Abstract
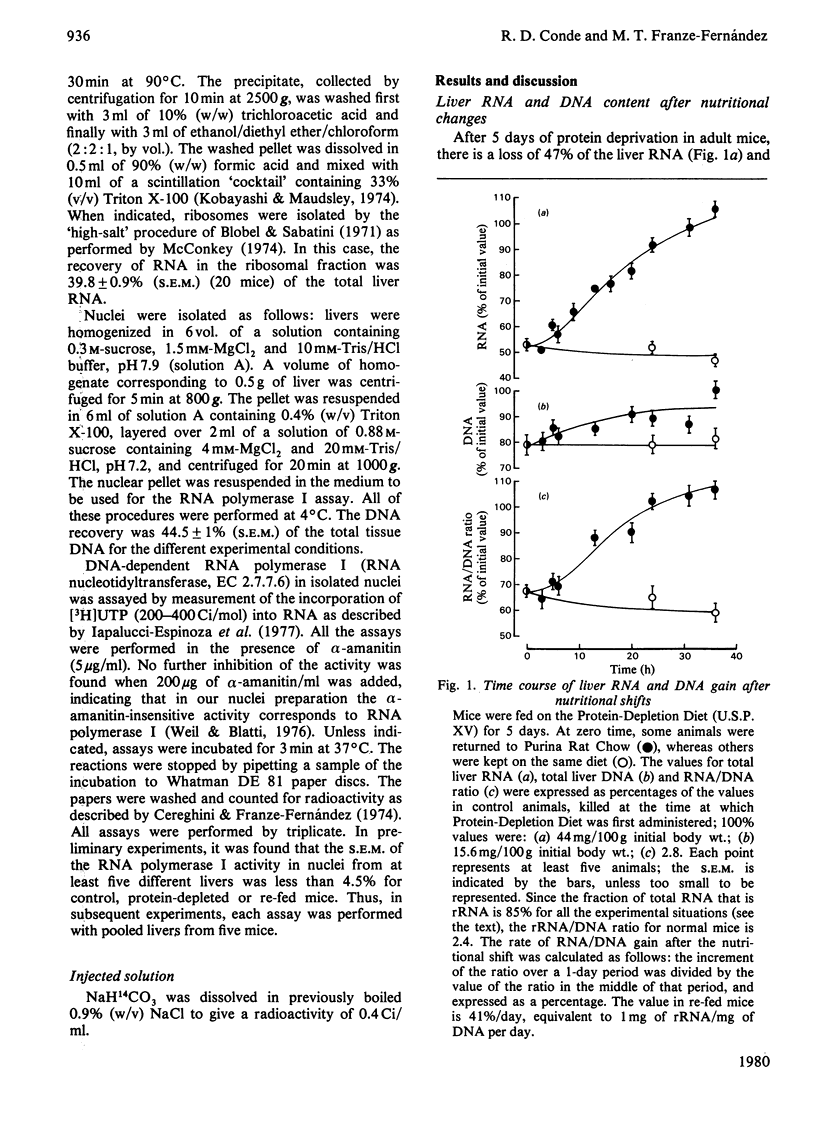
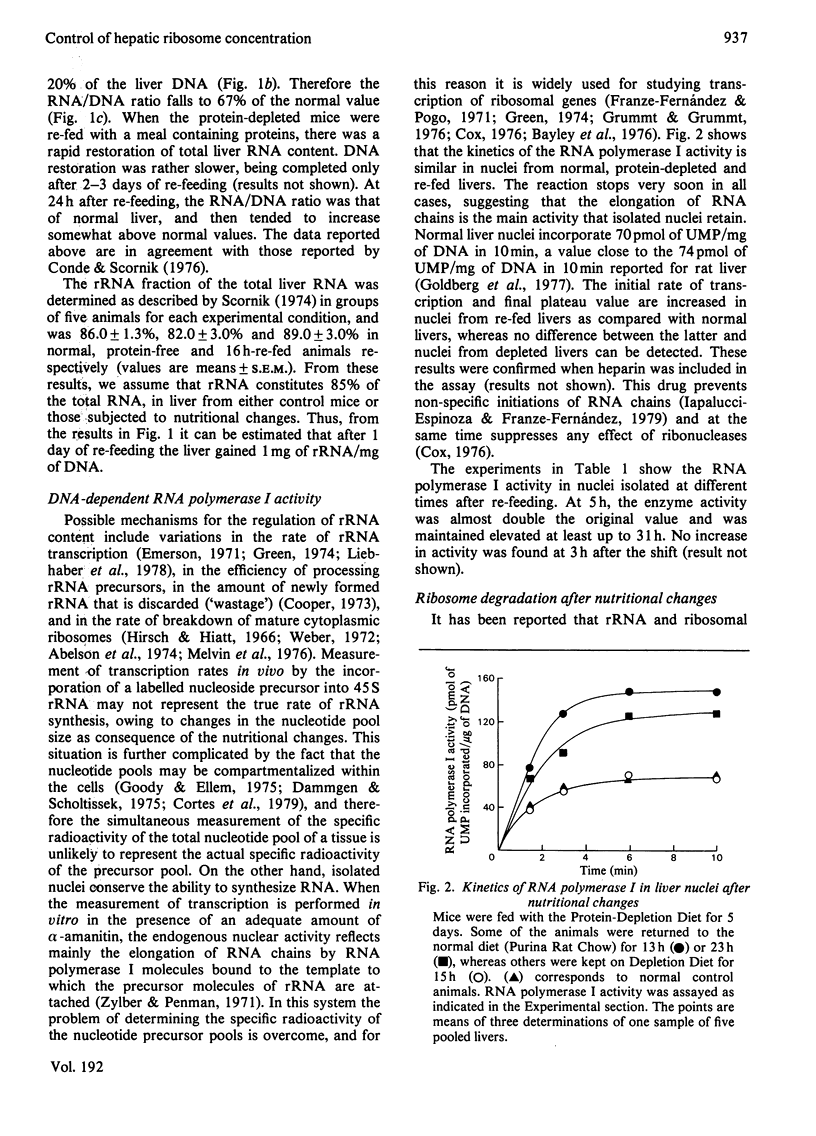
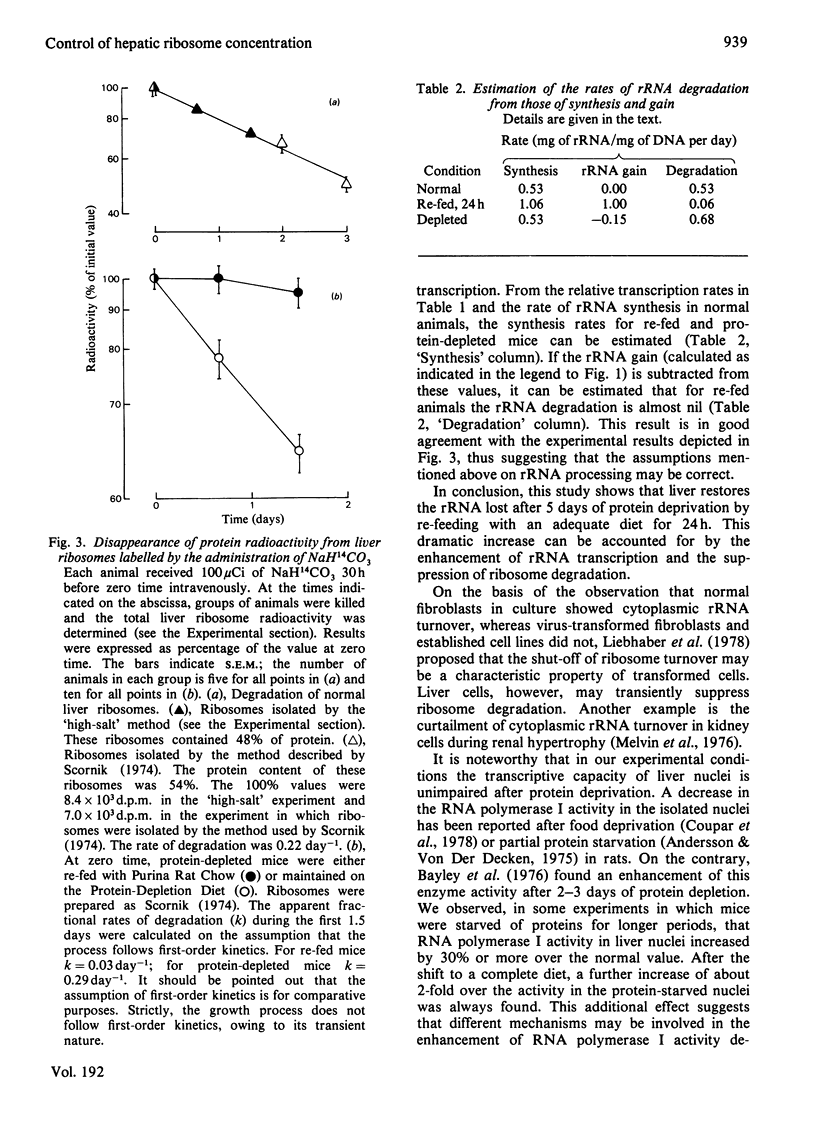
In the livers of 5-days-protein-depleted mice there is a decrease of 47% of the ribosome mass. When these animals are fed with an adequate diet, ribosome content is restored to the normal value after 1 day of re-feeding. The mechanisms underlying this phenomenon were studied. It was found that: (1) the activity of RNA polymerase I in the nuclei of livers from re-fed animals showed an enhancement of about 2-fold compared with the activity in normal and protein-depleted liver nuclei; (2) ribosome degradation, measured by the disappearance of radioactivity from ribosomal proteins previously labelled by the administration of NaH14CO3 to the mice, stopped during the first day after re-feeding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G. M., von der Decken A. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in rat liver after protein restriction. Biochem J. 1975 Apr;148(1):49–56. doi: 10.1042/bj1480049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. P., Vrooman M. J., Sawai Y., Tsukada K., Short J., Lieberman I. Amino acids and control of nucleolar size, the activity of RNA polymerase I, and DNA synthesis in liver. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3201–3205. doi: 10.1073/pnas.73.9.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Distribution of radioactivity between the acid-soluble pool and the pools of RNA in the nuclear, nonsedimethable and ribosome fractions of rat liver after a single injection of lebaled orotic acid. Biochim Biophys Acta. 1968 Aug 23;166(1):48–57. doi: 10.1016/0005-2787(68)90489-9. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Franze-Fernández M. T. Ehrlich ascites cells DNA-dependent RNA polymerases: effect of amino acids and protein synthesis inhibition. FEBS Lett. 1974 Apr 15;41(1):161–165. doi: 10.1016/0014-5793(74)80978-6. [DOI] [PubMed] [Google Scholar]
- Conde R. D., Scornik O. A. Role of protein degradation in the growth of livers after a nutritional shift. Biochem J. 1976 Aug 15;158(2):385–390. doi: 10.1042/bj1580385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L. Degradation of 28S RNA late in ribosomal RNA maturation in nongrowing lymphocytes and its reversal after growth stimulation. J Cell Biol. 1973 Oct;59(1):250–254. doi: 10.1083/jcb.59.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes P., Levin N. W., Dumler F., Venkatachalam K. K., Verghese C. P., Bernstein J. Incorporation of exogenous precursors into uridine nucleotides and ribonucleic acid. Nucleotide compartmentation in the renal cortex in vivo. Biochem J. 1979 Sep 15;182(3):677–686. doi: 10.1042/bj1820677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar B. E., Davies J. A., Chesterton C. J. Quantification of hepatic transcribing RNA polymerase molecules, polyribonucleotide elongation rates and messenger RNA complexity in fed and fasted rats. Eur J Biochem. 1978 Mar 15;84(2):611–623. doi: 10.1111/j.1432-1033.1978.tb12204.x. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Craig N. C. On the regulation of the synthesis of ribosomal proteins in L-cells. J Mol Biol. 1971 Jan 14;55(1):129–134. doi: 10.1016/0022-2836(71)90288-9. [DOI] [PubMed] [Google Scholar]
- Dämmgen J. W., Scholtissek C. Cellular RNA and influenza-virion RNA are synthesized from different pyrimidine-nucleoside-triphosphate pools in chick-embryo cells. Eur J Biochem. 1975 Nov 1;59(1):51–54. doi: 10.1111/j.1432-1033.1975.tb02423.x. [DOI] [PubMed] [Google Scholar]
- Emerson C. P. Regulation of the synthesis and the stability of ribosomal RNA during contact inhibition of growth. Nat New Biol. 1971 Jul 28;232(30):101–106. doi: 10.1038/newbio232101a0. [DOI] [PubMed] [Google Scholar]
- Enwonwu C. O., Munro H. N. Rate of RNA turnover in rat liver in relation to intake of protein. Arch Biochem Biophys. 1970 Jun;138(2):532–539. doi: 10.1016/0003-9861(70)90378-4. [DOI] [PubMed] [Google Scholar]
- Franze-Fernández M. T., Pogo A. O. Regulation of the nucleolar DNA-dependent RNA polymerase by amino acids in Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3040–3044. doi: 10.1073/pnas.68.12.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. I., Perriard J. C., Rutter W. J. Purification of rat liver and mouse ascites DNA-dependent RNA polymerase I. Biochemistry. 1977 Apr 19;16(8):1655–1665. doi: 10.1021/bi00627a021. [DOI] [PubMed] [Google Scholar]
- Goody H. E., Ellem K. A. Nutritional effects on precursor uptake and compartmentalization of intracellular pools in relation to RNA synthesis. Biochim Biophys Acta. 1975 Feb 24;383(1):30–39. doi: 10.1016/0005-2787(75)90243-9. [DOI] [PubMed] [Google Scholar]
- Grummt I., Grummt F. Control of nucleolar RNA synthesis by the intracellular pool sizes of ATP and GTP. Cell. 1976 Mar;7(3):447–453. doi: 10.1016/0092-8674(76)90175-6. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A. Studies on the turnover and messenger activity of rat-liver ribonucleic acids. Biochim Biophys Acta. 1966 Jun 22;119(3):547–556. doi: 10.1016/0005-2787(66)90131-6. [DOI] [PubMed] [Google Scholar]
- Hirsch C. A., Hiatt H. H. Turnover of liver ribosomes in fed and in fasted rats. J Biol Chem. 1966 Dec 25;241(24):5936–5940. [PubMed] [Google Scholar]
- Iapalucci-Espinoza S., Cereghini S., Franze-Fernández M. T. Regulation of ribosomal RNA synthesis in mammalian cells: effect of toyocamycin. Biochemistry. 1977 Jun 28;16(13):2885–2889. doi: 10.1021/bi00632a013. [DOI] [PubMed] [Google Scholar]
- Iapalucci-Espinoza S., Franze-Fernández M. T. Effect of protein synthesis inhibitors and low concentrations of actinomycin D on ribosomal RNA synthesis. FEBS Lett. 1979 Nov 15;107(2):281–284. doi: 10.1016/0014-5793(79)80390-7. [DOI] [PubMed] [Google Scholar]
- Kawada T., Fujisawa T., Imai K., Ogata K. Effects of protein deficiency on the biosynthesis and degradation of ribosomal RNA in rat liver. J Biochem. 1977 Jan;81(1):143–152. doi: 10.1093/oxfordjournals.jbchem.a131429. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Liebhaber S. A., Wolf S., Schlessinger D. Differences in rRNA metabolism of primary and SV40-transformed human fibroblasts. Cell. 1978 Jan;13(1):121–127. doi: 10.1016/0092-8674(78)90143-5. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Augustine S. L., Burk T. L., Swick R. W. A comparison of methods for the measurement of protein turnover in vivo. Biochem J. 1979 Nov 15;184(2):473–476. doi: 10.1042/bj1840473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J. C., McConkey E. H. Nucleolar protein metabolism in actinomycin D treated HeLa cells. J Mol Biol. 1971 Oct 14;61(1):251–255. doi: 10.1016/0022-2836(71)90221-x. [DOI] [PubMed] [Google Scholar]
- McConkey E. H. Composition of mammalian ribosomal subunits: a re-evaluation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1379–1383. doi: 10.1073/pnas.71.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin W. T., Kumar A., Malt R. A. Conservation of ribosomal RNA during compensatory renal hypertrophy. A major mechanism in RNA accretion. J Cell Biol. 1976 Jun;69(3):548–556. doi: 10.1083/jcb.69.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Tsurugi K., Morita T., Ogata K. Mode of degradation of ribosomes in regenerating rat liver in vivo. Eur J Biochem. 1974 Jun 1;45(1):119–126. doi: 10.1111/j.1432-1033.1974.tb03536.x. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Girard M., Latham H., Darnell J. E. Ribosome formation in HeLa cells in the absence of protein synthesis. J Mol Biol. 1966 Aug;19(2):373–382. doi: 10.1016/s0022-2836(66)80011-6. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Udem S. A. Temperature sensitive mutations affecting ribosome synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):243–257. doi: 10.1016/0022-2836(72)90280-x. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Ribosomal RNA turnover in contact inhibited cells. Nat New Biol. 1972 Jan 12;235(54):58–61. doi: 10.1038/newbio235058a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Blatti S. P. HeLa cell deoxyribonucleic acid dependent RNA polymerases: function and properties of the class III enzymes. Biochemistry. 1976 Apr 6;15(7):1500–1509. doi: 10.1021/bi00652a022. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Hoagland M. B. Physiology of rat-liver polysomes. The stability of messenger ribonucleic acid and ribosomes. Biochem J. 1967 May;103(2):556–566. doi: 10.1042/bj1030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Kumar A., Warner J. R. Ribosome formation is blocked by camptothecin, a reversible inhibitor of RNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3009–3014. doi: 10.1073/pnas.68.12.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]


