Abstract
Aims
This study aimed to evaluate the symptomatic efficacy and tolerability of three different radiotherapy (RT) regimens for patients with vertebral metastases in a low-middle-income country setting, focusing specifically on the effectiveness of single-fraction radiotherapy.
Methods
Conducted at the National Institute of Cancer Research and Hospital, Bangladesh, from July 1, 2020, to June 30, 2021, this prospective, non-randomized study enrolled 90 patients aged 18 to 75 years with histologically confirmed primary malignancies and vertebral metastases. Patients were allocated to one of three treatment arms: 8 Gy in a single fraction (Arm A), 20 Gy in 5 fractions (Arm B), or 30 Gy in 10 fractions (Arm C). The primary endpoint was pain response at 12 weeks, assessed by the Visual Analogue Scale and International Bone Metastases Consensus. Secondary endpoints included toxicity, measured by the Common Terminology Criteria for Adverse Events, and overall survival.
Results
Pain control at 12 weeks showed no significant differences among the treatment groups, with 70% of patients in Arm A, 67% in Arm B, and 70% in Arm C experiencing either partial or complete pain relief (p = 0.95). The overall survival rates were comparable across the groups (median survival, 7 months for arms A and C, 6 months for Arm B). Skin toxicity was significantly lower in Arm A (10% incidence) compared to arms B (30%) and C (47%) (p = 0.017). There were no reports of Grade 3 or higher toxicities.
Conclusion
The study confirms the efficacy and safety of single-fraction RT for spinal bone metastases, providing significant pain relief and lower skin toxicity relative to multiple fraction regimens. These results confirm the efficacy of single-fraction RT in the treatment of vertebral metastases also in resource-limited settings, suggesting its broader adoption to reduce toxicity and treatment burdens in low-middle-income countries.
Keywords: Single-fraction radiotherapy, Spinal bone metastases, Pain management, Radiotherapy regimens, Low-middle-income countries, Treatment efficacy and tolerability
Introduction
Bone metastases (BMs) are a common and debilitating complication in cancer patients, frequently occurring in the spine [1]. BMs can cause severe pain and significantly impair the quality of life. Radiotherapy (RT) has been established as a standard treatment modality for managing pain associated with BMs [2]. Over the years, several clinical studies have explored the efficacy of various RT regimens, particularly comparing single-fraction RT to multiple-fraction RT [3]. These studies consistently demonstrated that both regimens provide similar levels of pain relief [4–9].
Despite the robust data supporting the efficacy of single-fraction RT, the majority of existing research encompasses BMs across various body sites, thus providing a broad but not necessarily precise understanding of the best practices for specific locations such as vertebral metastases. Furthermore, most trials have been conducted in Western countries. In fact, there is a notable scarcity of data from low- and middle-income countries (LMICs), where healthcare resources and patient management strategies might differ significantly from those in higher-income settings [10]. In addition, the limited studies available from LMICs often suffer from small sample sizes and medium to high risk of bias and provide minimal information on the tolerability of different RT regimens [10].
Given these gaps in the literature, the aim of our study is to assess and compare the symptomatic efficacy and tolerability profiles of three distinct RT regimens in a homogeneously selected group of patients with spinal metastases, treated at a tertiary healthcare center in an LMIC. This research aims to provide useful information that could influence treatment protocols and improve patient outcomes in similar healthcare settings globally.
Materials and methods
Study design
This prospective, experimental, non-randomized study was conducted at the National Institute of Cancer Research and Hospital (NICRH), Bangladesh, from July 1, 2020, to June 30, 2021. NICRH is the only tertiary-level cancer care center in Bangladesh under government setup. The study received approval from the Institutional Review Board of the NICRH, adhering to the Declaration of Helsinki. Written informed consent was obtained from all participants.
Patients were enrolled in three consecutive cohorts and treated with three different regimens: 8 Gy in a single fraction (Arm A), 20 Gy in 5 fractions (Arm B), and 30 Gy in 10 fractions (Arm C). We utilized Simon’s minimax phase II trial design [11], structured in two stages, to calculate cohort sizes. This design tested the null hypothesis that the complete pain response rate would improve from 5.0% (baseline without RT) to 20.0%, with an α error of 0.0416 and a power of 0.8011. Initially, the plan was to include 13 patients in the first stage. If no patients showed a complete pain response, enrollment would be halted, and the study would be closed. However, if at least one symptomatic response was observed, the study would proceed to enroll an additional 14 patients, totaling 27. The treatment regimen would be deemed inactive if fewer than 4 out of the 27 patients achieved a symptomatic complete response. Anticipating a possible dropout rate of 10%, we decided to enroll 30 patients per cohort. A complete pain response was defined as a Visual Analogue Scale (VAS) score of 0 at the treated site without any increase in analgesic intake, maintaining or reducing the daily morphine equivalent dosage.
Patient selection
Eligible patients were aged 18 to 75 years and diagnosed with a histologically confirmed primary solid tumor and radiologically and/or histologically proven vertebral metastasis. Inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–3; absence of spinal cord compression, vertebral collapse, or compression fracture; metastasis in no more than two vertebrae; and no prior RT at the index site or systemic therapy within 28 days of RT initiation. A total of 90 patients were enrolled and equally divided into the three treatment arms.
Radiotherapy protocol
RT was administered using 2D conventional techniques in a prone position with a 6MV Linear Accelerator (LINAC). X-ray simulation was conducted, and the treatment field was delineated using bony landmarks. The field extended craniocaudally from one vertebra above to one below the lesion and laterally up to the vertebral transverse process. The maximum dose depth (Dmax) ranged from 5–7 cm in the cervical and thoracic regions to 7–8 cm in the lumbar region, based on body contour and guided by pre-treatment MRI which was primarily used to exclude cord compression. A single posterior field was used.
Study endpoints
The primary endpoint was pain response, assessed using the Visual Analogue Scale (VAS) pre-treatment and the International Bone Metastases Consensus response definition of 2012 [5]. Secondary endpoints included toxicity (measured by the Common Terminology Criteria for Adverse Events, version 5), symptom improvement other than pain (assessed using three items from the EORTC QLQ C-30 Questionnaire), and overall survival, calculated from the day of study assignment.
Patient assessment
Initial evaluation included a history review, physical examination, and relevant investigations (MRI of the entire spine and bone scan for all patients, with additional imaging and biochemical tests as needed). During treatment, patients were monitored daily through history and physical examination, along with ongoing investigations. Post-treatment follow-up assessments were conducted at 4, 8, and 12 weeks for toxicities and treatment response and then every 3 months.
Statistical analysis
Statistical analysis was performed using SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). Data were analyzed using the chi-square test for categorical variables. Survival curves were calculated with the Kaplan–Meier method and compared using the log-rank test. All reported p-values were two-sided, with a significance level set at less than 0.05.
Results
Patient characteristics
The study comprised patients with a mean age of 58.2 ± 6.8 years in Arm A, 57.6 ± 6.6 years in Arm B, and 58.3 ± 6.7 years in Arm C. The majority of patients were male across all groups. Lung cancer emerged as the most common primary cancer, with the lumbar region being the most frequently involved and treated vertebral area. Over fifty percent of patients in each arm had metastases at multiple sites (15 in Arm A, 17 in Arm B, and 16 in Arm C). No patients had an ECOG Performance Status of 0, with the majority presenting with a status of 2 or 3. The distribution of patient characteristics was balanced among the groups (Table 1).
Table 1.
Patient characteristics
| Characteristics | Arm A (8 Gy) | Arm B (20 Gy) | Arm C (30 Gy) | |
|---|---|---|---|---|
| Mean age | 58.2 ± 6.8 | 57.6 ± 6.6 | 58.3 ± 6.7 | |
| Sex | Male | 17 | 17 | 19 |
| Female | 13 | 13 | 11 | |
| Primary site | Lung | 10 | 11 | 11 |
| Breast | 8 | 8 | 7 | |
| Prostate | 6 | 5 | 6 | |
| Others | 6 | 6 | 6 | |
| Involved sites | Single | 15 | 13 | 14 |
| Multiple | 15 | 17 | 16 | |
| Vertebral site | Lumber | 25 | 22 | 14 |
| Thoracic | 18 | 19 | 16 | |
| Cervical | 5 | 6 | 6 | |
| ECOG PS | 1 | 4 | 4 | 3 |
| 2 | 14 | 13 | 11 | |
| 3 | 12 | 13 | 16 | |
Symptomatic improvement
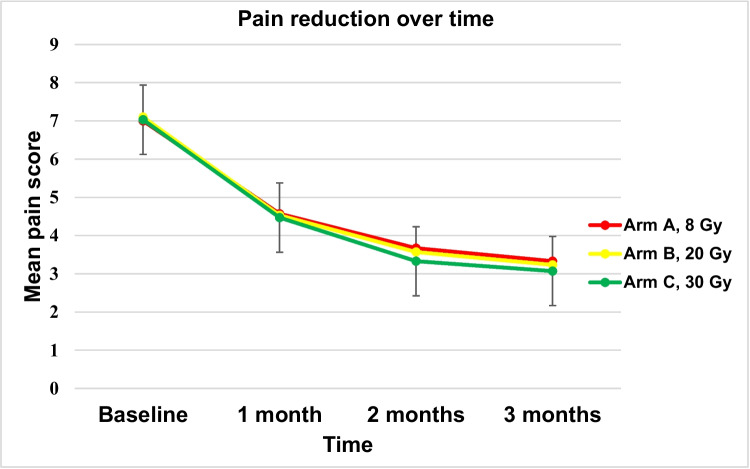
All treatment groups demonstrated similar effectiveness in terms of pain response at 12 weeks post-initial radiation, with 70% in Arm A, 67% in Arm B, and 70% in Arm C experiencing partial or complete pain relief (p = 0.95) (Table 2). The duration of the interval between initiation of therapy and initial reduction in reported pain was 9, 10, and 10 days in arms A, B, and C, respectively (p: 0.16). No treatment regimen provided superior early pain relief (Fig. 1). Most patients reported improvements in sleep and general daily activities post-RT, regardless of the fractionation regimen used. Improvement in walking outside the home was closely associated with the pain response (Table 3).
Table 2.
Comparison of pain response rate among different study Arm
| Response | Arm A (8 Gy), n (%) | Arm B (20 Gy), n (%) | Arm C (30 Gy), n (%) | p-value |
|---|---|---|---|---|
| Complete response | 4 (13) | 3 (10) | 3 (10) | 0.958 |
| Partial response | 17 (57) | 17 (57) | 18 (60) | |
| Overall response | 21 (70) | 20 (67) | 21 (70) |
Fig. 1.
Graphical representation of the mean pain scores for Arm A, Arm B, and Arm C at baseline, 1 month, 2 months, and 3 months. All three lines closely overlap, showing a pronounced decline in pain scores from baseline to 1 month, followed by a more gradual decrease. This illustrates that the pattern of pain reduction over time is remarkably similar across all three treatment regimens
Table 3.
Improvement of symptoms (other than pain) in different study arms
| Symptom | Arm A (8 Gy), n (%) | Arm B (20 Gy), n (%) | Arm C (30 Gy) n (%) |
p-value | |
|---|---|---|---|---|---|
| Walking outside home | Improved after RT | 19 (63) | 18 (60) | 20 (67) | 0.866 |
| Not improved | 11 (37) | 12 (40) | 10 (33) | ||
| Sleeping difficulty | Improved after RT | 25 (83) | 25 (83) | 26 (87) | 0.919 |
| Not improved | 5 (17) | 5 (17) | 4 (13) | ||
| General daily activities | Improved after RT | 23 (77) | 24 (80) | 25 (83) | 0.812 |
| Not improved | 7 (23) | 6 (20) | 5 (17) | ||
Overall survival
By the median follow-up of 6.5 months, 16 patients from Arm A, 18 from Arm B, and 15 from Arm C had died. The actuarial median survival was 7 months for arms A and C, and 6 months for Arm B, with no significant differences in survival rates between the groups.
Toxicity
No patients experienced Grade 3 or higher toxicity following RT. Gastrointestinal symptoms such as nausea, abdominal pain, vomiting, diarrhea, and dysphagia were the most common. While the incidence of gastrointestinal toxicity was somewhat higher in the 20 Gy and 30 Gy arms, these differences were not statistically significant, except for skin toxicity. In fact, arm A had significantly lower rates of skin toxicity compared to arms B and C (p = 0.017). Specifically, 10% of patients in Arm A experienced skin toxicity, compared to 30% in Arm B and an equivalent proportion in Arm C, with symptoms ranging from Grade 1 to Grade 2 skin toxicity (Table 4). During follow-up, four bone fractures were recorded at irradiated sites between 7 and 24 weeks after RT: one in Arm A, two in Arm B, and one in Arm C. These fractures were unlikely to be directly radiation-related, as their timing corresponded with disease progression.
Table 4.
Commonly observed acute toxicities in different study group
| Acute toxicity | Arm A (8 Gy), n | Arm B (20 Gy), n | Arm C (30 Gy), N | p-value |
|---|---|---|---|---|
| Skin toxicity | 0.017 | |||
| Grade 1 | 3 | 9 | 12 | |
| Grade 2 | 0 | 0 | 2 | |
| Absent | 27 | 21 | 16 | |
| Nausea | 0.628 | |||
| Grade 1 | 8 | 13 | 13 | |
| Grade 2 | 3 | 2 | 3 | |
| Absent | 19 | 15 | 14 | |
| Abdominal pain | 0.373 | |||
| Grade 1 | 8 | 10 | 10 | |
| Grade 2 | 3 | 6 | 8 | |
| Absent | 19 | 14 | 12 | |
| Vomiting | 0.216 | |||
| Grade 1 | 7 | 9 | 13 | |
| Grade 2 | 3 | 6 | 6 | |
| Absent | 20 | 15 | 11 | |
| Diarrhoea | 0.486 | |||
| Grade 1 | 3 | 6 | 7 | |
| Garde 2 | 1 | 2 | 3 | |
| Absent | 26 | 22 | 20 | |
| Dysphagia | 0.343 | |||
| Grade 1 | 2 | 5 | 6 | |
| Grade 2 | 0 | 0 | 1 | |
| Absent | 27 | 23 | 21 |
Discussion
This study aimed to compare the symptomatic efficacy and tolerability of three different RT regimens for patients with vertebral metastases in a LMIC setting. The study employed a prospective, non-randomized design at the National Institute of Cancer Research and Hospital, Bangladesh, enrolling patients aged 18 to 75 years with histologically confirmed primary malignant diseases and vertebral metastasis. Patients were assigned to one of three treatment arms: 8 Gy in a single fraction, 20 Gy in 5 fractions, and 30 Gy in 10 fractions. The results demonstrated that all treatment groups achieved similar pain relief at 12 weeks post-treatment, with no significant differences in overall survival or severe toxicity rates among the groups. Notably, skin toxicity was lower in the single-fraction arm compared to the multi-fraction arms. These findings suggest that single-fraction RT is equally effective for pain relief and has a better tolerability profile, making it a viable option for treating vertebral metastases in resource-constrained settings.
Our findings align closely with those from previous studies on the efficacy of single-fraction RT for spinal metastases. Notably, a subset analysis of the North American multicenter trial (RTOG 97–14) compared 8 Gy in a single fraction with 30 Gy in 10 fractions for painful vertebral metastases. This study, the largest of this type, demonstrated that both regimens were equally effective in alleviating pain from vertebral BMs [12]. Similarly, a Canadian study, conducted in the same setting, reported comparable pain control using a single fraction of 8 Gy in 117 patients [13]. These results are further supported by the findings of Majumder et al. (2012), who observed similar outcomes in a cohort of 64 patients treated with RT for spinal metastases [14].
The safety of single-fraction RT, particularly regarding nausea or emesis when lower dorsal spines are irradiated, remains a major concern. However, it has been proven safe in several randomized controlled trials [2, 4, 6, 9]. Our study reaffirms this safety profile, as no Grade 3 or higher adverse events were observed post-RT, and gastrointestinal (GI) or genitourinary (GU) toxicities in the single-fraction group were lower, albeit not statistically significant. Interestingly, our results also revealed significantly less skin toxicity with the 8 Gy regimen (p = 0.017), with only 10% of patients experiencing Grade 1–2 skin toxicity compared to 30% and 47% in the 20 Gy and 30 Gy groups, respectively.
This finding aligns with the results from Shuja et al. [15], who reported that 11% of patients developed skin toxicity (Grade 2 or less) within 3 months after receiving a single 8 Gy fraction. The subset analysis of RTOG 97–14 also indicated significantly higher acute toxicities in multifractionated regimens compared to single-fraction RT (20% vs. 10%, p = 0.01) [12].
Collectively, these findings underscore the safety of the 8 Gy single-fraction RT, addressing concerns regarding acute toxicities and supporting its use in clinical practice for the treatment of BMs in the spine. Moreover, our study contributes to the growing body of evidence confirming the efficacy and safety of single fraction RT in treating spinal BMs [16], further challenging the existing barriers to its broader adoption, particularly in LMICs. In fact, in the latter, single-fraction RT offers several advantages, including reduced treatment time and lower patient burden, making it especially beneficial in settings where healthcare resources are limited. Despite these benefits, several factors hinder the widespread adoption of single-fraction RT. These include concerns over its efficacy for complex or larger metastatic lesions, perceived higher rates of retreatment, and clinician and patient preferences for more traditional multi-fraction regimens, supposed to be safer and more effective.
In LMICs, additional challenges such as outdated treatment guidelines, lack of training among radiation oncologists in single fractions, and cultural beliefs about radiation safety further complicate its adoption [17, 18]. Our results, which demonstrate no significant differences in efficacy or safety between single-fraction and multi-fraction RT, provide evidence that could help overcome these barriers. By showing lower skin toxicity and equivalent pain control and survival outcomes, this study supports the viability of single-fraction RT as a practical option in LMICs, aligning with the need for cost-effective and accessible cancer care solutions. This evidence should encourage the revision of guidelines and the education of clinicians and patients about the benefits of single-fraction RT, suggesting this regimen as a standard care option for spinal metastases in diverse healthcare settings. Furthermore, our study confirms the effectiveness of palliative RT of BMs, regardless of the treatment technique used, as recently reaffirmed by European guidelines [19].
This study is not without its limitations. Primarily, the non-randomized design may introduce selection bias, affecting the generalizability of the results. Additionally, the limited follow-up period restricted our ability to assess long-term outcomes and survival, which are critical in evaluating the full impact of treatment regimens. Another significant limitation was the failure to evaluate the retreatment rate, an important factor in understanding the long-term efficacy and sustainability of pain control. In fact, while our study confirms the effectiveness of single-fraction RT in providing pain relief similar to multi-fraction regimens, we recognize that the need for retreatment tends to be higher with single-fraction RT. This has been reported in previous literature, where single-fraction regimens, though equally effective for initial pain management, show a higher rate of repeat treatments compared to multi-fraction RT [20]. Future studies in our setting may benefit from a design that includes an assessment of repeat irradiation rates to comprehensively evaluate the long-term efficacy of single-fraction RT.
Another limitation of our study is the lack of specific data on baseline analgesic use and any potential reductions in NSAID or opioid intake post-treatment. Including such data could provide a more comprehensive understanding of single-fraction RT impact not only on pain relief but also on reducing dependence on analgesics, which would be valuable for future studies in this field.
Finally, in our study, the simplified single posterior field technique was chosen due to resource constraints and the need to streamline patient access to RT services. Although this approach might contribute to higher rates of skin and gastrointestinal toxicity in specific regions, it reflects the reality in many LMICs, where advanced techniques and equipment are often unavailable. Therefore, our study results highlight the potential for single-fraction RT to serve as a viable treatment option in such settings, also with limitations on technique that should be noted when interpreting toxicity outcomes.
Despite these limitations, the study has several strengths. It is one of the few studies to focus specifically on the treatment of vertebral metastases in a LMIC, providing valuable data from a setting that is often underrepresented in global cancer research. The study also benefits from a well-balanced patient distribution across treatment arms and a consistent treatment protocol, which enhances the reliability of the comparative results. Additionally, the significantly lower skin toxicity with single fraction RT and comparable pain control across regimens contribute to the ongoing debate about the optimal RT approach in treating BMs.
Conclusion
This study reaffirms the efficacy and safety of single-fraction RT, even if delivered by conventional techniques (2D), for treating spinal BMs, demonstrating comparable pain relief and lower skin toxicity compared to multiple-fraction regimens. These findings highlight the potential of single-fraction RT as a practical and effective treatment option, particularly in LMIC healthcare resources that are limited.
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Author contribution
Conception and design: AH. Research and data collection: AH. Analysis and interpretation of data: AH, EG, AGM. Manuscript writing: AH, EG, AAZ, AGM. Approval of final article: All authors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the National Institute of Cancer Research and Hospital, Dhaka, Bangladesh.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Altaf Hossain, Email: riad2005.ahr@gmail.com.
Arina A. Zamfir, Email: arinaalexandra.zamfir@aosp.bo.it
References
- 1.Jiang W, Rixiati Y, Zhao B, Li Y, Tang C, Liu J (2020) Incidence, prevalence, and outcomes of systemic malignancy with bone metastases. J Orthop Surg 28(2):2309499020915989 [DOI] [PubMed] [Google Scholar]
- 2.Harris AA, Hartsell WF (2018) Perez and Brady’s principles and practice of radiatin oncology. Palliation of Bone Metastases. 7th edition. Philadelphia: Wolters Kluwer
- 3.Barrett A, Morris S, Dobbs J, Roques T (2009) Practical radiotherapy planning. CRC Press [Google Scholar]
- 4.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S (2012) Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol 24(2):112–124 [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Hoskin P, Mitera G, Zeng L, Lutz S, Roos D, Hahn C, van der Linden Y, Hartsell W, Kumar E (2012) International Bone Metastases Consensus Working Party. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat* Oncol Biol* Phys 82(5):1730–7 [DOI] [PubMed] [Google Scholar]
- 6.De Felice F, Piccioli A, Musio D, Tombolini V (2017) The role of radiation therapy in bone metastases management. Oncotarget 8(15):25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarnold JR (1999) 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-upOn behalf of the Bone Pain Trial Working Party. Radiother Oncol 52(2):111–121 [PubMed] [Google Scholar]
- 8.Arnalot PF, Fontanals AV, Galcerán JC, Lynd F, Latiesas XS, de Dios NR, Castillejo AR, Bassols ML, Galán JL, Conejo IM, López MA (2008) Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 89(2):150–155 [DOI] [PubMed] [Google Scholar]
- 9.Chow R, Hoskin P, Schild SE, Raman S, Im J, Zhang D, Chan S, Chiu N, Chiu L, Lam H, Chow E (2019) Single vs multiple fraction palliative radiation therapy for bone metastases: cumulative meta-analysis. Radiother Oncol 1(141):56–61 [DOI] [PubMed] [Google Scholar]
- 10.Kaganda Bomboka V, Galietta E, Donati CM, Cellini F, Rossi R, Buwenge M, Wondemagegnehu T, Deressa BT, Uddin AK, Sumon MA, Vadalà M, Maltoni M, Morganti AG (2024) Assessing the effectiveness of palliative radiotherapy for painful bone metastases in low- and middle-income countries: a systematic review. J Med Imaging Radiat Oncol 68:495–504 [DOI] [PubMed] [Google Scholar]
- 11.Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 12.Howell DD, James JL, Hartsell WF, Suntharalingam M, Machtay M, Suh JH, Demas WF, Sandler HM, Kachnic LA, Berk LB (2013) Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases—equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97–14. Cancer 119(4):888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen J, Chow E, Zeng L, Zhang L, Culleton S, Holden L, Mitera G, Tsao M, Barnes E, Danjoux C, Sahgal A (2011) Palliative response and functional interference outcomes using the Brief Pain Inventory for spinal bony metastases treated with conventional radiotherapy. Clin Oncol 23(7):485–491 [DOI] [PubMed] [Google Scholar]
- 14.Majumder D, Chatterjee D, Bandyopadhyay A, Mallick SK, Sarkar SK, Majumdar A (2012) Single fraction versus multiple fraction radiotherapy for palliation of painful vertebral bone metastases: a prospective study. Indian J Palliat Care 18(3):202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuja M, Elghazaly AA, Iqbal A, Mohamed R, Marie A, Tunio MA, Aly MM, Balbaid A, Asiri M (2018) Efficacy of 8 Gy single fraction palliative radiation therapy in painful bone metastases: a single institution experience. Cureus 10(1). 10.7759/cureus.2036 [DOI] [PMC free article] [PubMed]
- 16.Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich SE, Wong R, Hahn C (2017) Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol 7(1):4–12 [DOI] [PubMed] [Google Scholar]
- 17.Sharma V, Gaye PM, Wahab SA, Ndlovu N, Ngoma T, Vanderpuye V, Sowunmi A, Kigula-Mugambe J, Jeremic B (2008) Patterns of practice of palliative radiotherapy in Africa, Part 1: Bone and brain metastases. Int J Radiat Oncol Biol Phys 70(4):1195–1201. 10.1016/j.ijrobp.2007.07.2381 [DOI] [PubMed] [Google Scholar]
- 18.Jeremic B, Vanderpuye V, Abdel-Wahab S, Gaye P, Kochbati L, Diwani M, Emwula P, Oro B, Lishimpi K, Kigula-Mugambe J, Dawotola D, Wondemagegnehu T, Nyongesa C, Oumar N, El-Omrani A, Shuman T, Langenhoven L, Fourie L (2014) Patterns of practice in palliative radiotherapy in Africa - case revisited. Clin Oncol (R Coll Radiol) 26(6):333–343. 10.1016/j.clon.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 19.van der Velden J, Willmann J, Spałek M, Oldenburger E, Brown S, Kazmierska J, Andratschke N, Menten J, van der Linden Y, Hoskin P (2022) ESTRO ACROP guidelines for external beam radiotherapy of patients with uncomplicated bone metastases. Radiother Oncol 173:197–206 [DOI] [PubMed] [Google Scholar]
- 20.Rich SE, Chow R, Raman S, Liang Zeng K, Lutz S, Lam H, Silva MF, Chow E (2018) Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol 126:547–557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.