Abstract
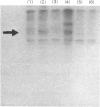
1. The ability of a range of phenothiazines to inhibit activation of brain phosphodiesterase by purified calmodulin was studied. Trifluoperazine, prochlorperazine and 8-hydroxyprochlorperazine produced equipotent dose-dependent inhibition with half-maximum inhibition at 12μm. When tested at 10 or 50μm, 7-hydroxyprochlorperazine was a similarly potent inhibitor. However, trifluoperazine-5-oxide and N-methyl-2-(trifluoromethyl)phenothiazine were ineffective at concentrations up to 50μm, and produced only a modest inhibition at 100μm. 2. The same phenothiazines were tested for their ability to inhibit activation of brain phosphodiesterase by boiled extracts of rat islets of Langerhans. At a concentration of 20μm, 70–80% inhibition was observed with trifluoperazine, prochlorperazine, 7-hydroxyprochlorperazine or 8-hydroxyprochlorperazine, whereas trifluoperazine-5-oxide and N-methyl-2-(trifluoromethyl)phenothiazine were less effective. 3. The effect of these phenothiazines on insulin release from pancreatic islets was studied in batch-type incubations. Insulin release stimulated by glucose (20mm) was markedly inhibited by 10μm-trifluoperazine or -prochlorperazine and further inhibited at a concentration of 20μm. 8-Hydroxyprochlorperazine (20μm) was also a potent inhibitor but 7-hydroxyprochlorperazine (20μm) elicited only a modest inhibition of glucose-stimulated insulin release; no inhibition was observed with trifluoperazine-5-oxide or N-methyl-2-(trifluoromethyl)phenothiazine. 4. Trifluoperazine (20μm) markedly inhibited insulin release stimulated by leucine or 4-methyl-2-oxopentanoate in the absence of glucose, and both trifluoperazine and prochlorperazine (20μm) decreased insulin release stimulated by glibenclamide in the presence of 3.3mm-glucose. 5. None of the phenothiazines affected basal insulin release in the presence of 2mm-glucose. 6. Trifluoperazine (20μm) did not inhibit islet glucose utilization nor the incorporation of [3H]leucine into (pro)insulin or total islet protein. 7. Islet extracts catalysed the incorporation of 32P from [γ-32P]ATP into endogenous protein substrates. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis resolved several phosphorylated bands, but incorporation was slight. However, calmodulin in the presence of Ca2+ greatly enhanced incorporation: the predominant phosphorylated band had an estimated mol.wt. of 55000. This enhanced incorporation was abolished by trifluoperazine, but not by cyclic AMP-dependent protein kinase inhibitor protein. 8. These results suggest that islet phosphodiesterase-stimulating activity is similar to, although not necessarily identical with, calmodulin from skeletal muscle; that islet calmodulin may play an important role in Ca2+-dependent stimulus–secretion coupling in the β-cell; and that calmodulin may exert part at least of its effect on secretion via phosphorylation of endogenous islet proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Ashcroft S. J., Bunce J., Lowry M., Hansen S. E., Hedeskov C. J. The effect of sugars on (pro)insulin biosynthesis. Biochem J. 1978 Aug 15;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Crossley J. R. The effects of glucose, N-acetylglucosamine, glyceraldehyde and other sugars on insulin release in vivo. Diabetologia. 1975 Aug;11(4):279–284. doi: 10.1007/BF00422392. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Coll-Garcia E., Gill J. R. Insulin release by isolated pancreatic islets of the mouse incubated in vitro. Diabetologia. 1969 Apr;5(2):61–66. doi: 10.1007/BF01211999. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Totsuka T., Asano M. Selective inhibitors of Ca2+-binding modulator of phosphodiesterase produce vascular relaxation and inhibit actin-myosin interaction. Mol Pharmacol. 1979 Jan;15(1):49–59. [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966 Jun 18;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H. Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978 Jan 10;17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G., Marichal M., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XV. Participation of cations in the recognition of glucose by the beta-cell. Endocrinology. 1973 Nov;93(5):1012–1018. doi: 10.1210/endo-93-5-1012. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Erlichman J., Fleischer N. The role of calmodulin in the regulation of protein phosphorylation and insulin release in hamster insulinoma cells. J Biol Chem. 1980 May 10;255(9):4120–4124. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W., Theoharides T. C., Alper S. L., Douglas W. W., Greengard P. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature. 1978 Sep 28;275(5678):329–331. doi: 10.1038/275329a0. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Ashcroft S. J., Sugden P. H. Protein kinase activities in rat pancreatic islets of Langerhans. Biochem J. 1979 Apr 15;180(1):219–229. doi: 10.1042/bj1800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Christie M. R., Ashcroft S. J. Presence and possible role of calcium-dependent regulator (calmodulin) in rat islets of Langerhans. FEBS Lett. 1979 Sep 1;105(1):95–100. doi: 10.1016/0014-5793(79)80894-7. [DOI] [PubMed] [Google Scholar]
- Valverde I., Vandermeers A., Anjaneyulu R., Malaisse W. J. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979 Oct 12;206(4415):225–227. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Teo T. S., Ho H. C., Stevens F. C. Bovine heart protein activator of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:179–194. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]