Abstract
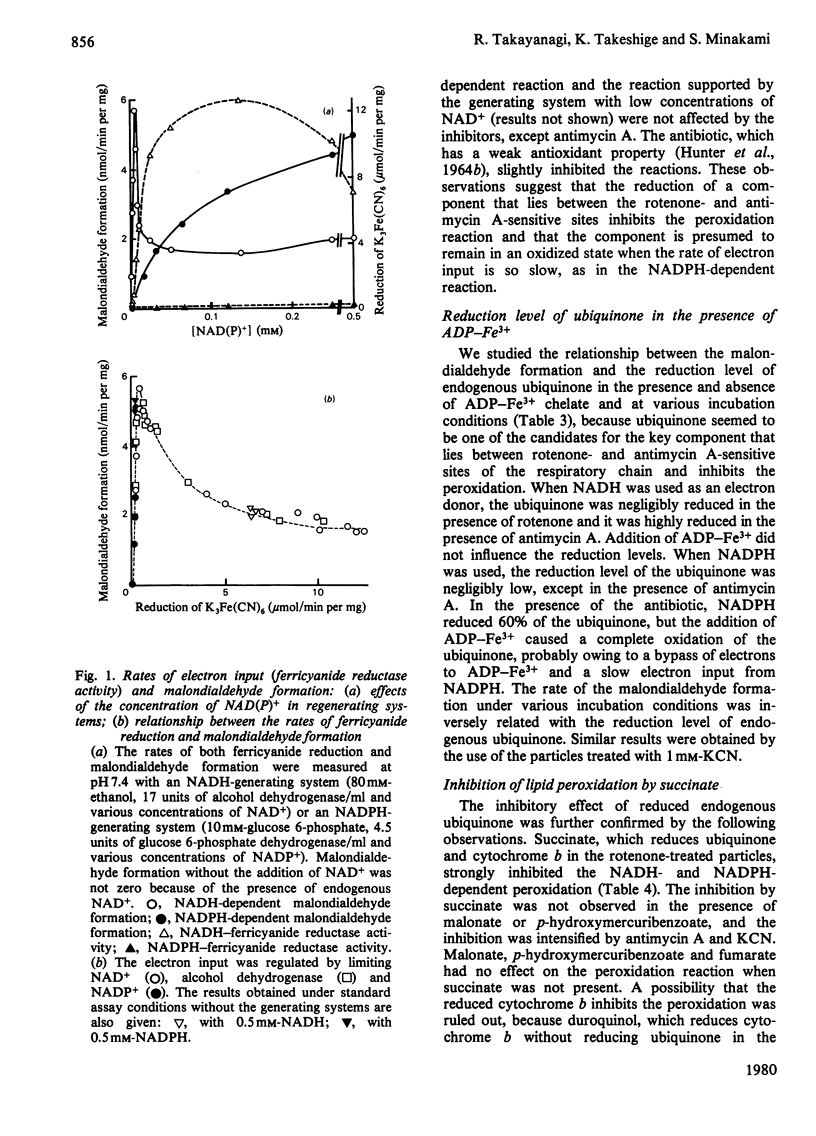
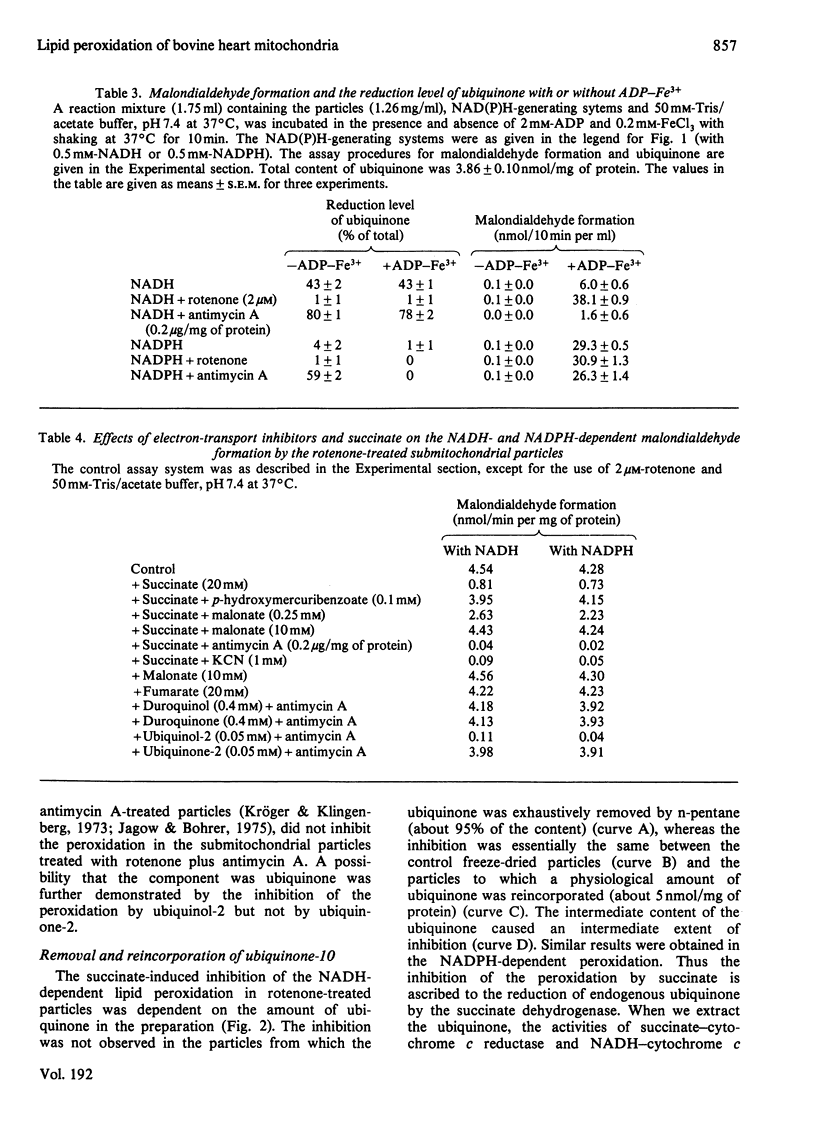
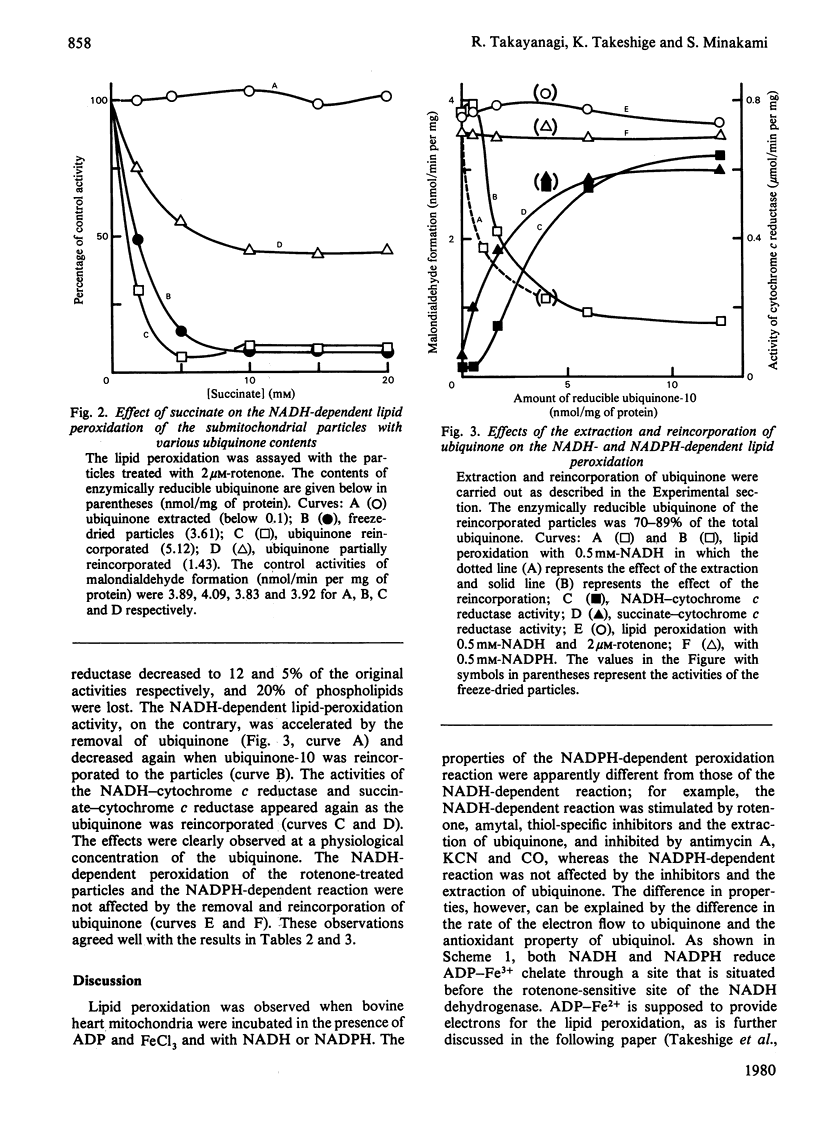
Malondialdehyde formations by bovine heart submitochondrial particles supported by NADH or NADPH in the presence of ADP and FeCl3 was studied. The NADH-dependent reaction was maximal at very low rate of electron input from NADH to the respiratory chain and it decreased when the rate became high. The reaction was stimulated by rotenone and inhibited by antimycin A when the input was fast, whereas it was not affected by the inhibitors when the input was slow. The input rate of the electrons from NADPH was also so low that the reaction supported by NADPH was not affected by the inhibitors. Most of the endogenous ubiquinone in the particles treated with antimycin A was reduced by NADH even in the presence of ADP-Fe3+ chelate, but uniquinone was not reduced by NADPH when ADP-Fe3+ was present. Succinate strongly inhibited both NADH- and NADPH-dependent lipid peroxidation. The inhibition was abolished when uniquinone was removed from the particles, and it appeared again when uniquinone was reincorporated into the particles. Reduced uniquinone-2 also inhibited the peroxidation, but duroquinol, which reduces cytochrome b without reducing endogenous uniquinone, did not. Thus the malondialdehyde formation appeared to be inversely related to the extent of the reduction of endogenous uniquinone. These observations suggest that both NADH- and NADPH-dependent liquid-peroxidation reactions are closely related to the respiratory chain and that the peroxidation is controlled by the concentration of reduced ubiquinone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkers K., Liu M., Watanabe T., Porter T. H. Inhibition by adriamycin of the mitochondrial biosynthesis of coenzyme Q10 and implication for the cardiotoxicity of adriamycin in cancer patients. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1536–1542. doi: 10.1016/s0006-291x(77)80152-6. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, SCOTT A., HOFFSTEN P. E., GEBICKI J. M., WEINSTEIN J., SCHNEIDER A. STUDIES ON THE MECHANISM OF SWELLING, LYSIS, AND DISTINTEGRATION OF ISOLATED LIVER MITOCHONDRIA EXPOSED TO MIXTURES OF OXIDIZED AND REDUCED GLUTATHIONE. J Biol Chem. 1964 Feb;239:614–621. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, SCOTT A., HOFFSTEN P. E., GUERRA F., WEINSTEIN J., SCHNEIDER A., SCHUTZ B., FINK J., FORD L., SMITH E. STUDIES ON THE MECHANISM OF ASCORBATE-INDUCED SWELLING AND LYSIS OF ISOLATED LIVER MITOCHANDRIA. J Biol Chem. 1964 Feb;239:604–613. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, SCOTT A., WEINSTEIN J., SCHNEIDER A. EFFECTS OF PHOSPHATE, ARSENATE, AND OTHER SUBSTANCES ON SWELLING AND LIPID PEROXIDE FORMATION WHEN MITOCHONDRIA ARE TREATED WITH OXIDIZED AND REDUCED GLUTATHIONE. J Biol Chem. 1964 Feb;239:622–630. [PubMed] [Google Scholar]
- Hatefi Y., Bearden A. J. Electron paramagnetic resonance studies on the reduction of the components of complex I and transhydrogenase-inhibited complex I by NADH and NADPH. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1032–1038. doi: 10.1016/0006-291x(76)90476-9. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Lipid oxidation in biological membranes. I. Lipid oxidation in submitochondrial particles and microsomes induced by chaotropic agents. Arch Biochem Biophys. 1970 May;138(1):73–86. doi: 10.1016/0003-9861(70)90286-9. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973 Nov 15;39(2):313–323. doi: 10.1111/j.1432-1033.1973.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKnight R. C., Hunter F. E., Jr Mitochondrial membrane ghosts produced by lipid peroxidation induced by ferrous ion. II. Composition and enzymatic activity. J Biol Chem. 1966 Jun 25;241(12):2757–2765. [PubMed] [Google Scholar]
- Mellors A., Tappel A. L. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J Biol Chem. 1966 Oct 10;241(19):4353–4356. [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Gellerfors P. Characterization and resolution of complex III from beef heart mitochondria. Methods Enzymol. 1978;53:80–91. doi: 10.1016/s0076-6879(78)53016-4. [DOI] [PubMed] [Google Scholar]
- Norling B., Glazek E., Nelson B. D., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Quantitative incorporation of small amounts of ubiquinone and its effects on the NADH and succinate oxidase activities. Eur J Biochem. 1974 Sep 16;47(3):475–482. doi: 10.1111/j.1432-1033.1974.tb03715.x. [DOI] [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. A method for determining the concentration of ubiquinone in mitochondrial preparations. Biochem J. 1960 Jul;76:61–64. doi: 10.1042/bj0760061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer P. M., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. VI. Structural changes in mitochondria associated with inactivation of electron transport activity. J Biol Chem. 1972 Nov 10;247(21):6763–6769. [PubMed] [Google Scholar]
- Ragan C. I. The interaction of reduced nicotinamide--adenine dinucleotide phosphate with reduced nicotinamide--adenine dinucleotide--ubiquinone reductase from bovine heart mitochondria. Biochem J. 1976 Jul 15;158(1):149–151. doi: 10.1042/bj1580149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydström J., Montelius J., Bäckström D., Ernster L. The mechanism of oxidation of reduced nicotinamide dinucleotide phosphate by submitochondrial particles from beef heart. Biochim Biophys Acta. 1978 Mar 13;501(3):370–380. doi: 10.1016/0005-2728(78)90105-6. [DOI] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Takeshige K., Minadami S. Reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation by beef heart submitochondrial particles. J Biochem. 1975 May;77(5):1067–1073. doi: 10.1093/oxfordjournals.jbchem.a130807. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979 Apr 15;180(1):129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Takayanagi R., Minakami S. Lipid peroxidation and the reduction of ADP-Fe3+ chelate by NADH-ubiquinone reductase preparation from bovine heart mitochondria. Biochem J. 1980 Dec 15;192(3):861–866. doi: 10.1042/bj1920861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON Jagow G., Bohrer C. Inhibition of electron transfer from ferrocytochrome b to ubiquinone, cytochrome c1 and duroquinone by antimycin. Biochim Biophys Acta. 1975 Jun 17;387(3):409–424. doi: 10.1016/0005-2728(75)90082-1. [DOI] [PubMed] [Google Scholar]


