Abstract
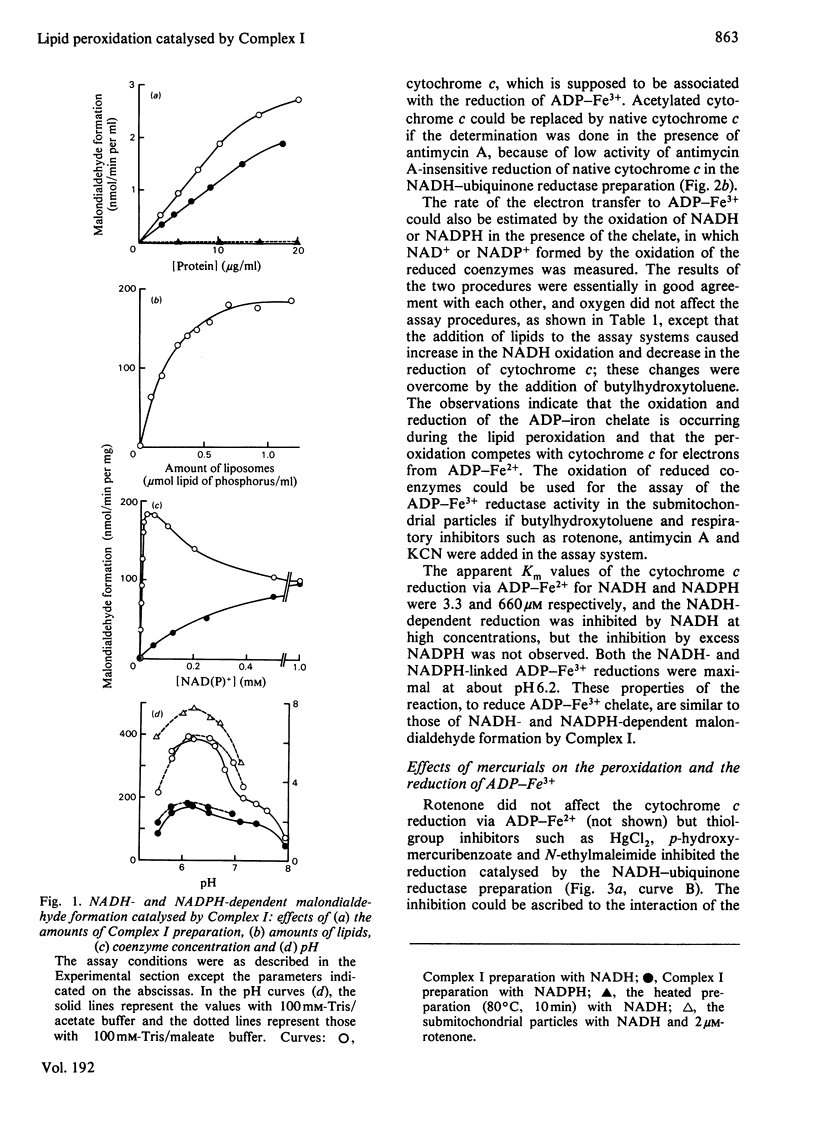
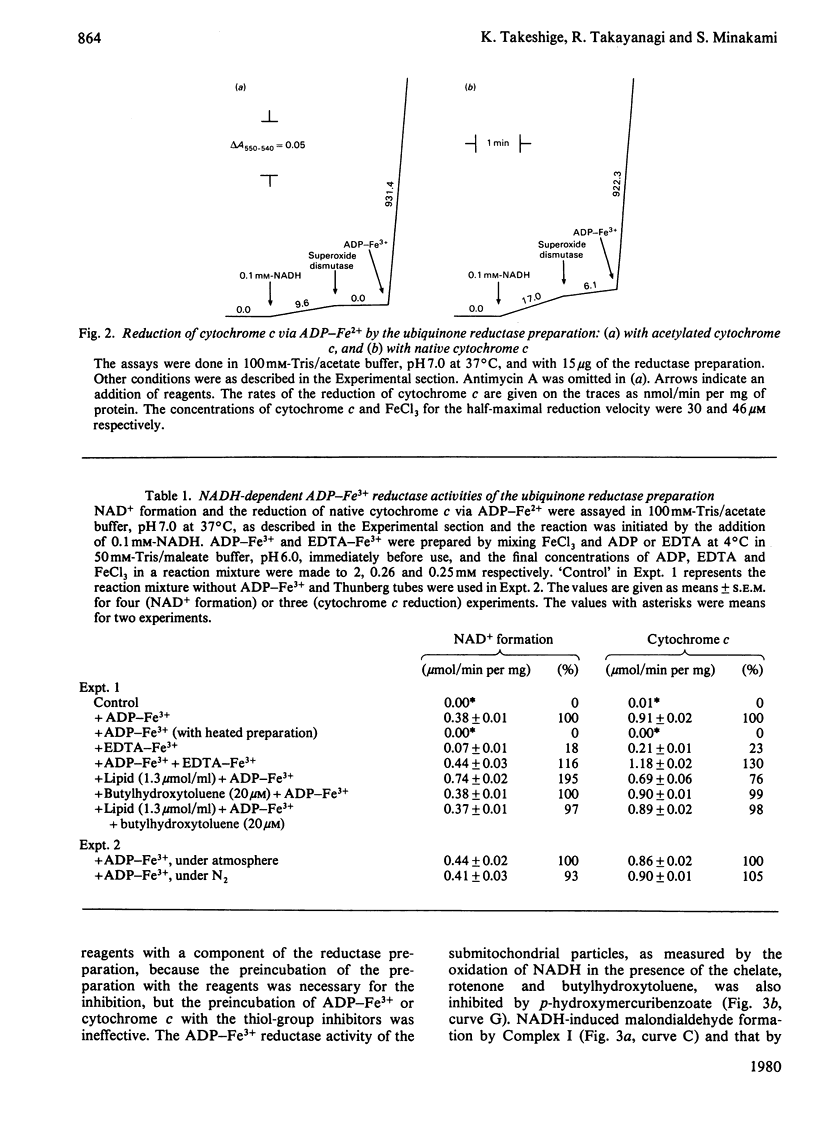
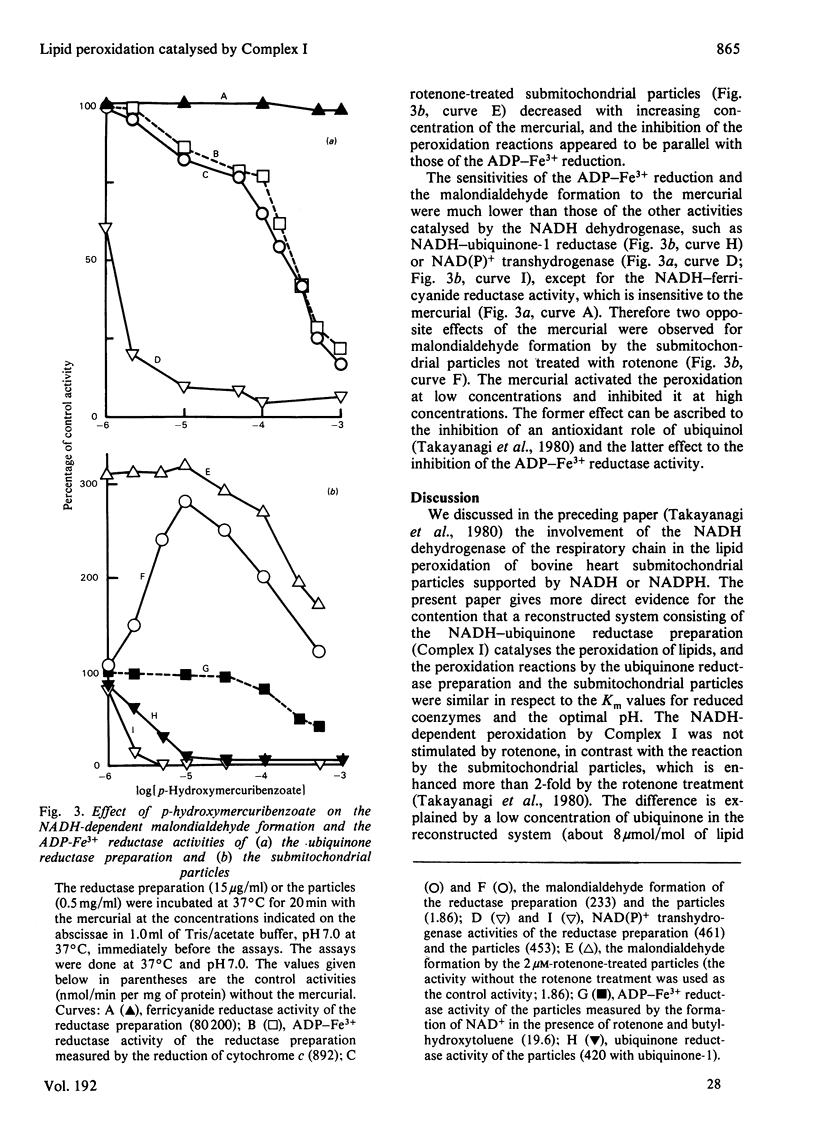
The NADH-ubiquinone reductase preparation (Complex I) of bovine hart mitochondria catalysed in the presence of reduced coenzymes and ADP-Fe3+ the lipid peroxidation of liposomes prepared from mitochondrial lipids. The apparent Km values for the coenzymes and the optimal pH of the reactions agreed well with those of the lipid peroxidation of the submitochondrial particles treated with rotenone. On assay of the reduction of ADP-Fe3+ chelate by the reduction of cytochrome c in the presence of superoxide dismutase and antimycin A or by the oxidation of reduced coenzymes, the reactions were not affected by rotenone but were inhibited by thiol-group inhibitors. The properties of the ADP-Fe3+ reductase activity were highly consistent with those of the lipid-peroxidation reaction. These observations suggest that electrons from reduced coenzymes are transferred to ADP-Fe3+ chelate from a component between a mercurial-sensitive site and the rotenone-sensitive one of the NADH dehydrogenase and that the reduction of ADP-Fe3+ chelate by the NADH dehydrogenase is an essential step in the lipid peroxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- MINAKAMI S., RINGLER R. L., SINGER T. P. Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem. 1962 Feb;237:569–576. [PubMed] [Google Scholar]
- MINAKAMI S., SCHINDLER F. J., ESTABROOK R. W. HYDROGEN TRANSFER BETWEEN REDUCED DIPHOSPHOPYRIDINE NUCLEOTIDE DEHYDROGENASE AND THE RESPIRATORY CHAIN. I. EFFECT OF SULFHYDRYL INHIBITORS AND PHOSPHOLIPASE. J Biol Chem. 1964 Jun;239:2042–2048. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. NADPH-dependen lipid peroxidation catalyzed by purified NADPH-cytochrome C reductase from rat liver microsomes. Biochem Biophys Res Commun. 1972 Aug 21;48(4):789–795. doi: 10.1016/0006-291x(72)90676-6. [DOI] [PubMed] [Google Scholar]
- Pederson T. C., Buege J. A., Aust S. D. Microsomal electron transport. The role of reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase in liver microsomal lipid peroxidation. J Biol Chem. 1973 Oct 25;248(20):7134–7141. [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- TYLER D. D., BUTOW R. A., GONZE J., ESTABROOK R. W. EVIDENCE FOR THE EXISTENCE AND FUNCTION OF AN OCCULT, HIGHLY REACTIVE SULPHYDRYL GROUP IN THE RESPIRATORY CHAIN DPNH DEHYDROGENASE. Biochem Biophys Res Commun. 1965 May 3;19:551–557. doi: 10.1016/0006-291x(65)90161-0. [DOI] [PubMed] [Google Scholar]
- Takayanagi R., Takeshige K., Minakami S. NADH- and NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem J. 1980 Dec 15;192(3):853–860. doi: 10.1042/bj1920853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Minadami S. Reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation by beef heart submitochondrial particles. J Biochem. 1975 May;77(5):1067–1073. doi: 10.1093/oxfordjournals.jbchem.a130807. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979 Apr 15;180(1):129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]


