialty addiction consultation hospital services that offer medication for OUD (MOUD) have also been shown to increase linkage to outpatient treatment and decrease the severity of OUD illness.10–12
Inpatient addiction consultation services are feasible to implement in care settings where there is access to addiction specialists, but this type of service may not be realistic for smaller hospitals or settings where no specialist is available.13,14 Given the high prevalence of OUD in the general population and the frequency of hospitalizations among this patient population, OUD is being reconsidered as an illness that a general medical practitioner should address in the hospital setting.15,16 Evidence supports non-addiction specialists providing MOUD in primary care settings of equal quality to specialty care, yet little evidence exists for non-addiction specialists providing MOUD in the acute hospital setting.17–19
We analyzed electronic health records of patients with OUD who were admitted during a 2.5-year period to an urban safety-net teaching hospital and were cared for on general medicine inpatient services. We sought to: (1) describe the patient population with OUD in our hospital who were not on MOUD, (2) identify demographic and clinical associations with hospital treatment initiation, and (3) examine associations with MOUD initiation by non-addiction specialists and subsequent patient healthcare utilization and mortality within 90-days of discharge.
METHODS
Setting
The study took place at Zuckerberg San Francisco General Hospital (ZSFG), an academic, urban county hospital with 397 inpatient beds. The hospital serves as the safety-net facility and only level 1 trauma center in San Francisco. Hospitalizations on the family medicine service, faculty hospitalist service, and internal medicine teaching services were included in the dataset. These services are staffed by the University of California, San Francisco, residents, and clinical faculty members. We focused on these services because MOUD prescribing was more common and there is a greater precedent for inpatient generalists to treat OUD based on evidence for this practice in the outpatient setting.17–19 Discharge planning on these services is coordinated between the treating physicians and a team social worker. Discharge planning nurses and case managers were involved on a case-by-case basis. There was no postdischarge support for connecting to OUD treatment.
During this time period there was no addiction consult service or active research studies on inpatient treatment of substance use disorders. There was informal teaching on MOUD on some services and the hospital hosted Drug Addiction Treatment Act 2000 Waiver Trainings several times during the study period, but there were no formal hospital protocols for initiating MOUD. Physicians in the health system with expertise in MOUD were available for informal peer-to-peer guidance on buprenorphine initiation. Currently, about 27 faculty who staff the services in this study have a Drug Addiction Treatment Act 2000 Waiver, which is estimated to be 20%. We do not have historical data on when providers obtained their waiver. The number of residents with a waiver is unknown.
Study Population
Patients 18 and older who were admitted to one of the above-mentioned services for any primary diagnosis between January 1, 2016 and June 30, 2018 who also had at least 1 OUD-related ICD-10 diagnosis code for the encounter were included in the study. Coding for OUD was based on the admission note, progress notes, and/or discharge summary for the encounter. OUD diagnosis codes included root codes F11 (opioid-related) and F19 (other psychoactive substance). A subset of hospitalizations with an F11 code was manually reviewed and it was confirmed that coding was an accurate marker of an OUD diagnosis by the documenting provider. F19 codes were included to capture patients with polysubstance use and those who were classified into a nonspecific substance use category. All of these hospitalizations were manually chart reviewed for keywords to determine if the use disorder included opioids. Patients who were pregnant at the time of admission were excluded because many were admitted for OUD management in the setting of high-risk pregnancy, unlike non-pregnant patients who are not admitted routinely for OUD management.
Demographic and Index Admission Measures
The index admission was defined as the first hospitalization with an OUD-related code during the study period for each patient. For each index encounter, pharmacy records were assessed to determine whether MOUD was newly initiated during the encounter, was an outpatient medication continued by the inpatient treatment team, or was not prescribed. A new hospital MOUD initiation was defined as any patient who was not on MOUD for at least 7-days before admission because patients in our hospital were clinically treated as new starts after this amount of time, meaning they were initiated on starting doses and titrated up. This was determined by matching inpatient pharmacy and hospitalization records to determine which patients were on MOUD during the visit, then reviewing pharmacy documentation to determine if it was a new initiation. Where pharmacy documentation did not clearly indicate the number of days the patient was off of MOUD, a manual chart review of the admission notes was performed. The highest dose of methadone or buprenorphine that the patient received on any 1 day during the hospitalization was captured. For patients initiated on buprenorphine, the incidence of precipitated withdrawal was determined by manual chart view. For patients not prescribed MOUD in the hospital, their outpatient MOUD treatment status was unknown.
Age, sex, race, ethnicity, primary language, housing status, insurance carrier, primary discharge diagnosis, severity of illness score, length of hospital stay, and co-morbidities were identified using electronic medical record data for the index encounter. Race and ethnicity were self-reported to hospital intake coordinators and eligibility workers and for this study were organized into four categories: White, Black, Hispanic and other/unknown (Asian, Native American, Native Hawaiian/Pacific Islander, other race, and unspecified; combined due to small sample size). Insurance type was divided into Medicaid, Medicare, and other (private pay, jail coverage, commercial, Veteran’s Affairs Health Benefits, and Tricare were combined due to the small sample size). The severity of illness score for the admission is on a one-to-four scale with 4 being the most severe,20 assigned by the hospital billing department for every hospitalization. The score is based on the provider’s assessment of the extent of physiologic decompensation and responsiveness to treatment, or the need for ongoing life-sustaining measures. For measures with missing data, the totals are shown separately in Table 1.
TABLE 1.
Patient Characteristics and Index Admission Measures
| Variable | Total (n = 757) | No MOUD (n = 516) | Started MOUD (n = 241) | Bivariate P Value |
|---|---|---|---|---|
| Age, median (IQR), years | 47 (34–56) | 50 (36–57.5) | 40 (30–53) | <0.001 |
| Male sex | 546 (72.13) | 383 (74.22) | 163 (67.63) | 0.060 |
| Race/ethnicity | <0.001 | |||
| White, non-Hispanic | 356 (47.79) | 215 (42.24) | 141 (59.75) | |
| Black, non-Hispanic | 231 (31.01) | 178 (34.97) | 53 (22.46) | |
| Hispanic | 105 (14.09) | 79 (15.52) | 26 (11.02) | |
| Other/unknown | 53 (7.11) | 37 (7.27) | 16 (6.78) | |
| English as primary language | 713 (94.19) | 476 (92.25) | 237 (98.34) | 0.001 |
| Insurance type | 0.086 | |||
| Medicaid | 566 (74.77) | 375 (72.67) | 191 (79.25) | |
| Medicare | 127 (16.78) | 97 (18.80) | 30 (12.45) | |
| Other | 64 (8.45) | 44 (8.53) | 20 (8.30) | |
| Homelessness (n = 754) | 377 (50.07) | 233 (45.51) | 144 (59.75) | <0.001 |
| Medication for OUD | N/A | |||
| Methadone (median dose: 30 mg) | – | – | 219 (90.87) | |
| Buprenorphine (median dose: 10 mg) | – | – | 22 (9.13) | |
| Co-occurring disorders | ||||
| Cocaine or other stimulant use | 521 (68.82) | 352 (68.22) | 169 (70.12) | 0.598 |
| Alcohol use | 237 (31.31) | 180 (34.88) | 57 (23.65) | 0.002 |
| Psychotic disorder | 125 (16.51) | 97 (18.80) | 28 (11.62) | 0.013 |
| Anxiety disorder | 233 (30.78) | 164 (31.78) | 69 (28.63) | 0.381 |
| Mood disorder | 288 (38.04) | 221 (42.83) | 67 (27.80) | <0.001 |
| Severity of illness score, mean (SD) (n = 740) | 2.33 (0.92) | 2.35 (0.95) | 2.26 (0.87) | 0.238 |
| Length of hospital stay, median (IQR), days | 4 (3–7) | 4 (2–6.5) | 5 (3–8) | 0.028 |
All values shown are n (%) for the column unless otherwise indicated.
IQR, interquartile range; MOUD, medication for opioid use disorder; OUD, opioid use disorder; SD, standard deviation.
The comorbidities we examined were alcohol use disorder, cocaine and stimulant use disorder, psychotic disorder, mood disorder, and anxiety disorder. Co-morbidities were selected on hypothesized relevance to OUD outcomes.21–23 Patients were considered to have a co-morbid condition if they had a relevant ICD-10 code at any encounter during the study time period. This approach was chosen because chronic and/or psychiatric conditions are not always captured during medical hospitalizations and outpatient billing in our system limits the number of billing codes assigned per encounter. Additionally, many mental health disorders are diagnosed over the course of several visits. For these reasons, we were concerned that there would be an underestimation of the prevalence of comorbidities due to under documentation if only the hospital encounter or shorter time frames were used.
Postdischarge Outcomes
Outcomes assessed were emergency department (ED) presentation, hospital readmission, presentation to an opioid treatment program (OTP), and postdischarge mortality within 30-days and 90-days after discharge from the index encounter. Patients who died in the hospital were transferred to another facility or service, enrolled in hospice, or were incarcerated were not included in the postdischarge data analysis because we were unable to follow them to discharge or their follow-up care differed from the general hospital population. We re-ran our analysis of mortality with hospice patients and planned to report it if there were any changes in the significance that would change the interpretation of our results. ED presentations and hospital readmissions were determined using medical record data at ZSFG Hospital. Linkage to outpatient OUD treatment was assessed using data from the Department of Public Health’s (DPH) network of OTPs throughout the city. One encounter within the first 30-days after discharge and another encounter between 31 and 90-days was considered linkage to care for each time frame. Identified patient data was provided to the DPH, but due to data sharing restrictions, the OTP data were only available in aggregated, de-identified form. Therefore, analysis requiring matching to the primary dataset could not be performed. OTPs provide both methadone and buprenorphine treatment, however, this outcome does not include buprenorphine prescriptions from primary care settings or OTP encounters outside of San Francisco. Postdischarge mortality was collected from vital records.
Data Analysis
Patient and index hospital encounter characteristics for patients who initiated MOUD and those who did not receive MOUD treatment were analyzed using descriptive statistics. Differences in patient and index hospital encounter characteristics and postdischarge outcomes were assessed using χ2 tests.
Unadjusted and adjusted logistic regression models were used to examine associations with the initiation of MOUD in-hospital. Patient and index-hospitalization characteristics were included as independent variables based on hypothesized clinical importance and previously published findings on predictors of treatment initiation. Gender, age, race and ethnicity, insurance type, housing status, psychiatric disorders, and alcohol use have been shown to be predictors of outpatient treatment entry21,23–27 and housing status and methamphetamine use have been shown to have associations with inpatient treatment initiation.22 All statistics were performed using STATA version 16.0.
This study was approved by the University of California, San Francisco Institutional Review Board (IRB #18–25148) and the San Francisco DPH. Informed consent by participants was not required.
RESULTS
There were 16,404 hospitalizations on the 3 services during the study time period. There were 1,417 unique patients with opioid or psychoactive substance-related ICD-10 code. 1,284 were found to have OUD after excluding a subset of patients with psychoactive substance use that did not include opioids. Of patients with OUD, 527 were on buprenorphine or methadone in the community and were continued on treatment during their index hospital stay. Of the other 757 patients, 241 (31.84%) were initiated on MOUD during their index hospital stay whereas 516 (68.16%) received no inpatient MOUD (Fig. 1).
FIGURE 1.
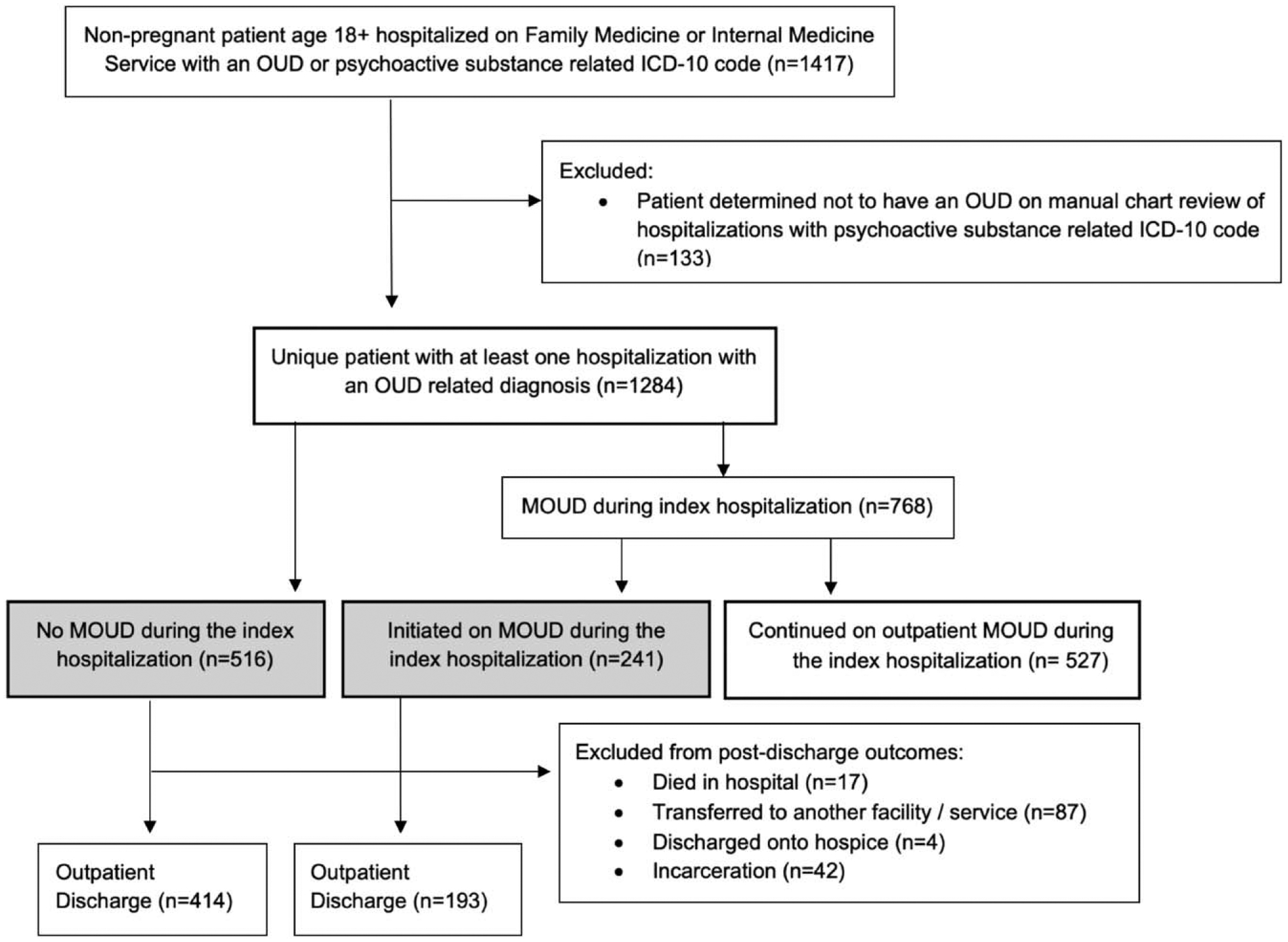
Participant flowchart. Comparison groups are shaded in grey. MOUD, medication for opioid use disorder; OUD, opioid use disorder.
The patients who were not on MOUD during their index hospital stay (n = 757) were predominantly male (72.13%), English speaking (94.19%), and had Medicaid (74.77%). Half were experiencing homelessness and 68.82% had a comorbid cocaine or stimulant use disorder (Table 1). The three most common groups of primary discharge diagnoses were skin and soft tissue infection (22.46%), sepsis and bacteremia (15.46%), and opioid-related disorders (5.94%). Of patients initiated on MOUD during the index hospitalization, the median highest daily dose of methadone was 30 mg interquartile range (IQR 20–40 mg) and the median highest daily dose of buprenorphine was 10 mg (IQR 8–16 mg) (Table 1). None of the patients initiated on buprenorphine experienced precipitated withdrawal. No patients received naltrexone for OUD treatment.
In bivariate analysis, initiation of MOUD was associated with differences in age, race and ethnic group, language, homelessness, length of stay, alcohol use disorder, psychotic disorder, and mood disorder (Table 1.). In multivariable analysis assessing associations with in-hospital MOUD initiation, being aged 25 to 34 (aOR 2.32, 95% CI 1.09–4.94) compared to a reference group of age 18–24, experiencing homelessness (aOR 1.63, 95% CI 1.14–2.32), and having a severity of illness score of 2 (aOR 1.77, 95% CI 1.11–2.81) or 3 (aOR 1.75, 95% CI 1.03–2.95) compared to 1, were positively associated with MOUD initiation. Black race (aOR 0.59, 95% CI 0.39–0.88) compared to White race, and co-occurring mood disorder (aOR 0.51, 95% CI 0.34–0.75), alcohol use disorder (aOR 0.67, 95% CI 0.45–0.98), and psychotic disorder (aOR 0.58, 95% CI 0.35–0.96) were negatively associated with MOUD initiation (Table 2).
TABLE 2.
Unadjusted and Adjusted Logistic Regression of MOUD Initiation in the Hospital
| Independent Variable | Unadjusted Odds Ratios (95% CI) | Adjusted Odds Ratios (95% CI) |
|---|---|---|
| Age 18–24* | 1 | 1 |
| 25–34 | 2.27 (1.14–4.49) | 2.32 (1.09–4.94) |
| 35–44 | 1.37 (0.69–2.70) | 1.65 (0.76–3.56) |
| 45–54 | 1.05 (0.53–2.07) | 1.25 (0.58–2.70) |
| 55–64 | 0.70 (0.34–1.41) | 0.89 (0.40–2.00) |
| 65–74 | 0.67 (0.28–1.62) | 1.10 (0.36–3.34) |
| Sex | 1.38 (0.99–1.92) | 1.39 (0.96–2.00) |
| Race/ethnicity | ||
| White, non-Hispanic* | 1 | 1 |
| Black, non-Hispanic | 0.45 (0.31–0.66) | 0.59 (0.39–0.88) |
| Hispanic | 0.51 (0.31–0.84) | 0.63 (0.38–1.07) |
| Other/unknown | 0.70 (0.40–1.22) | 0.73 (0.40–1.34) |
| Insurance type | ||
| Medicaid* | 1 | 1 |
| Medicare | 0.61 (0.39–0.95) | 1.03 (0.57–1.84) |
| Other/commercial | 0.89 (0.51–1.56) | 0.85 (0.45–1.63) |
| Homelessness | 1.78 (1.30–2.43) | 1.63 (1.14–2.32) |
| Severity of illness score | ||
| 1* | 1 | 1 |
| 2 | 1.33 (0.87–2.04) | 1.77 (1.11–2.81) |
| 3 | 1.00 (0.63–1.60) | 1.75 (1.03–2.95) |
| 4 | 0.78 (0.43–1.41) | 1.31 (0.68–2.53) |
| Co-occurring disorders | ||
| Alcohol use | 0.58 (0.41–0.82) | 0.67 (0.45–0.98) |
| Cocaine and other stimulant use | 1.09 (0.78–1.52) | 1.10 (0.76–1.61) |
| Mood | 0.51 (0.37–0.72) | 0.51 (0.34–0.75) |
| Psychotic | 0.57 (0.36–0.89) | 0.58 (0.35–0.96) |
| Anxiety | 0.86 (0.62–1.20) | 1.01 (0.68–1.52) |
Reference group.
MOUD, medication for opioid use disorder.
In bivariate analysis, initiation of MOUD was not associated with subsequent ED presentation, hospital admission or all-cause mortality at 30- and 90-days postdischarge. Initiation of MOUD in the hospital was associated with presenting to an OTP within 30 (27.98% vs 10.63%, P < 0.001) and 90-days (30.57% vs 12.80%, P < 0.001) (Table 3).
TABLE 3.
Mortality and Healthcare Utilization 30- and 90-days Postdischarge
| Total | No MOUD During Index Hospitalization | Started MOUD During Index Hospitalization | Bivariate P Value | |
|---|---|---|---|---|
| Patients followed post-discharge, n | 607 | 414 | 193 | – |
| ED presentations, n (%) | ||||
| 30 days | 113 (18.62) | 79 (19.08) | 34 (17.62) | 0.666 |
| 90 days | 201 (33.11) | 138 (33.33) | 63 (32.64) | 0.866 |
| Hospital admissions, n (%) | ||||
| 30 days | 78 (12.85) | 51 (12.32) | 27 (13.99) | 0.567 |
| 90 days | 137 (22.57) | 92 (22.22) | 45 (23.32) | 0.764 |
| All-cause mortality, n (%) | ||||
| 30 days | 10 (1.65) | 7 (1.69) | 3 (1.55) | 0.902 |
| 90 days | 19 (3.13) | 15 (3.62) | 4 (2.07) | 0.307 |
| Outpatient OTP presentation, n (%) | ||||
| 30 days | 98 (16.14) | 44 (10.63) | 54 (27.98) | <0.001 |
| 90 days | 112 (18.45) | 53 (12.80) | 59 (30.57) | <0.001 |
All values shown are n (%) for the column.
ED, emergency department; MOUD, medication for opioid use disorder; OTP, opioid treatment program.
DISCUSSION
To our knowledge, this is the first study to examine real-world hospital MOUD treatment outside of addiction specialty consultation. We found that about 60% of patients received MOUD and about a third were newly initiated. Treatment initiation was associated with certain demographic and clinical characteristics that could be important for targeted interventions. Patients who were initiated on MOUD in-hospital were more likely to engage with an OTP postdischarge, despite that most patients were not presenting to the hospital for OUD treatment and there was no formalized protocol for treatment linkage.
The rate of treatment initiation found in this study is higher than previously reported in the general hospital population at Veterans Health Administration (31.8% vs 8.4%).3 The treatment initiation rate for those who received a hospital addiction consultation has ranged from 31% to 87% in previous studies.11,22,28–30 Our study initiation rates may differ from addiction consultation rates due to the expertise provided by these care teams. Differences could also reflect a selection bias where addiction consultation services were more likely to be consulted for patients who are interested in starting treatment.
Our findings support the notion that general hospital medicine providers can increase the number of patients on MOUD without the assistance of addiction medicine specialists. Specialty addiction consultation services implemented at hospitals across the country, mainly tertiary academic centers, bring myriad benefits.10–12 Nonetheless, not all hospital systems can implement an addiction consultation service due to the limited availability of addiction specialists in many communities and lack of financial resources to staff a consulting service.13,14 Furthermore, hospital addiction consultation services may not be able to staff every case. Management of OUD can be conceptualized similarly to the management other common illnesses such as diabetes,31 where an endocrinology consultation is not required for every patient with diabetes who requires insulin or needs to start a new medication for better glucose control. Some patients will require specialty care, but training hospitalists to prescribe MOUD and refer to outpatient care is a practical conjunctive or alternative solution for increasing access to MOUD that merits further research.
This study also highlights demographic and clinical characteristics associated with initiating MOUD in the hospital. The higher rate of initiating treatment among patients experiencing homelessness,22 in contrast to outpatient settings in which homelessness is associated with not initiating MOUD,21,24,26,32 suggests a unique opportunity during hospitalization for MOUD initiation. In contrast, mood disorder, psychotic disorder, and alcohol use disorder were associated with not initiating MOUD, suggesting that complex patient cases may require addiction specialists or additional provider education.
Lower rates of MOUD initiation among Black patients raise concerns about equity, stigma, or lack of trust. This finding is consistent with previous evidence that racial and ethnic minorities often receive lower-quality care compared to White patients.33 The literature on racial differences in substance use disorder treatment in the outpatient setting is mixed, with some studies showing higher odds of MOUD treatment among people of Black race or Hispanic ethnicity compared to White race wheras others showing the opposite.25,34,35 Situations in which there is cognitive overload, such as busy hospital settings, are more susceptible to provider implicit bias.36 The consequences of stigma related to seeking treatment also might be more pronounced in some Black communities37 and previous research on methadone maintenance therapy demonstrated that some minority communities have negative perceptions of methadone, the primary medication utilized in this study.38
We did not identify an association between initiating MOUD in the hospital and presenting to the ED after discharge, being readmitted, or dying within 30- or 90-days of discharge. Studies of MOUD initiation by addiction medicine services have been mixed, with some demonstrating reduced ED and hospital utilization and others showing no reduction.8,12,28,29,39 Expanded and well-tailored services, including patient navigation, may be needed to help to reduce postdischarge ED use and hospital readmissions in this population. Regardless of these outcomes, MOUD is an effective treatment that should be offered to all patients with OUD.40
We found lower rates of postdischarge OUD treatment in this study than previously reported.10,11,41 Although many factors could contribute to the failure of linkage to outpatient care, the absence of hospital protocols, and our inability to fully capture postdischarge OUD treatment, may explain some of the differences. We also found low median doses of MOUD, which could indicate that medications were used for management of withdrawal symptoms only, without intention to up titrate to manage cravings or link to ongoing OUD care. Although this may represent a missed opportunity to adequately treat OUD, hospital initiation of MOUD solely to manage withdrawal is still important for patient comfort and will help with the maintenance of tolerance, mitigating the theoretical risk of overdose postdischarge.42,43 Given that methadone titrations can take days to weeks to reach therapeutic doses, the low median dose could also reflect incomplete medication titrations due to short lengths of stay.
More research is needed to better understand hospital OUD care by non-addiction specialists and to determine if our findings are replicated in other hospital systems. There are some strategies that can be considered to increase OUD care by inpatient providers and target undertreated populations. Provider education on OUD treatment and implicit bias and standardized hospital protocols for screening and starting patients on MOUD could help fill gaps for when no addiction specialists are available while mitigating health care disparities. Signage that makes patients aware of the availability of treatment in the hospital may normalize MOUD and help patients recognize that there could be a benefit to discussing their opioid use. The California Bridge Project (bridgetotreatment.org) and Oregon Health and Sciences University IMPACT Team both have published resources to help hospitals adopt standardized practices for substance use treatment.44
Limitations
Given that this was a single-site study with unknown levels of provider training on MOUD, the generalizability of the study may be limited. Our findings may be unique due to health policy, hospital, provider, and community factors that can influence MOUD prescribing. We use ICD-10 billing codes, which are not intended for research or clinical assessments. Use of ICD-10 codes to identify patients with OUD likely underestimates the number of patients with OUD, especially those who did not receive MOUD, because it only includes people with an identified diagnosis and likely a more severe disorder. Co-morbid conditions were identified using relevant ICD-10 codes for any encounter during the study time period. We chose this approach to avoid underestimation, however, it is possible that the co-morbidity developed after the index hospitalization, which would overestimate prevalence. We also lack measures such as the severity of OUD illness, engagement in primary care, and past patient experiences with MOUD which would potentially confound the MOUD initiation associations we identified.
The analysis of postdischarge data is unadjusted and could be confounded. It is also limited because ED presentations and hospital readmissions only include those at our site, not at other acute care hospitals in the community. Buprenorphine prescribed outside of an OTP and any MOUD treatment outside of our city were not captured. Therefore, ED presentation, hospital readmission, and outpatient OUD treatment rates, especially for buprenorphine, are likely underestimated and we cannot draw conclusions about post-discharge linkage to office-based OUD care.
CONCLUSIONS
This study demonstrates that inpatient generalists can increase the number of patients on MOUD without addiction specialty consultation. Hospital MOUD initiation varied by patient race, age, severity of illness score, housing status, and co-morbidities and was positively associated with presenting to an OTP after discharge. In unadjusted analysis, there was no association between hospital MOUD initiation and post-discharge ED presentation, hospital readmission, or mortality. More research is needed to understand the feasibility and effectiveness of OUD treatment without specialty consultation.
ACKNOWLEDGMENTS
We would like to thank the staff at the ZSFG inpatient pharmacy, Monica Rose, PhD, and Sherry Lam, MPH for help with data collection and D. Andrew Tompkins, MD, MHS and Oanh Nguyen, MD, MAS for their input on the study design.
This work was investigator led. The UCSF Primary Care Leadership Academy supported Hannah Tierney’s time for this project. The time of the other authors was supported by the City and County of San Francisco. Outside of the submitted work, Dr. Coffin has received grant support from the Center for Disease Control and National Institutes of Health. Dr. Snyder has received grant support from the Substance Abuse and Mental Health Services Administration. Dr. Coffa has received grant support from the California Office of Statewide Planning and Development and from the Health Resources and Services Administration.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35(5):832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson C, Xu L, Mikosz CA, Florence C, Mack KA. US hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011–2015. J Subst Abuse Treat. 2018;92:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priest KC, Lovejoy TI, Englander H, Shull S, McCarty D. Opioid agonist therapy during hospitalization within the Veterans Health Administration: a pragmatic retrospective cohort analysis. J Gen Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saloner BKS. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013. JAMA. 2015;314:1515–1517. [DOI] [PubMed] [Google Scholar]
- 5.Merchant E, Burke D, Shaw L, et al. Hospitalization outcomes of people who use drugs: one size does not fit all. J Subst Abuse Treat. 2020;112:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White AG, Birnbaum HG, Mareva MN, Daher M, Vallow S, Schein J, Katz N. Direct cost of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Toole TP, Strain EC, Wand G, McCaul ME, Barnhart M. Outpatient treatment entry and healthcare utilization after a combined medical/substance abuse intervention for hospitalized medical patients. J Gen Intern Med. 2002;17:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J Gen Intern Med. 2019;34(12):2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services –linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan O, Murphy D, Krom L. Vital signs: taking the pulse of the addiction treatment profession, a national report, Version 1. Addiction Technology Transfer Center National Office in residence at the University of Missouri-Kansas City. 2012. [Google Scholar]
- 14.Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;14:E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanucchi LC, Lofwall MR. Putting parity into practice – integrating opioid-use disorder treament into the hospital setting. N Engl J Med. 2016;375(9):809–811. [DOI] [PubMed] [Google Scholar]
- 17.Bhatraju EP, Grossman E, Tofighi B, et al. Public sector low threshold office-based buprenorphine treatment: outcomes at year 7. Addict Sci Clin Pract. 2017;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad MS, Zelenev A, Altice FL. Buprenorphine maintenance treatment retention improves nationally recommended preventive primary care screenings when integrated into urban federally qualified health centers. J Urban Health. 2015;92(1):193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertner AK, Robertson AG, Powell BJ, Jones H, Silberman P, Domino ME. Primary care providers and specialists deliver comparable buprenorphine treatment quality. Health Aff (Millwood). 2020;39(8):1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn SD, Horn RA, Sharkey PD. The Severity of Illness Index as a severity adjustment to diagnosis-related groups. Health Care Financ Rev. 1984;(Suppl (Suppl)):33–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Damian AJ, Mendelson T, Agus D. Predictors of buprenorphine treatment success of opioid dependence in two Baltimore City grassroots recovery programs. Addict Behav. 2017;73:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englander H, King C, Nicolaidis C, et al. Predictors of opioid and alcohol pharmacotherapy initiation at hospital discharge among patients seen by an inpatient addiction consult service. J Addict Med. 2020;14.(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantone RE, Garvey B, O’Neill A, et al. Predictors of medication-assisted treatment initiation for opioid use disorder in an interdisciplinary primary care model. J Am Board Fam Med. 2019;32(5):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer B, Cruz MF, Patra J, Rehm J. Predictors of methadone maintenance treatment utilization in a multisite cohort of illicit opioid users (OPICAN). J Subst Abuse Treat. 2008;34(3):340–346. [DOI] [PubMed] [Google Scholar]
- 25.Krawczyk N, Feder KA, Fingerhood MI, Saloner B. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. national sample. Drug Alcohol Depend. 2017;178:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsui JI, Burt R, Thiede H, Glick SN. Utilization of buprenorphine and methadone among opioid users who inject drugs. Substance Abus. 2018;39(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pro G, Utter J, Haberstroh S, Baldwin JA. Dual mental health diagnoses predict the receipt of medication-assisted opioid treatment: associations moderated by state Medicaid expansion status, race/ethnicity and gender, and year. Drug Alcohol Depend. 2020;209:107952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68(11):1935–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordeck CD, Welsh C, Schwartz RP, et al. Rehospitalization and substance use disorder (SUD) treatment entry among patients seen by a hospital SUD consultation-liaison service. Drug Alcohol Depend. 2018;186:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki J Medication-assisted treatment for hospitalized patients with intravenous-drug-use related infective endocarditis. Am J Addict. 2016;25(3):191–194. [DOI] [PubMed] [Google Scholar]
- 31.McLellan AT, Starrels JL, Tai B, et al. Can substance use disorders be mangaed using the chronic care model? Review and recommendations from a NIDA consensus group. Public Health Reviews. 2015;35:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prangnell A, Daly-Grafstein B, Dong H, et al. Factors associated with inability to access addiction treatment among people who inject drugs in Vancouver, Canada. Subst Abuse Treat Prev Policy. 2016;11.(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson A Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666. [PMC free article] [PubMed] [Google Scholar]
- 34.Lundgren LM, Amodeo M, Ferguson F, Davis K. Racial and ethnic differences in drug treatment entry of injection drug users in Massachusetts. J Subst Abuse Treat. 2001;21:145–153. [DOI] [PubMed] [Google Scholar]
- 35.Pinedo M A current re-examination of racial/ethnic disparities in the use of substance abuse treatment: do disparities persist? Drug Alcohol Depend. 2019;202:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess DJ, Phelan S, Workman M, Hagel E, Nelson DB, Fu SS, Widome R, van Ryn M. The effect of cognitive load and patient race on physicians’ decisions to prescribe opioids for chronic low back pain: a randomized trial. Pain Medicine. 2014;15:965–974. [DOI] [PubMed] [Google Scholar]
- 37.Alvidrez J, Snowden LR, Kaiser DM. The experience of stigma among Black mental health consumers. J Health Care Poor Underserved. 2008;19(3):874–893. [DOI] [PubMed] [Google Scholar]
- 38.Zaller ND, Bazazi AR, Velazquez L, Rich JD. Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities. Int J Environ Res Public Health. 2009;6(2):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-Day and 90-Day hospital readmission among patients with opioid use disorder. J Addict Med. 2019; 13(4):306–313. [DOI] [PubMed] [Google Scholar]
- 40.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravndal E, Amundsen EJ. Mortality among drug users after discharge from inpatient treatment: an 8-year prospective study. Drug Alcohol Depend. 2010;108(1–2):65–69. [DOI] [PubMed] [Google Scholar]
- 43.White SR, Bird SM, Merrall EL, Hutchinson SJ. Drugs-related death soon after hospital-discharge among drug treatment clients in Scotland: record linkage, validation, and investigation of risk-factors. PLoS One. 2015;10(11):e0141073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85–89. [DOI] [PubMed] [Google Scholar]


