Abstract
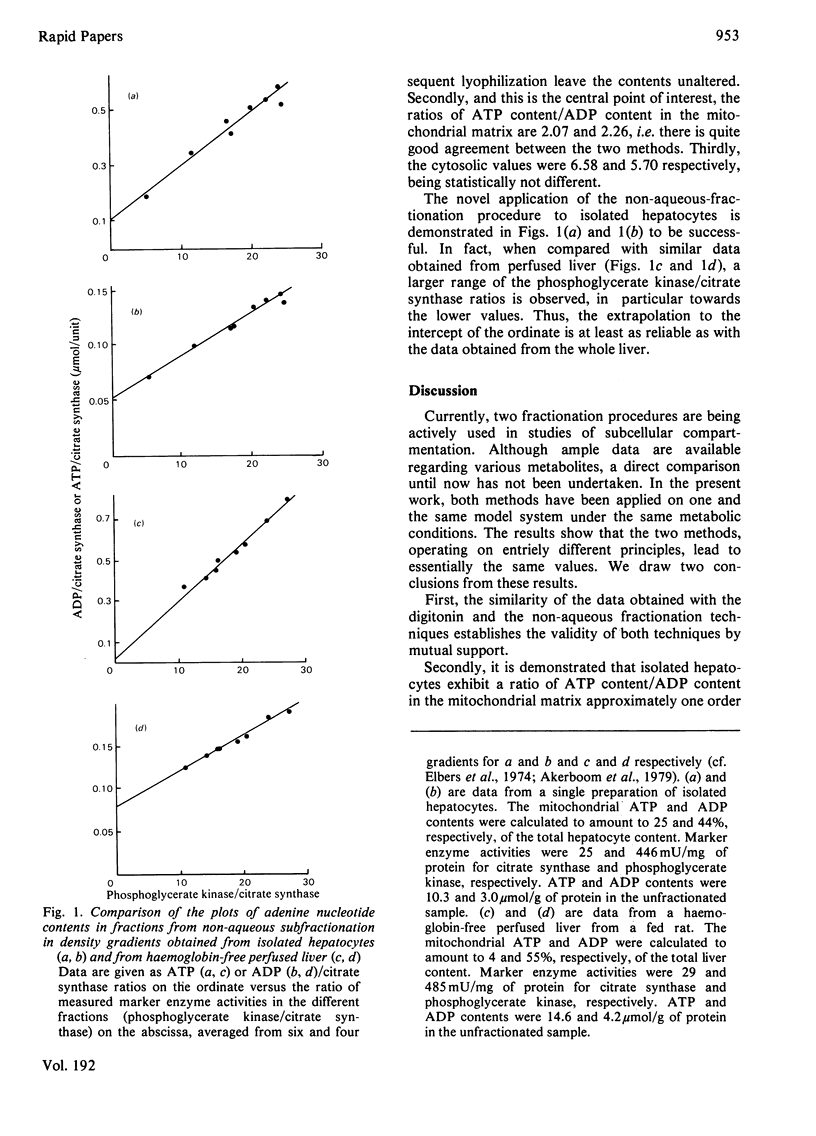
The ratio of ATP content/ADP content in the mitochondrial matrix was found to be 2.07 +/- 0.21 and 2.26 +/- 0.22 as determined with six different preparations of isolated hepatocytes subfractionated with the digitonin and non-aqueous-fractionation procedures, respectively. In contrast, the mitochondrial matrix ATP/ADP determined with isolated haemoglobin-free perfused liver by using the non-aqueous-fractionation procedure was about 0.2, whereas the cytosolic values obtained with isolated cells and with the intact organ were similar. It is concluded that the relatively higher ATP/ADP ratio in the mitochondrial matrix of isolated hepatocytes represents a biochemical difference due to properties of the model rather than a methodological artifact.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers R., Heldt H. W., Schmucker P., Soboll S., Wiese H. Measurement of the ATP/ADP ratio in mitochondria and in the extramitochondrial compartment by fractionation of freeze-stopped liver tissue in non-aqueous media. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):378–393. doi: 10.1515/bchm2.1974.355.1.378. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M. Differences between the reactivity of endogenous and exogenous adenine nucleotides in mitochondria as studied at low temperature. Eur J Biochem. 1968 Mar;4(1):1–8. doi: 10.1111/j.1432-1033.1968.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Hensgens H. E., Meijer A. J. Inhibition of urea-cycle activity by high concentrations of alanine. Biochem J. 1980 Jan 15;186(1):1–4. doi: 10.1042/bj1860001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Akerboom T. P., Tager J. M. Mitochondrial and cytosolic NADPH systems and isocitrate dehydrogenase indicator metabolites during ureogensis from ammonia in isolated rat hepatocytes. Eur J Biochem. 1977 Jan;72(2):301–307. doi: 10.1111/j.1432-1033.1977.tb11253.x. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboll S., Scholz R., Heldt H. W. Subcellular metabolite concentrations. Dependence of mitochondrial and cytosolic ATP systems on the metabolic state of perfused rat liver. Eur J Biochem. 1978 Jun 15;87(2):377–390. doi: 10.1111/j.1432-1033.1978.tb12387.x. [DOI] [PubMed] [Google Scholar]
- Zuurendonk P. F., Tischler M. E., Akerboom T. P., Van Der Meer R., Williamson J. R., Tager J. M. Rapid separation of particulate and soluble fractions from isolated cell preparations (digitonin and cell cavitation procedures). Methods Enzymol. 1979;56:207–223. doi: 10.1016/0076-6879(79)56023-6. [DOI] [PubMed] [Google Scholar]


