Abstract
In this transdisciplinary study, we investigated, using genomic tools and physico-chemical parameters, the effect of Moringa oleifera seed (MOS) on the removal of microorganisms and pharmaceutic residues (antibiotics), and also the development of antibiotic-resistant genes (ARGs) in water samples from a domestic wastewater treatment plant (WWTP) prototype. Water samples were analyzed with and without the addition of powder of MOS. The results showed that MOS addition reduced the total bacterial load from 1.73 × 1010 ± 3.21 × 109 to 6.67 × 106 ± 5.77 × 106 CFU/L, while fecal coliforms and Escherichia coli were removed with efficiencies of 99% and 57%, respectively. Furthermore, MOS treatment resulted in a reduction in fecal coliforms and E. coli resistant to ampicillin by about 100% and 96%, respectively. The results indicated that ciprofloxacin removal efficiencies at 29 °C were over 93% (fecal coliforms) and 68% (E. coli) with doxycycline. Adding MOS significantly reduced the copy number of the 16S rRNA gene and the genes conferring resistance to β-lactam (blaCTX-M, blaSHV, and blaTEM). However, MOS does not reveal real effectiveness on removal of pollutants (phosphorus and nitrates) contrary to what was expected. Additional studies are needed for confirmation from our observations. The findings of this study, whatever the functioning conditions (not optimal) of the prototype followed over 4 years, confirmed that MOS is potentially an effective natural and environmentally friendly coagulant that could be applied to wastewater treatment in low-income countries to remove or minimize multiple pollutants and control ARG spread. To promote sustainable development, this small-scale study provides guidance for designing infrastructure in resource-limited locations to take advantage of MOS effects in wastewater treatments.
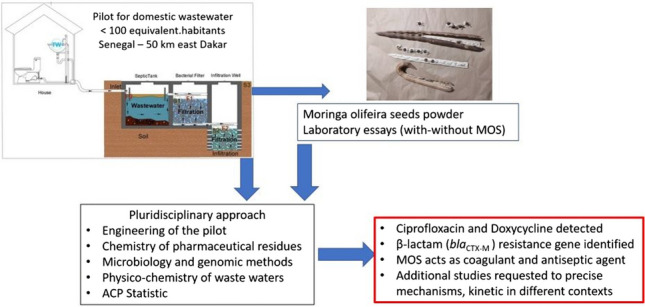
Graphical Abstract
An individual WWTP prototype was installed in Senegal in 2018 and evaluated in 2020 and 2022. MOS powder addition is supposed to reduce bacterial loads and pharmaceutical residues in wastewater from the WWTP. Evaluation of treatment efficiency was carried out based on using chemistry, microbiology, genomics, physic-chemistry, and statistics analysis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-024-35362-8.
Keywords: Moringa oleifera seeds, Wastewater, Pollutants, Pharmaceutic residues, Antibiotic resistant, Resistant genes, Genomic, Prototype, Natural coagulant
Introduction
Domestic wastewater treatment poses a major problem for developing countries due to the costs and energy involved in conventional wastewater treatment (De Anda et al. 2018). In wastewaters, different chemical, biological, and molecular compounds are derived from various uses of water by the community. However, treating wastewater using cost-effective and environmentally friendly methods has become a priority for many sub-Saharan African nations. In Senegal, about 21% of the population has access to safe sanitation services (UN-Water 2021). For the remaining population, a good sanitation service is essential to maintain solid and liquid waste management. Therefore, exploring an appropriate solution is critical, especially in terms of efficiency and maintenance of solution selected (Sané et al. 2022). Climate change associated with deficient politics and practices in terms of continental waters leads to droughts and shortages of freshwater and used waters. Population exposed to used waters without or poor treatment of these waters induces high risk on human health, environment and foods quality. Therefore, reuse of treated wastewater remains a viable alternative and sustainable solution to meet agriculture’s water needs. While there are several methods for disinfecting wastewater (UV, active carbons, and ozone), these are ineffective for removing antibiotic-resistant bacteria (ARBs) and antibiotic-resistant genes (ARGs) (Rizzo et al. 2013).
The spread of ARGs into the receiving natural water systems through wastewater discharge is therefore a key issue and could threaten human health. Also, irrigating agricultural products with contaminated water can spread ARGs back to humans and promote the development of new resistant infections (Manaia 2017). Consequently, ARGs are considered emerging environmental pollution and have significant human health implications (Pruden et al. 2006). This explains the need for more collaborative research considering the concept of one health which connects humans, animals, and the environment (Kahn 2017). This is why this study used genomic approaches and tools to evaluate ARG. The proliferation of ARGs in the environment has been shown to be influenced by several abiotic factors, including antibiotics and heavy metals (Gupta et al. 2022; Larsson and Flach 2022). Further, antibiotics, which exert direct selection pressure, can affect the fate of ARGs in the environment (Marti et al. 2013). In addition, socioeconomic status and infrastructure factors have been reported to influence antimicrobial resistance (AMR) proliferation (Collignon et al. 2018). With the growing use of antibiotics in health care and veterinary medicine, new antibiotic-resistant bacteria and ARGs are emerging (Holmes et al. 2016). In Senegal, 25% of medical prescriptions include antibiotics (Camara 2015). There is also massive use of antibiotics through self-medication according to several surveys carried out in the country, indicating inappropriate and uncontrolled use of antibiotics (Sylla 2014). Added to this is the issue of fake medicines, estimated at 25% of medicines used in developing countries, particularly antibiotics, which at the global level 5% are listed as fake or of inferior quality by the WHO (Gueye 2007). Therefore, antibiotics should be used prudently, and on the other hand, a WWTP should be designed to remove antibiotics effectively before releasing them into the receiving ecosystem. In our previous study (Sané et al. 2022), a protype has been evaluated for its efficiency (with or without Moringa seeds). Two years after, we evaluate it again, despite the lack of maintenance and use in non-optimal conditions. Thus, it interrogates about the interest and potential of used water treatment plants of small capacity or individual (less than 200 eq. habitants) in case of lack of maintenance and regular cleaning. This study by interdisciplinary approach provide ligths and data on the efficiency of a prototype and MOS powder to avoid or decrease the anti microbial resistance (AMR) of used waters issued from human activities, especially in low incomes countries.
The issues related to AMR are poorly understood in Africa (Eholié et al. 2014). It has been reported by the World Health Organization that sub-Saharan African countries have a lack of data on AMR. Although a relatively limited number of clinical studies have demonstrated the prevalence of AMR in Senegal (Breurec et al. 2016; Chereau et al. 2015), environmental antibiotic resistance, particularly in wastewater, remains understudied.
The treatment of wastewater in Africa can be largely based on individual sanitation using natural filters such as coconut fiber, zeolite, vegetable charcoal, pumice stone, anthracite, quartz sand, and gravel as well as natural coagulants such as Moringa oleifera seeds (Adeniran et al. 2017). Moringa oleifera seeds (MOS) contain cationic polymers, making it an effective natural coagulant for removing turbidity from wastewater (Nouhi et al. 2019). A recent study demonstrated that MOS are highly effective at removing turbidity and chemical oxygen demand (COD) from wastewater (Desta and Bote 2021). However, it remains to be studied whether MOS can effectively remove ARGs from treated wastewater. Given this, in this study, Moringa oleifera seeds were used as a natural coagulant in the WWTP prototype. The physical and chemical parameters, as well as the ARGs, of untreated and treated effluents were investigated. Additionally, the correlation between ARGs and wastewaters influencing abiotic factors was examined. Finally, this study aims to answer about the potential of a small WWTP prototype even in non-optimal operating conditions in terms of used water quality and its potential impact on groundwater quality, in such cases.
Materials and methods
Sampling procedure
According to the previous description, the wastewater treatment plant prototype, located in Keur Moussa in Thies (Senegal), consists of a septic tank, a gravel filter, and an infiltration well (Sané et al. 2022). It was inaugurated in 2017 and pre-evaluated in 2018 by measuring physico-chemical parameters such as temperature, pH, biological oxygen demand (BOD), COD, and suspended solids. After preliminary studies in 2018 based essentially on physico-chemical analysis, a first campaign for evaluation of bacteria and antibiotic-resistant bacteria loads was conducted in 2020. A second campaign to confirm previous results on physico-chemistry (2018) and microbiology (2020) has been achieved in 2022. The last campaign aims to confirm the capacity of the pilot, whatever its state and its functionalities.
On checking the condition of the prototype, it became apparent that it had not been regularly maintained, nor had it collected any flows for which it is optimized. This led us to classify it as non-optimal. However, to our knowledge, there is no study of wastewater quality in the case of monitoring a low-capacity WWTP in West Africa, especially about antibiotic resistance. This raises the question of the value of such equipment in countries with few facilities (maintenance and control) from the point of view of its effectiveness in reducing the load of contaminants and water discharged at the outlet.
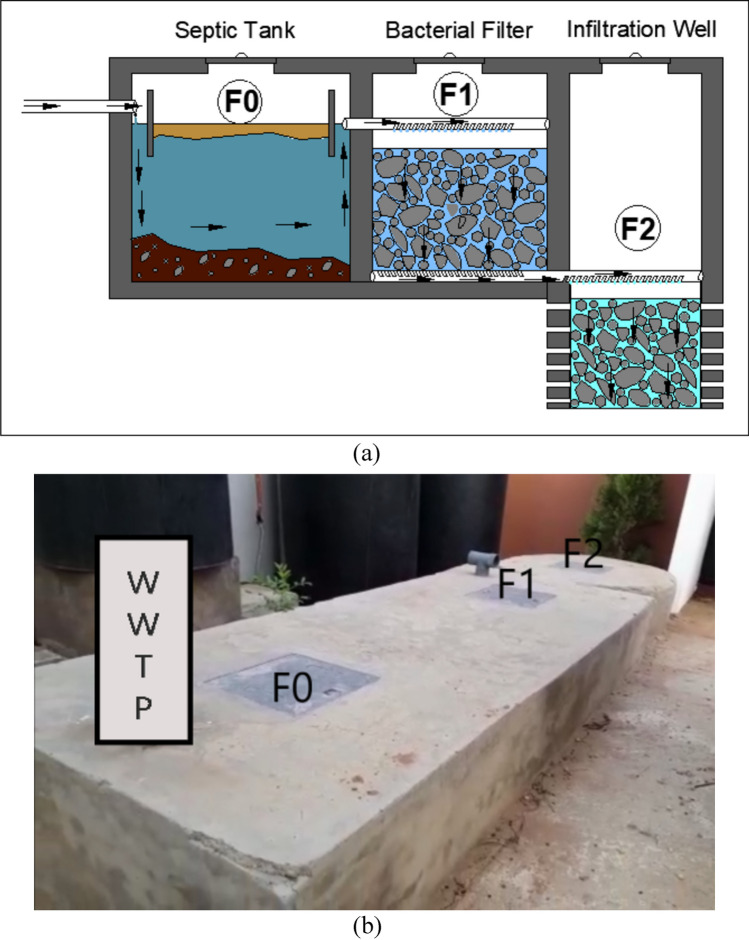
Samples were taken at three points (F0, F1, and F2) (Fig. 1) before and after the addition of MOS in F0. F0 represents a septic tank in which the wastewaters coming from the house are collected and stored. In F0, a settling phase coupled with transformation of organic matters into mineral under the effect of bacteria is noted. The WWTPP operates in overflow, which means that as soon as the useful height (1.5 m) in F0 is reached, the settled water flows into F1 which is a vertical filter made up of basalts with dimensions varying between 3 and 40 mm in diameter. After filtration, the treated waters flow toward F2 which is an infiltration well. Since MOS powder contains an active agent which demonstrated effectiveness in the coagulation flocculation process, it was added onsite in F0 (concentration = 300 mg/L) for optimal settling of colloidal particles for which sedimentation speed is very low. However, during the 2020 campaign, the contact between the MOS and wastewaters from the WWTP prototype was carried out on a laboratory scale on small sampled volumes of around 250 mL. It is therefore important to mention the difference in process during the second campaign, with the addition of MOS powder onsite and on a large scale for a storage volume of 3000 L. The mixture wastewater + MOS was carried out as described by LY (2016). Thus, 900 g of MOS powder was added in F0 followed by manual homogenization in two times: a quick one for 90 s to spread the MOS and destabilize the colloids (Bouazza 2011), then a slow one for 5 min to allow the discharged particles to form micro-flocs. After these homogenization phases, a settling period of 2 h was observed and a new sampling was carried out for physico-chemical, biological, and molecular analyses.
Fig. 1.
a Longitudinal section of wastewater treatment plant prototype. b Wastewater treatment plant prototype implemented onsite of Keur Moussa (Senegal)
Physico-chemical analysis
Physico-chemical parameters of wastewaters were measured onsite and in the laboratory. Temperature, pH, conductivity, and dissolved oxygen were measured onsite with a HACH HQ2200 multiparameter and PHC10105, CDC40105, and LDO10105 electrodes. The BOD was measured by the BOD-System BD 600 (LOVIBOND) installed in a chamber at 20 °C. The COD, suspended solids, total nitrogen, and phosphorus were measured by a HACH DR6000 spectrophotometer equipped with its tub test system. Standard tests have been carried out and the parameters measured comply with the Senegalese normalization NS 05–061.
Microbiological analysis
After wastewater sampling, microbiological analysis was conducted within 48 h. The wastewater samples were analyzed for Escherichia coli, fecal coliforms, Enterococcus sp., total flora, and Vibrio cholera as previously described (Rodier et al. 2009). Analyses were performed in triplicate for dilutions up to 10−6 for E. coli, fecal coliforms, fecal streptococci, and Vibrio and up to 10−9 for total flora. Tryptone Bile X Glucuronide (BIOKAR) was used to culture E. coli at 44 °C for 24 h. Fecal coliform was cultured on Violet Red Bile Lactose (BIOKAR) at 44 °C for 24 h. Enterococcus sp. was cultured on Bile Esculin Sodium Azide Agar (BIOKAR) at 44 °C for 24 h. Vibrio cholerae was isolated on Thiosulfate Citrate Bile Saccharose (BIOKAR) at 37 °C for 24 h. Total flora was cultured on Reasoner’s 2A agar (BIOKAR) at room temperature for 5 days. The nutrient agar plate supplemented with ampicillin was used to test the sensitivity of bacteria according to M100 sheet of the CLSI with a minimal inhibitory concentration (MIC) that falls in the range in which specific microbial resistance mechanisms are likely (Clinical and Laboratory Standards Institute 2017). MIC of ampicillin used were 32 mg/L for E. coli and fecal coliform sensitivity tests and 16 mg/L for Enterococcus sp., Vibrio cholerae, and total flora sensitivity tests.
Passive sampler extraction
The AttractSPE® hydrophilic-lipophilic balance (HLB) disks were used to collect pharmaceutical residual molecules (doxycycline, ciprofloxacin, enoxacin, enrofloxacin, norfloxacin, ofloxacin, amoxicillin, ampicillin) in wastewater. After activation of the AttractSPE® Disks and mounting of the SST housing, the passive samplers were placed in the wastewater treatment plant prototype at three points (F0, F1, F2). These were in the septic tanks, on the gravel filter, and in the infiltration well. Two immersion campaigns were made and three passive samplers were placed for each point. During the immersion experiment, passive samplers were exposed continuously for 7 days before recovery and extraction which follow the recommended procedures provided by AFFINISEP (2023). Additionally, the passive sampler with a disk was exposed to sterile MQ water as a negative control. Following sampling, each disk was immersed in 6 mL of acetone, and then shaken at room temperature for 60 min. The supernatant was collected and the extraction was repeated a second time with 6 mL of methanol for 1 h and a third time with 6 mL of acetone for 1 h. Following extraction, all supernatants were pooled together in Falcon tubes. For evaporation, the tubes were placed in a water bath at 40 °C under a chemical hood and a final volume of around 5 mL of each extract was reserved. The extracts were stored at 4 °C until antibiotic quantification.
Analysis of antibiotics
The antibiotic residues were determined by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS–MS, Agilent Technologies, Inc., USA). The spectrometry analysis was performed using positive mode ESI + with an ion voltage of 5 kV. An aliquot of 2.5 mL filtrate from each water sample was used for solid phase extraction (SPE). The samples were then treated with 5 µL of ammonium thiosulfate, and 1.5 µL of acetic acid. An isotope dilution was performed in the next step. Isotopically labeled internal standards were used (ciprofloxacin D8, enrofloxacin D5, norfloxacin D5, and carbamazepine D10). The separation was carried out on a Waters Xbridge C18 analytical column (100 mm × 4.6 mm, 3.5-µm particle size). The mobile phase consisted of methanol and water with 0.5 mM ammonium fluoride and 0.3% acetic acid as the mobile phase additives. The flow rate was 0.5 mL/min. The injection volume was 0.002 mL.
Extraction of DNA
Water samples collected before and after MOS treatment were filtered through a 0.22-µm membrane (Merck Millipore, Germany) using a vacuum filtration apparatus. The frozen membrane filters at − 20 °C were then used to extract DNA with the DNeasy PowerSoil Kit, QIAGEN, according to the manufacturer’s instructions. The concentration of extracted DNA was then determined using the Quant-iT™ Picogreen™ dsDNA assay Kit (Life Technologies, Zug, Switzerland). The extracted DNA was stored at − 20 °C until further analysis.
Quantification of ARGs by quantitative polymerase chain reaction (qPCR)
qPCR was conducted using the diluted DNA. Quantification of 16S rRNA, blaSHV, blaCTX-M, and blaTEM was performed as previously described (Laffite et al. 2020). The target genes were quantified using an Eco qPCR system (Illumina, Switzerland) using SensiFAST™ SYBR® Kit (Bioline, London, UK). All analyses were performed in triplicate. The primers used in this study are shown in Table 1. In each sample, the relative abundance of ARGs was calculated as a percentage of “copy number of a gene relative to 16S rRNA copy number.”
Table 1.
Primers used in this study
| Target gene | Sequence (5′–3′) | Amplicon size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|
| blaTEM | GCKGCCAACTTACTTCTGACAACG | 247 | 55 | Sidrach Cardona et al. (2014) |
| CTTTATCCGCCTCCATCCAGTCTA | ||||
| blaSHV | TCAGCGAAAAACACCTTG | 110 | 60 | Xi et al. (2009) |
| TCCCGCAGATAAATCACCA | ||||
| blaCTX-M | ATTCCRGGCGAYCCGCGTGATACC | 227 | 62 | Fujita et al. (2011) |
| ACCGCGATATCGTTGGTGGTGCCAT | ||||
| 16S rRNA | ACTCCTACGGGAGGCAGCAG | 197 | 55 | Ovreas et al. (1997) |
| ATTACCGCGGCTGCTGG |
Statistical analysis
Data analysis was carried out not only to describe the reaction observed during the different treatment processes, but also to estimate the error in values and finally have a visualization through tables and figures. Statistical analysis was performed using the R software package 3.6.1 (https://www.r-project.org). Spearman’s rank correlation and principal component analysis (PCA) were performed with R 3.6.1. Statistical significance was determined with a P value < 0.05.
Results and discussion
Effect of WWTP treatment coupled with MOS on physico-chemical parameters
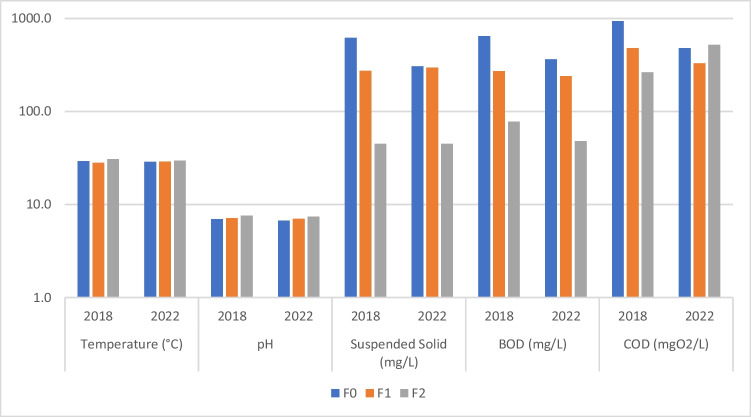
The average concentrations of BOD, COD, suspended solid, total nitrogen, phosphorus, temperature, pH, conductivity, and dissolved oxygen were 48 ± 16 mg/L, 520 ± 608 mg/L, 45 ± 13 mg/L, 57 ± 60 mg/L, 18 ± 0.2 mg/L, 29.6 ± 0.2 °C, 7.4 ± 0.1 (pH), 1103 ± 54 µS/cm, and 0.2 ± 0.1 mg/L (DO), respectively, after treatment with the WWTP prototype. We have noticed a reduction of 14% of the phosphorus and 8% of the conductivity after treatment by the WWTP prototype. A reduction in the amount of BOD and suspended solid was also observed in treated wastewater by 87% and 85%, respectively. In 2018, when the WWTP was started, preliminary analysis was carried out and parameters such as BOD, COD, suspended solid, temperature, and pH were measured (Table A, Supplementary Material). We noted abatement rates that could reach 97% for suspended solids and 94% for BOD. If we refer to Fig. 2 and Table A and Table B (supplementary material), we can see that the average value of MES after treatment by the WWTP prototype is the same for 2018 and 2022, which was equal to 45 mg/L < 50 mg/L before discharge as indicated by the Senegalese discharge standard NS 05–061 (ISN 2001) presented in Table C (supplementary material). According to NS 05–061, BOD value of treated wastewater before rejection in the nature should be ≤ 80 mg/L. However in 2018 and 2022, average values of BOD in F2 are under the limit of rejection (80 mg/L), respectively equal to 78 ± 20 mg/L and 48 ± 16 mg/L. When it comes to COD, the values obtained during the 2018 and 2022 campaigns are well above the discharge limit set at 200 mg/L by NS 05–061. However, in F0, the average value of MES in 2018 is two times higher than values obtained in 2022; hence, the reduction rate noted for 2018 (93%) is higher than the value obtained in 2022 (85%). In the same trend, BOD and COD in F0 are also halved between 2018 and 2022. This clearly shows us that the WWTP prototype as it currently stands is not being used optimally.
Fig. 2.
Comparative histogram of physico-chemical parameters of WWTP of 2018 and 2022
In addition to the 2022 campaign, MOS were added to the untreated water to enhance water quality before discharge but MOS seeds have not demonstrated their effectiveness as an adsorptive natural coagulant to reduce all physico-chemical parameters. COD, total nitrogen, and dissolved oxygen in MOS-treated water were higher than those in untreated water. The increased DO in the treated water was probably due to organic matter degradation. Furthermore, the elevated levels of COD and total nitrogen could be attributed to the presence of organic compounds after treatment as well as the addition of MOS as a natural coagulant (Baptista et al. 2017; Sánchez-Martín et al. 2010). The results were similar to those of Shan et al. (2017), who found that the COD increased after MOS were added. Furthermore, the higher organic content values could cause bacterial regrowth in the treated effluent. It is evident that studies have reported that physico-chemical factors might have a role in the bacterial community and their diversity (D. Li et al. 2022a, b). Thus, after MOS treatment, certain parameters such as total nitrogen were under the limit of rejection equal to 30 mg/L by the Senegalese standardization NS 05–061. That is not the case of the concentration of total phosphorus after MOS treatment with a value equal to 19.25 mg/L > 10 mg/L the limit of rejection set by Senegalese standardization (Table C, Supplementary material). The results of physico-chemical analysis of MOS-treated wastewater do not indicate the effectiveness of improving physico-chemical parameters, so optimization of WWTP treatment is still requested before water can be reused for unrestricted agricultural irrigation of processed food crops in accordance with French Standard NF ISO 16075 (AFNOR 2021). Sensitization and survey are essential especially in the case of small villages or communities that are poorly informed of guidelines and maintenance.
Removal effect of WWTP prototype coupled with MOS on bacterial load
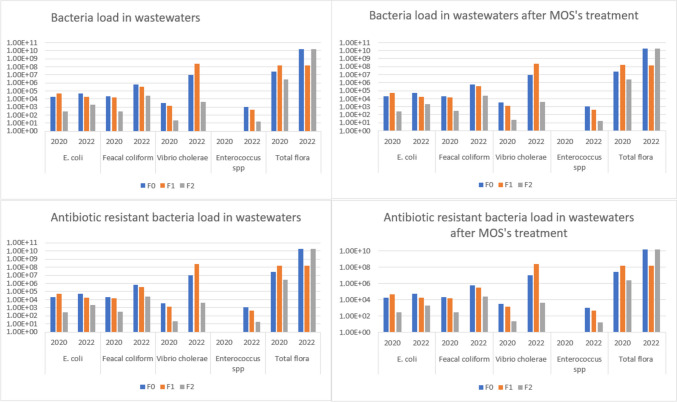
Figure 3 summarizes the results of the microbiological analysis for 2022 and 2020 campaigns. In 2022, WWTP treatment markedly reduced E. coli (96%), fecal coliforms (95%), Enterococcus sp. (99%), Vibrio cholerae (100%), and total flora (99%). Before rejection into the soil, water treated by WWTP contained average concentrations of 2.0 × 103 ± 2.65 × 102, 2.50 × 104 ± 5.77 × 103, 1.67 × 101 ± 2.89 × 101, 4.34 × 103 ± 1.76 × 103, and 1.73 × 1010 ± 2.08 × 109 respectively for E. coli, fecal coliform, Enterococcus sp., Vibrio cholerae, and total flora. As presented in Table D (supplementary material), results of the previous study, conducted in 2020, show that the treated waters contained 2.73 × 102 ± 3.79 × 101, 2.97 × 102 ± 2.52 × 101, 2.17 × 101 ± 4.93, and 2.65 × 106 ± 1.37 × 105 respectively for E. coli, fecal coliform, Vibrio cholerae, and total flora. This confirms that the WWTP after 2 years without monitoring or maintenance is no longer 100% optimal. With reference to total flora removal efficiency, 99.9% was achieved after MOS treatment. Similar results have also been reported previously. For example, Madsen et al. (1987) found that MOS treatment removed the largest percentage of total flora in wastewater. However, they have also noted a regrowth of the total flora after 24 h. It is therefore worth noting that the contact time could be an important factor when MOS are used in wastewater treatment processes as natural coagulants. The Senegalese standard NS 05–061 sets a maximum concentration of fecal coliforms of 20 CFU/mL in discharge water and a maximum of 1 CFU/mL is imposed by NF ISO 16075 French and International regulation before water reuse. Despite MOS treatment, these standard values were not obtained. In 2022, we found that the untreated water contained 6.43 × 105 ± 6.03 × 104 CFU/mL of ampicillin-resistant E. coli, 6.69 × 107 ± 1.14 × 107 CFU/mL of ampicillin-resistant fecal coliforms, and > 300 × 106 CFU/mL of ampicillin-resistant total flora. A total absence of Enterococcus sp. and Vibrio cholerae resistant to ampicillin was noted in F0. MOS treatment increased ampicillin-resistant bacteria removal efficiency to 96.3% for resistant E. coli and 99.8% for fecal coliforms. In comparison to the non-MOS treatment, significant improvements in microbiological parameters were observed. Thus, it is important to note that MOS have remarkable effects on the elimination of most of E. coli, fecal coliforms, and Vibrio cholerae strain. Similar results have been reported by other authors such as Vega Andrade et al. (2021), who noted that MOS aqueous extract treatment dosed at 600 mg/L showed significant effectiveness on the removal of E. coli (99.7%) and total coliforms (99.6%). The authors concluded that MOS are an efficient and low-cost water treatment alternative. A possible reason for the elimination of E. coli during MOS treatment might be the interaction of cationic proteins in Moringa and potential damage to E. coli (Shebek et al. 2015). As a result, this study confirms and emphasizes the benefits of using MOS as a low-cost and eco-friendly coagulant (with significative bactericidal properties) in resource-limited settings.
Fig. 3.
Comparative (2020, 2022) histogram of bacterial load and antibiotic-resistant bacterial load in WWTP
Antibiotics removal
The results of antibiotics quantification in the WWTP prototype are shown in Table 2. In this study, the main targeted antibiotics are from the beta-lactam, fluoroquinolone, and tetracycline families. In Senegal, beta-lactams remain the most prescribed class of antibiotics in general medicine (Camara 2015; Chamor 2021; Ndour et al. 2014), followed by quinolones (Guissé 2017). It has also been demonstrated by scientists that in the case of watery diarrhea treatment, the use of antibiotics in addition to rehydration increased the emergence of resistance to beta-lactam, fluoroquinolone, and tetracycline (Eholié et al. 2014), hence the choice of antibiotics we quantified.
Table 2.
Antibiotic concentration (ng/L) in WWTP
| Tank | Ciprofloxacin (ng/L) | Doxycycline (ng/L) | Enoxacin (ng/L) | Enrofloxacin (ng/L) | Norfloxacin (ng/L) | Ofloxacin (ng/L) | Amoxicillin (ng/L) | Ampicillin (ng/L) |
|---|---|---|---|---|---|---|---|---|
| F0 | 564 | 85 | LQ | LQ | LQ | LQ | LQ | LQ |
| F1 | 38.9 | LQ | LQ | LQ | LQ | LQ | LQ | LQ |
| F2 | 145 | 27 | LQ | LQ | LQ | LQ | LQ | LQ |
LQ, limit of quantification
Ciprofloxacin was detected in wastewater at the highest concentration of all targeted antibiotics. Untreated water contained higher concentrations of both ciprofloxacin and doxycycline. Fluoroquinolones have been reported to be frequently used to treat infections in clinical practice (MacDougall et al. 2005). The average concentrations of ciprofloxacin were 564 ± 482 ng/L in the septic tank, 39 ± 9 ng/L in the filter, and 145 ± 103 ng/L in the infiltration well. Ciprofloxacin concentrations in the different tanks are highly variable. This variability could be due to either the nature, quantity, or retention time of the incoming flux. It is also important to mention that the POCIS position can vary from one sampling point to another regarding the direction of the main stream of used water. This is one of the limitations of the passive samplers used in this study. We noticed an average removal of ciprofloxacin of 93% between the septic tank and the filter and of 74% between the septic tank and the infiltration well. High concentrations of ciprofloxacin in wastewater could be correlated with frequent usage of this antibiotic by the inhabitants. Ciprofloxacin concentrations detected in this study were similar to those reported in Africa (Kelly and Brooks 2018). These results indicate that WWTPs do not completely remove antibiotics from effluents. Furthermore, results showed that the concentration of ciprofloxacin between the filter and the infiltration well, however, increased. It might confirm the non-optimal aspect of the studied WWTP protype. Thus, the increased concentration probably contributed to the low retention time in the filter. Similarly, in the septic tank, the average concentration of doxycycline was 85 ± 37 ng/L; in the infiltration well, 27 ± 2 ng/L; and in the filter, < 20 ng/L. Between the septic tank and the infiltration well, doxycycline concentration was reduced by 68%.
In the case of other fluoroquinolone antibiotics tested, the concentrations during the treatment process were quite low, with values of < 20 ng/L for enoxacin, enrofloxacin, and norfloxacin and < 50 ng/L for ofloxacin. For other penicillin compounds (amoxicillin and ampicillin), the concentrations were lower than 100 ng/L. Despite a non-optimal state, the prototype shows a relative trend to reduce the amount of trace antibiotics analyzed. If the flows and usage have remained similar over the 2 years and the prototype has deteriorated, then it remains partially effective against the targeted antibiotics.
Abundance of ARGs in wastewater
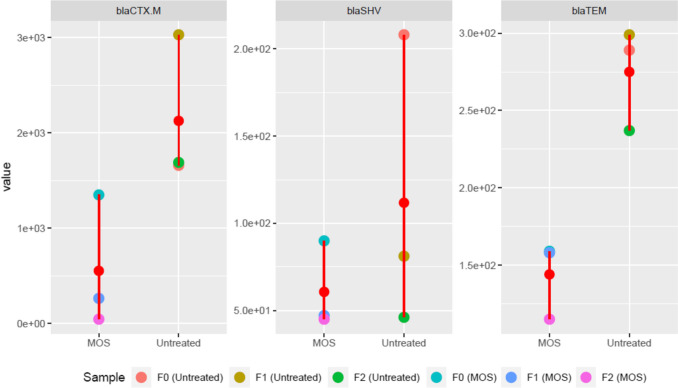
ARBs and ARGs have been shown to accumulate in WWTPs and are considered the intermediate channel between humans and their environment in the spread of antibiotic resistance (Proia et al. 2016). Four ARGs with clinical relevance were quantified in wastewater in this study. The relative concentrations of the analyzed ARGs in untreated and MOS-treated wastewater are shown in Fig. 4. This study examined ARGS in the inlet unit, filter unit, and infiltration well. In all samples, blaKPC was not a quantifiable ARG. The most abundant gene in wastewater samples was blaCTX-M, followed by blaTEM. ARGs’ relative abundance ranged between 1.66 × 103 and 1.69 × 103, 2.08 × 102 and 4.62 × 101, and 2.89 × 102 and 2.37 × 102 copies/16S rRNA gene for blaCTX-M, blaSHV, and blaTEM, respectively, in untreated wastewater. However, Fig. 4 shows a certain reduction between F0 (untreated with MOS) and F2 (untreated with MOS) evidence of a slight efficiency of the WWTP in eliminating ARGs. In wastewater treatment, MOS have not been extensively studied for their impact on the removal of antibiotic-resistant bacteria and ARGs. For blaCTX-M, blaSHV, and blaTEM, the relative abundances were between 1.35 × 103 and 4.51 × 101, 9.0 × 101 and 4.51 × 101, and 1.59 × 102 and 1.15 × 102 copies/16S rRNA gene respectively in MOS-treated wastewater. The abundance of blaCTX-M, blaTEM, and blaSHV decreased to some extent after MOS treatment of wastewater (Fig. 4). It is clear, therefore, that WWTP that had undergone MOS treatment influenced the ARGs significantly. The dominant β-lactam resistance genes may be associated with antibiotic consumption, which may cause selection pressure on wastewater microbial communities. Past studies have shown that removing ARGs from WWTPs is still inefficient (Rizzo et al. 2013). The results of this study indicated that, if considering the methodologies and cost-effectiveness of wastewater treatment, the MOS could be useful material for treating wastewater in sub-Saharan countries to some extent and reducing the load of bacterial density, ARB, and ARGs. While technologies and systematic management have advanced, cost increases have rendered them inaccessible to advanced WWTPs in developing countries, where control and maintenance of existing sanitation systems are already a huge challenge. In spite of this, a long-term study is highly encouraged to get a clearer picture of MOS treatment’s effect on ARG reduction, particularly at different seasons of the year.
Fig. 4.
Relative abundance of ARGs in wastewater (blaKPC was not presented because of being below the quantification limit)
Correlation between physico-chemical parameters and ARGs
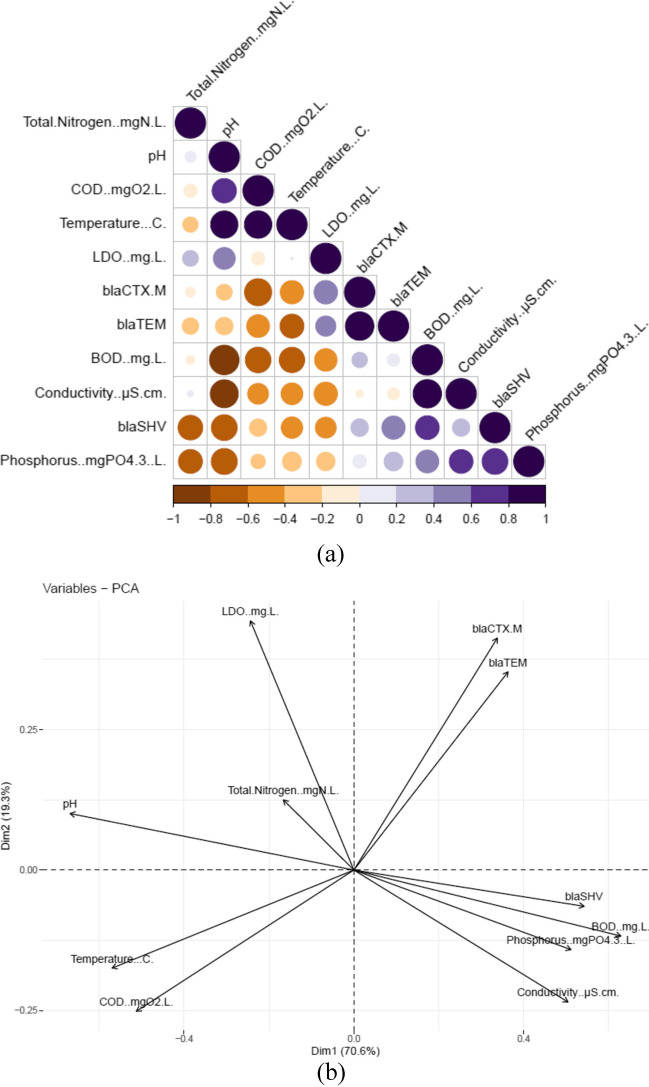
In this WWTP, we evaluated parameters that displayed a correlation with ARGs. ARG abundance in wastewater may be related to physico-chemical parameters, as observed by statistical correlations. We found positive correlations between COD and pH, temperature and COD, conductivity and BOD, phosphorus and conductivity, blaSHV and BOD, blaSHV and phosphorus, and blaSHV and conductivity, indicating that environmental factors are contributing to the abundance and propagation of ARGs in WWTP (Fig. 5a and b). A recent study by Li et al. (2022a, b) suggested that conductivity may be related to the availability of nutrients in the environment. Additionally, the positive correlation between blaCTX-M and blaTEM suggests co-occurrences of ARGs in WWTPs (Fig. 5b). The temperature is strongly correlated with COD, indicating that temperature could be an important factor in bacterial growth (Fig. 5b). There was a direct effect of total nitrogen and DO on the WWTP (Fig. 5b). There have also been previous studies indicating that the abiotic factors influence ARG occurrences in WWTPs (S. Li et al. 2022a, b).
Fig. 5.
a Correlation between ARGs and physico-chemical variables in the wastewater. b Principal component analysis (PCA)
Conclusion
In this study, pollution removal was investigated in a non-optimal WWTP prototype treated with MOS, revealing a moderate effect on the physico-chemical parameters bacterial densities, antibiotics, and ARGs.
A sampling campaign was carried out in 2022 following the first one in 2020 and a preliminary study in 2018 when the WWTP prototype was started. During the 2022 campaign, we have noticed that the WWTP is no longer used in 100% (fewer people use the toilets connected to the prototype), and moreover, the flow transmitted between F1 and F2 is random due to a counter-slope. This can explain the results obtained in 2022. In fact, during the 2022 campaign, we have noted reductions of 14% of phosphorus, 8% of conductivity value, 87% of BOD, and 85% of suspended solids after treatment by the WWTP prototype. However, parameters, such as BOD, COD, and suspended solids, were two times higher in F0 during the preliminary study than during the 2022 campaign. It shows that the WWTP is currently not used in 100% and that was confirmed by the comparative study between microbiological results from 2020 and 2022 campaigns. MOS show effectiveness to reduce total flora, with 99.9% removal after treatment. MOS was also effective in antibiotic bacteria removal with percentages that reach 96.3% for resistant E. coli and 99.8% for resistant fecal coliforms. In comparison to the non-MOS treatment, significant improvements in microbiological parameters were observed. Concerning antibiotics quantification, high concentrations of ciprofloxacin and doxycycline were detected and an average removal of 74.3% for ciprofloxacin and 68% for doxycycline was noticed between F0 and F2. For amoxicillin and ampicillin despite the fact that they are prescript in priority for many bacterial infections, their concentration was lower than the detection limit. The presence of antibiotics in treated waters could increase bacterial resistance; we therefore quantified resistance genes by qPCR. The most abundant gene was blaCTX-M, followed by blaTEM, and we noticed that after MOS treatment, the abundance of blaCTX-M, blaTEM, and blaSHV decreased.
This study, also, confirms that residues of ciprofloxacin at a low concentration persist in the environment and may lead to multi-drug resistance in wastewater. Based on the results, blaCTX-M appears to be the most abundant resistance in our wastewaters. The results showed that MOS act as an alternative multi-functional (coagulant and antiseptic) sustainable natural additive for the treatment of personal used water in developing countries like Senegal. As a small-scale study, our current focus is on the genes associated with resistance to β-lactam in wastewater. Our study reveals that ARG removal can be improved with further optimization by renovating the WWTP prototype and testing other filtration materials such as coconut fiber, sand, organic charcoal, zeolite, and pozzolan. However, a more extensive study is recommended to confirm the long-term abundance of different ARGs. In addition, this study has shown that in the absence of maintenance and regular use by the population, the prototype tested weakly complies with the various standards (NS 05–061, NF ISO 16075). This raises the question of how to manage collecting individual wastewater in sub-Saharan zones in small treatment units that are not monitored. This is a key question, in the case of scattered or low-density housing, with limited access to technical resources for the maintenance and monitoring of WWTP. Even a simple sanitation installation requires minimal maintenance, and up-to-date information on the risks associated with the discharge or use of wastewater. Alongside technical and technological innovations based on local resources, it is therefore necessary to inform and educate all interested parties and stakeholders. If major campaigns are envisaged by the state, the private sector, and international organizations, we believe it is necessary to base them on the needs (objective, realistic, and achievable over a long period) of the population concerned.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the partners, University of Bordeaux, Cheikh Anta Diop University, and University of Geneva, for the collaboration. Thanks to the Applied Biotechnology and Environmental Bioprocesses Research Group (Ecole Supérieure Polytechnique, Université Cheikh Anta Diop) for making human and material resources available for molecular extraction. Thanks to AFFINISEP (https://www.affinisep.fr/) which provides recommendations and procedure for activation and extraction of the passive samplers.
Author contribution
All authors wrote and have read, reviewed, and approved the manuscript before submission. Nini Sané, Philippe Le Coustumer, John Poté, and Serge Stoll conceived and designed the research. Nini Sané, Malick Mbengué, Periyasamy Sivalingam, John Poté, and Milan Koželuh performed sampling and laboratory analysis. Nini Sané, Periyasamy Sivalingam, John Poté, and Philippe Le Coustumer controlled, analyzed, and valorized data. Serge Stoll, John Pote, Periyasamy Sivalingam, and Philippe Le Coustumer provided recommendations and suggestions by reviewing the manuscript.
Funding
This work received support from the Schlumberger Foundation which allowed a grant to Miss N. Sané as a part of the faculty of Women of the Future program. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Data availability
Data are available on request by sending a demand to Ms. Nini Sané (ninisane@gmail.com) who centralized and stored the data. An option is to send also a demand to the associate professor Malik Mbengue (mailtomailtomalick.mbengue@ucad.edu.sn/mbenguemalick@hotmail.com), the co-supervisor who collected the data for the UCAD.
Declarations
Ethical approval
All authors declared that they follow the COPE guidelines.
Ethics approval
We confirm that the field studies and sampling did not involve misunderstanding.
Consent to participate
Not applicable.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images. The participants have consented to the submission of the case report to the journal. Patients signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare no competing interests.
Disclaimer
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Research highlight
• Wastewater treatment plant prototype was evaluated after 6 years in function.
• Purification efficiency of the personal WWTP and Moringa oleifera seeds was evaluated.
• After 2 years without monitoring, the prototype is no longer 100% functional.
• Moringa oleifera seeds show effectiveness in reducing bacterial load, antibiotic-resistant bacteria, and antibiotic-resistant genes.
• Moringa oleifera seeds due to their bactericidal effects could be integrated into wastewater treatment plants.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adeniran KA, Akpenpuun TD, Akinyemi BA, Wasiu RA (2017) Effectiveness of Moringa oleifera seed as a coagulant in domestic wastewater treatment. Afr J Sci Technol Innov Dev 9(3):323–328. 10.1080/20421338.2017.1327475 [Google Scholar]
- AFFINISEP (2023) Passive sampling for the analysis of 12 endocrine disruptors in river water using AttractSPE® POCIS – EDC+, Retrieved 6 April 2023. https://www.affinisep.fr/wp-content/uploads/2022/11/an-0018-01-passive-sampling-for-the-analysis-of-12-endocrine-disruptors-in-river-water-using-attractspe-pocis--edc_compressed.pdf
- AFNOR (2021) Lignes directrices pour l’utilisation des eaux usées traitées dans les projets d’irrigation - Partie 1 : les bases d’un projet de réutilisation pour l’irrigation. Afnor EDITIONS. Retrieved 6 April 2023. https://www.boutique.afnor.org/fr-fr/norme/nf-iso-160751/lignes-directrices-pour-lutilisation-des-eaux-usees-traitees-dans-les-proje/fa198212/238186
- Anda De, José A-L, Villegas-García E, Valdivia-Aviña K (2018) High-strength domestic wastewater treatment and reuse with onsite passive methods. Water 10(2):99. 10.3390/w10020099 [Google Scholar]
- Andrade PV, Palanca CF, de Oliveira MAC, Ito CYK, dos Reis AG (2021) Use of Moringa oleifera seed as a natural coagulant in domestic wastewater tertiary treatment: physicochemical, cytotoxicity and bacterial load evaluation. J Water Process Eng 40:101859. 10.1016/j.jwpe.2020.101859 [Google Scholar]
- Baptista AT, Alves MO, Silva RG, Gomes RB, Vieira MF, Vieira AMS (2017) Protein fractionation of seeds of Moringa oleifera Lam and its application in superficial water treatment. Sep Purif Technol 180:114–124. 10.1016/j.seppur.2017.02.040 [Google Scholar]
- Bouazza L (2011) Effet de la coagulationf-loculation sur la qualité des eaux épurées de la STEP de Ain El Houtz. Mémoire pour l’obtention du diplôme de magister, Université Abou Bekr Belkaid – TLEMCEN, Algérie
- Breurec S, Bouchiat C, Sire J-M, Moquet O, Bercion R, Cisse MF, Glaser P, Ndiaye O, Ka S, Salord H, Seck A, Sy HS, Michel R, Garin B (2016) High third-generation cephalosporin resistant Enterobacteriaceae prevalence rate among neonatal infections in Dakar, Senegal. BMC Infect Dis 16(1):587. 10.1186/s12879-016-1935-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara ML (2015) Evaluation de la prescription médicamenteuse en consultation externe au service pédiatrique de l'hôpital Aristide Le Dantec. Thèse pour Obtenir le grade de Docteur en Médecine, Université Cheikh Anta Diop de Dakar, Dakar
- Chamor I (2021) Profil de résistance aux antibiotiques des bactéries isolées au CHNU de FANN. Mémoire pour l’obtention du diplôme d’études spécialisées en biologie clinique, Université Cheikh Anta Diop de Dakar, Dakar
- Chereau F, Herindrainy P, Garin B, Huynh B-T, Randrianirina F, Padget M, Piola P, Guillemot D, Delarocque-Astagneau E (2015) Colonization of extended-spectrum-β-lactamase- and NDM-1-producing Enterobacteriaceae among pregnant women in the community in a low-income country: a potential reservoir for transmission of multiresistant Enterobacteriaceae to neonates. Antimicrob Agents Chemother 59(6):3652–3655. 10.1128/AAC.00029-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2017) Performance standards for antimicrobial susceptibility testing. USA
- Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R (2018) Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health 2(9):e398-405. 10.1016/S2542-5196(18)30186-4 [DOI] [PubMed] [Google Scholar]
- Desta WM, Bote ME (2021) Wastewater treatment using a natural coagulant (Moringa oleifera seeds): optimization through response surface methodology. Heliyon 7(11):e08451. 10.1016/j.heliyon.2021.e08451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eholié S-P, Bissagnéné E, Crémieux A-C, Girard P-M (2014) Momento du bon usage des antibiotiques en Afrique sub-saharienne
- Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124(4):509–25. 10.1007/s10265-011-0412-3 [DOI] [PubMed]
- Gueye AK (2007) Contrôle de qualité des antibiotiques vendus au Sénégal. Thèse pour obtenir le grade de Docteur en pharmacie, Université Cheikh Anta Diop de Dakar, Dakar
- Guissé S (2017) Analyse de la prescription des antibiotiques : enquête dans dix officines de Dakar. Thèse pour obtenir le grade de Docteur en pharmacie, Université Cheikh Anta Diop de Dakar, Dakar
- Gupta S, Graham DW, Sreekrishnan TR, Ahammad SZ (2022) Effects of heavy metals pollution on the co-selection of metal and antibiotic resistance in urban rivers in UK and India. Environ Pollut 306:119326. 10.1016/j.envpol.2022.119326 [DOI] [PubMed] [Google Scholar]
- Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJV (2016) Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet 387(10014):176–187. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- ISN (2001) EAUX USEES: NORMES DE REJET. Retrieved 13 July 2023. https://www.fao.org/faolex/results/details/fr/c/LEX-FAOC054265/
- Kahn LH (2017) Antimicrobial resistance: a one health perspective. Trans R Soc Trop Med Hyg 111(6):255–260. 10.1093/trstmh/trx050 [DOI] [PubMed] [Google Scholar]
- Kelly KR, Brooks BW (2018) Chapter Three - Global aquatic hazard assessment of ciprofloxacin: exceedances of antibiotic resistance development and ecotoxicological thresholds. In: Teplow DB (ed) Progress in molecular biology and translational science, vol 159. Academic Press, pp 59–77 [DOI] [PubMed] [Google Scholar]
- Laffite A, Dhafer MM, Salah Al, Slaveykova VI, Otamonga J-P, Poté J (2020) Impact of anthropogenic activities on the occurrence and distribution of toxic metals, extending-spectra β-lactamases and carbapenem resistance in sub-Saharan African urban rivers. Sci Total Environ 727:138129. 10.1016/j.scitotenv.2020.138129 [DOI] [PubMed] [Google Scholar]
- Larsson DG, Flach C-F (2022) Antibiotic resistance in the environment. Nat Rev Microbiol 20(5):257–69. 10.1038/s41579-021-00649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Xingjie Li Yu, Tao ZY, Ao Y (2022) Deciphering the bacterial microbiome in response to long-term mercury contaminated soil. Ecotoxicol Environ Saf 229:113062. 10.1016/j.ecoenv.2021.113062 [DOI] [PubMed] [Google Scholar]
- Li S, Ondon BS, Ho S-H, Jiang J, Li F (2022) Antibiotic resistant bacteria and genes in wastewater treatment plants: from occurrence to treatment strategies. Sci Total Environ 838:156544. 10.1016/j.scitotenv.2022.156544 [DOI] [PubMed] [Google Scholar]
- LY A (2016) Etude de la biodépollution des eaux usées avec un consortium bactérien et caractérisation bactériologique lors du traitement avec les graines de Moringa oleifera. Mémoire d’ingénieur, Université Cheikh Anta Diop de Dakar, Dakar
- MacDougall C, Patrick Powell J, Johnson CK, Edmond MB, Polk RE (2005) Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis 41(4):435–440. 10.1086/432056 [DOI] [PubMed] [Google Scholar]
- Madsen M, Schlundt J, Omer EFE (1987) Effect of water coagulation by seeds of Moringa oleifera on bacterial concentrations. J Trop Med Hyg 90:101–109. 10.1016/0378-8741(88)90285-1 [PubMed] [Google Scholar]
- Manaia CM (2017) Assessing the risk of antibiotic resistance transmission from the environment to humans: non-direct proportionality between abundance and risk. Trends Microbiol 25(3):173–181. 10.1016/j.tim.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Marti E, Jofre J, Balcazar JL (2013) Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PLoS ONE 8(10):e78906. 10.1371/journal.pone.0078906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndour M, Ghaddou Y, Seydi M, Sarr A, Diédhiou D, Sow D, Touré M, Ka-Cissé M, Diop SN (2014) Evaluation de la qualité de prescription des antibiotiques au centre hospitalier Abass Ndao. Dakar-Med
- Nouhi S, Kwaambwa HM, Gutfreund P, Rennie AR (2019) Comparative study of flocculation and adsorption behaviour of water treatment proteins from Moringa peregrina and Moringa oleifera seeds. Sci Rep 9(1):17945. 10.1038/s41598-019-54069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovreås L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63(9):3367–73. 10.1128/aem.63.9.3367-3373.1997 [DOI] [PMC free article] [PubMed]
- Proia L, von Schiller D, Sànchez-Melsió A, Sabater S, Borrego CM, Rodríguez-Mozaz S, Balcázar JL (2016) Occurrence and persistence of antibiotic resistance genes in river biofilms after wastewater inputs in small rivers. Environ Pollut 210:121–128. 10.1016/j.envpol.2015.11.035 [DOI] [PubMed] [Google Scholar]
- Pruden A, Pei R, Storteboom H, Carlson KH (2006) Antibiotic resistance genes as emerging contaminants: studies in Northern Colorado. Environ Sci Technol 40(23):7445–7450. 10.1021/es060413l [DOI] [PubMed] [Google Scholar]
- Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360. 10.1016/j.scitotenv.2013.01.032 [DOI] [PubMed] [Google Scholar]
- Rodier J, Legube B, Merlet N (2009) L’analyse de l’eau. Vol. 9e édition. DUNOD Paris. Paris: DUNOD
- Sánchez-Martín J, Ghebremichael K, Beltrán-Heredia J (2010) Comparison of single-step and two-step purified coagulants from Moringa oleifera seed for turbidity and DOC removal. Biores Technol 101(15):6259–6261. 10.1016/j.biortech.2010.02.072 [DOI] [PubMed] [Google Scholar]
- Sané N, Mbengue M, Laffite A, Stoll S, Poté J, Le Coustumer P (2022) Development of a process for domestic wastewater treatment using Moringa oleifera for pathogens and antibiotic-resistant bacteria inhibition under tropical conditions. Water 14(15):2379. 10.3390/w14152379 [Google Scholar]
- Shan TC, Matar MA, Makky EA, Ali EN (2017) The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl Water Sci 7(3):1369–1376. 10.1007/s13201-016-0499-8 [Google Scholar]
- Shebek K, Schantz AB, Sines I, Lauser K, Velegol S, Kumar M (2015) The flocculating cationic polypetide from Moringa oleifera seeds damages bacterial cell membranes by causing membrane fusion. Langmuir 31(15):4496–4502. 10.1021/acs.langmuir.5b00015 [DOI] [PubMed] [Google Scholar]
- Sidrach-Cardona R, Hijosa-Valsero M, Martí E, Balcazar J, Becares E (2014) Prevalence of antibiotic-resistant fecal bacteria in a river impacted by both an antibiotic production plant and urban treated discharges. Sci Total Environ 488–489:220–227. 10.1016/j.scitotenv2014.04.100 [DOI] [PubMed]
- Sylla A (2014) L’automédication par les antibiotiques : étude menée dans la commune de Guédiawaye. Thèse pour obtenir le grade de Docteur en pharmacie, Université Cheikh Anta Diop de Dakar, Dakar
- UN-Water (2021) Summary progress update 2021: SDG 6 — water and sanitation for all. UN-Water. Retrieved 1 June 2021. https://www.unwater.org/publications/summary-progress-update-2021-sdg-6-water-and-sanitation-for-all/
- Xi C, Zhang Y, Marrs CF, Ye W, Simon C, Foxman B et al (2009) Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Microbiol 75:5714–5718. 10.1128/AEM.00382-09 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request by sending a demand to Ms. Nini Sané (ninisane@gmail.com) who centralized and stored the data. An option is to send also a demand to the associate professor Malik Mbengue (mailtomailtomalick.mbengue@ucad.edu.sn/mbenguemalick@hotmail.com), the co-supervisor who collected the data for the UCAD.