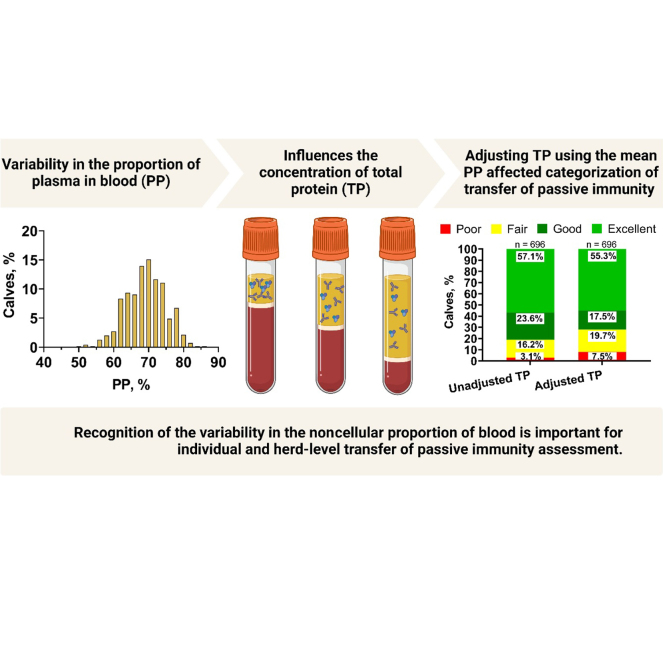
Graphical Abstract
Summary: Individual blood samples from newborn calves show variability in the noncellular proportion of blood during the time of transfer of passive immunity (TPI) assessment. Categorization of TPI using unadjusted total protein concentration (uTP) was compared with total protein concentration adjusted using the sample average proportion of plasma in blood (aTP). Adjusting total protein increased the proportion of calves with poor or fair TPI and decreased the proportion of calves with good or excellent TPI. Created with BioRender.com.
Highlights
-
•
Calves showed individual variability in the degree of hemoconcentration.
-
•
Adjusting total protein increased the proportion of calves with poor and fair TPI.
-
•
Variability in hemoconcentration should be considered when assessing TPI.
Abstract
Assessing transfer of passive immunity (TPI) is a critical management strategy to evaluate colostrum management and feeding; however, variability in hemoconcentration or serum or plasma volume in calves might influence TPI assessment. The objectives of this study were to (1) describe the variability in hemoconcentration as well as TPI in Holstein calves in New York State and (2) describe the effect of adjusting total protein (TP) for the degree of hemoconcentration by applying a sample average proportion of plasma in blood (PP) on TPI assessment. Records of TP and PP from 703 Holstein calves 1 to 9 d of age from 19 commercial dairy farms were analyzed. The PP was determined by centrifugation of microhematocrit tubes and serum and plasma TP was determined by digital refractometry. Transfer of passive immunity was categorized using unadjusted TP (uTP) as excellent = ≥6.2, good = 5.8–6.1, fair = 5.1–5.7, and poor <5.1 g/dL. Individual calf TP concentrations were adjusted to the sample average PP and TPI categories were reassessed using the adjusted TP value (aTP). The sample mean ± SD (range) PP was 68.8% ± 5.8% (50.5% to 86.0%). The PP was lower on d 1 compared with d 7 of age. Using uTP to categorize TPI, 22 (3.1%) calves had poor, 113 (16.2%) calves had fair, 164 (23.6%) calves had good, and 397 (57.1%) calves had excellent TPI, respectively. After adjusting TP for hemoconcentration, TPI determined using aTP resulted in 52 (7.5%, +4.4 percentage points) calves in poor, 137 (19.7%, +3.5 percentage points) calves in fair, 122 (17.5%, −6.1 percentage points) calves in good, and 385 (55.3%, −1.8 percentage points) calves in excellent. The mean (range) proportion of calves with TPI determined using uTP by farm was 3.9% (0% to 16%) for poor, 19.0% (2% to 36%) for fair, 25.3% (10% to 42%) for good, 51.8% (26% to 83%) for excellent. When categorized using aTP, the proportion of calves by farm was 8.1% (0% to 21%) in poor, 20.5% (8% to 42%) in fair, 19.1% (6% to 33%) in good, and 52.4% (27% to 83%) in excellent TPI. In conclusion, PP was variable in calves during the time of TPI assessment and this variability should be considered when assessing TPI at the calf- or herd level.
Neonatal calves rely on colostrum ingestion to acquire passive immunity as well as for nutrients, hormones, and bioactive factors (Fischer-Tlustos et al., 2021; Lopez and Heinrichs, 2022). Achieving adequate passive immunity has been recognized as a key management factor to reduce preweaning morbidity and mortality as well as improve ADG and increase the likelihood to reach first insemination and calving (Crannell and Abuelo, 2023; Sutter et al., 2023). Transfer of passive immunity (TPI) can be directly assessed by measuring IgG concentrations in serum or plasma or indirectly assessed using a refractive index measurement in 1- to 9-d-old calves (Wilm et al., 2018). Estimation of total protein (TP) in serum and plasma by refractometry has shown strong correlation (r = 0.74 to 0.93) with the gold standard measurement, radial immunodiffusion (Deelen et al., 2014; Elsohaby et al., 2015, 2019; McCracken et al., 2017), providing a rapid method for on-farm TPI assessment. Although the prevalence of poor TPI has decreased (USDA, 1993; Urie et al., 2018), the benefits of achieving higher blood concentrations of IgG in the calf have been emphasized (Lombard et al., 2020). Using the latest recommendation for TPI categories, Lombard et al. (2020) proposed >40%, 30%, 20%, and <10% of calves in a herd should achieve excellent, good, fair, and poor TPI (categorized as ≥6.2, 5.8 to 6.1, 5.1 to 5.7, and <5.1 g/dL unadjusted TP [uTP]), respectively. Using records from a commercial dairy farm in Michigan, Crannell and Abuelo (2023) reported TPI proportions that met the aforementioned recommendations; however, reports of the prevalence of TPI using the recommendations introduced by Lombard et al. (2020) on multiple dairy farms remain scarce to date.
Serum and plasma volume comprises approximately 9% to 10% of BW in neonatal calves (Quigley et al., 1998). Circulating blood contains a cellular component, made up mostly of red blood cells, and the plasma/serum fraction. Packed cell volume (PCV) is a quantitative measurement to determine the cellular proportion of blood and has a moderate positive correlation with dehydration (r = 0.51; R2 = 0.77) in calves (Constable et al., 1998). Variability in the proportion and volume of serum or plasma in blood of 1- to 9-d-old calves has been demonstrated (Quigley et al., 1998; Panousis et al., 2018). This variability could influence the concentration of solutes in serum and plasma including IgG and TP and therefore affect the classification of TPI. Previous authors have observed higher concentrations of TP in dehydrated calves (Walker et al., 1998; Singh et al., 2014), but our current methods to assess TPI do not consider the influence of the volume or proportion of serum/plasma in blood.
Because of the variability in the serum or plasma fraction of blood in calves, we investigated the variability in hemoconcentration in a cohort of calves assessed for TPI and considered the possible changes in interpretation of the obtained values and resulting categories of TPI success when adjusting TP by the degree of hemoconcentration determined by the proportion of plasma (PP) in blood. We hypothesized that adjusting TP would affect the individual calf TPI categorization and the proportion of calves within a TPI category. Our objectives were to (1) describe the variability in PP as well as TPI in newborn Holstein calves in New York State and (2) describe the effect of adjusting TP by applying the sample average PP on TPI assessment.
Records of TP and PCV were compiled from 703 Holstein calves aged 1 to 9 d from 19 commercial dairy farms enrolled in 2 previous studies (Molano et al., 2020; Westhoff et al., 2023). Procedures for each study were approved by the Cornell University Institutional Animal Care and Use Committee. Calf management and colostrum feeding were previously described in detail (Molano et al., 2020; Westhoff et al., 2023) and practices were representative of those used in calf research and on commercial dairy farms in New York, respectively. In brief, in the first study, 39 male and 39 female calves born at the Cornell University Ruminant Center (farm code A) were offered 2 colostrum meals. Within 2 h of birth, calves received 4 L of fresh maternal colostrum (Brix ≥21.0%) and an additional 2 L of maternal colostrum (Brix ≥21.0%; Molano et al., 2020) was fed to all calves at 12 h of age. On d 2, blood was collected by jugular venipuncture into evacuated collection tubes containing sodium heparin. Blood was placed on ice until analysis.
In the second study, Holstein female calves (n = 625) from 18 New York dairy farms (farm codes B–U) enrolled in an observational study (Westhoff et al., 2023) were selected by convenience as calves between 1 and 9 d of age present on the farm at the time of the visit. Calf management and colostrum feeding were performed according to existing farm protocols. The dairy producers reported feeding 3.78 L of maternal colostrum at first feeding (n = 18; 100%) and offering one (n = 5; 27.8%), 2 (n = 10; 55.5%), or 3 (n = 3; 16.7%) colostrum meals to heifer calves. Each farm was visited 4 times, approximately 3 mo apart and blood from calves was collected into plain serum tubes and those containing K2 EDTA. Samples containing K2 EDTA were placed on ice immediately and whole blood was allowed to clot at ambient temperature for 30 min before being placed on ice for transportation to the laboratory.
Within 12 h of collection, K2 EDTA or heparinized whole blood was centrifuged at 13,000 or 13,200 × g in microhematocrit tubes at room temperature for 5 or 13 min, respectively. Packed cell volume percentage was determined as the interface between the cellular and noncellular fractions and PP was calculated as (100 − PCV%). Serum and plasma were harvested by centrifugation at 2,300 × g at 4°C for 15 min and at 3,000 × g at 4°C for 20 min, respectively, to estimate TP using a digital refractometer (Palm Abbe, Misco). Refractometers were zero-set with distilled water and calibrated (refractometer calibration fluid, Misco) according to the manufacturer's instructions. Calves were categorized by TP into 4 categories to assess TPI according to the cut points described using uTP in serum by Lombard et al. (2020): excellent = ≥6.2, good = 5.8–6.1, fair = 5.1–5.7, poor <5.1 g/dL. To describe how consideration of hemoconcentration would affect categorization of TPI, TP was subsequently adjusted to the average sample PP using the equation where aTP = adjusted TP, uTP = individual unadjusted TP, µPP = sample mean PP, and PP = individual PP. Category of TPI was again assessed by using the aTP. A chi-squared test (JMP Pro v. 17.0.0; SAS Institute Inc.) was used to investigate the difference in TPI categorized by uTP and aTP. Mixed effects ANOVA were conducted in JMP Pro to explore differences in PP, uTP, and aTP by age of sampling. Models included the fixed effect of age and the random effects of farm and birth month. Tukey's post hoc test was used to adjust for multiple comparisons.
A total of 696 calves remained in the final dataset. Two and five calves were removed for missing data and being sampled outside of 1 to 9 d of age, respectively. Of the 696 calves, 19 (2.7%) were sampled on d 1, 164 (23.6%) on d 2, 121 (17.4%) on d 3, 92 (13.2%) on d 4, 90 (12.9%) on d 5, 67 (9.6%) on d 6, 61 (8.8%) on d 7, 49 (7.1%) on d 8, and 33 (4.7%) on d 9 of age. Sample mean ± SD (range) was 68.8% ± 5.8% (50.5% to 86.0%) for PP, 6.3 ± 0.7 (4.2 to 9.2) g/dL for uTP, and 6.4 ± 0.9 (4.0 to 9.6) g/dL for aTP (Figure 1).
Figure 1.
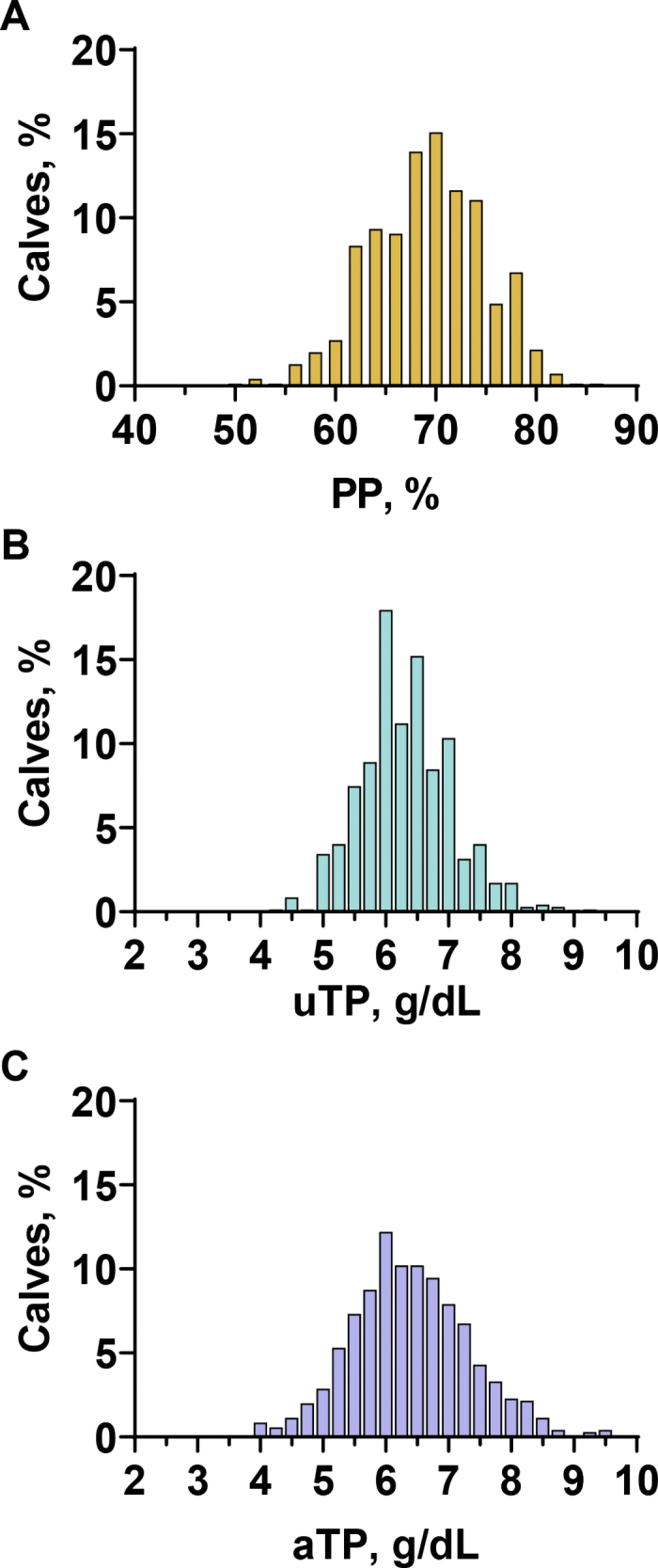
Distribution of the proportion of plasma in blood (PP; A), unadjusted total protein concentration (uTP; B), and total protein concentration adjusted to the sample mean PP (aTP; C) from 696 Holstein calves aged 1 to 9 d old from 19 commercial dairy farms.
The results for PP, uTP, and aTP by age of the calf are shown in Figure 2. Plasma proportion was associated with age (P = 0.05) such that PP on d 1 was lower than on d 7 (66.0% ± 1.4% vs. 70.7% ± 1.0%; P = 0.05), respectively. Unadjusted TP was greater on d 2 (6.6 ± 0.1 g/dL) compared with d 4 (6.1 ± 0.1 g/dL; P < 0.01), d 5 (6.1 ± 0.1 g/dL; P < 0.01), d 6 (6.1 ± 0.1 g/dL; P < 0.01), d 7 (6.2 ± 0.1 g/dL; P < 0.01), and d 9 (6.0 ± 0.1 g/dL; P < 0.01), respectively. Adjusted TP was higher on d 2 (6.6 ± 0.1 g/dL) compared with d 5 (6.2 ± 0.1 g/dL; P < 0.01) and d 6 (6.1 ± 0.1 g/dL; P = 0.01), respectively.
Figure 2.
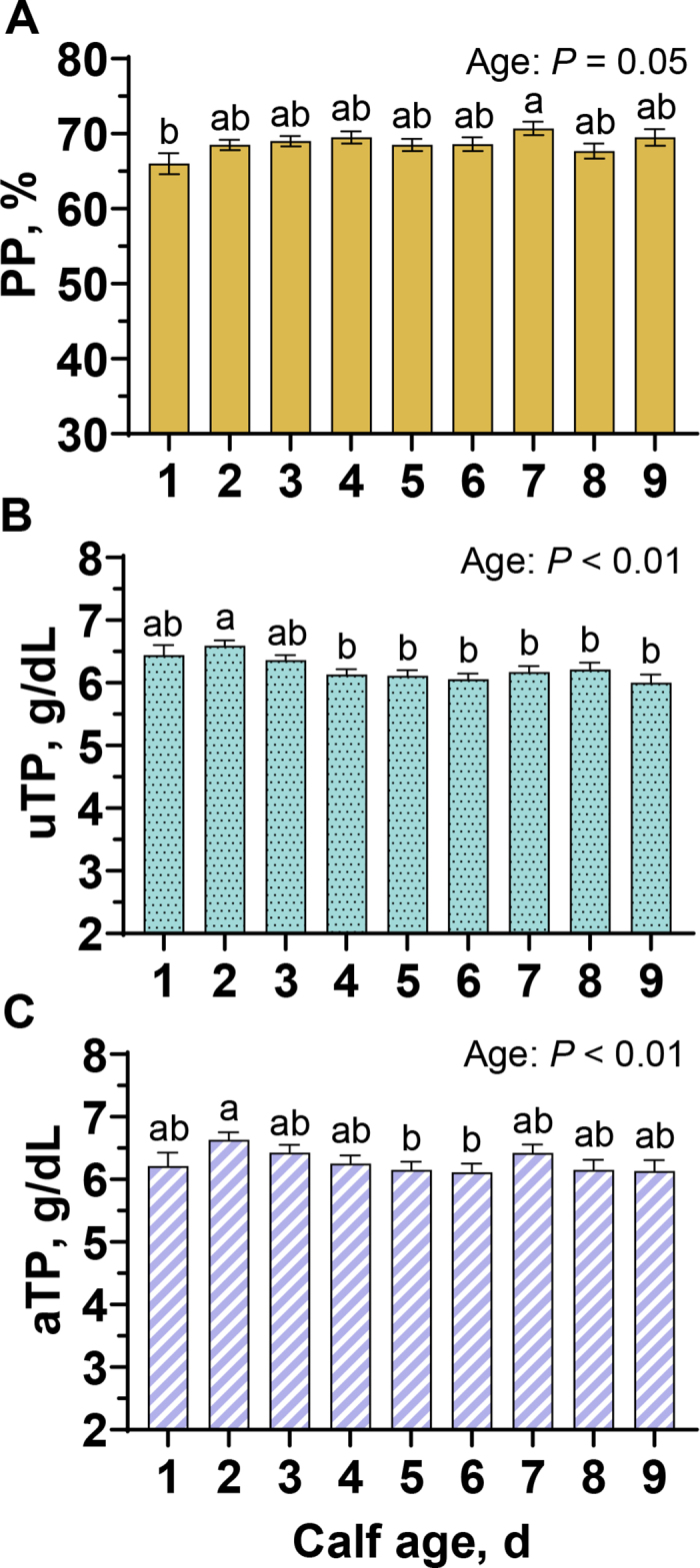
Proportion of plasma in blood (PP; A), unadjusted total protein concentration (uTP; B), and total protein concentration adjusted to the sample mean PP (aTP; C) from 696 Holstein calves aged 1 to 9 d old from 19 commercial dairy farms. Data presented as LSM ± SEM. Least squares means with different letters differ (P ≤ 0.05; Tukey's test).
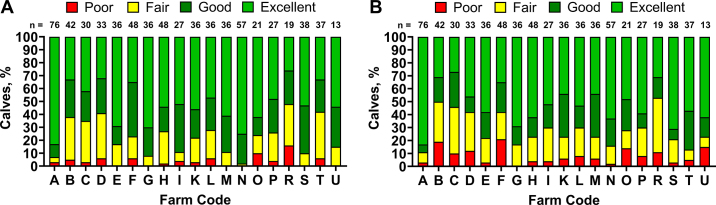
The proportion of calves in each TPI category, determined via uTP and aTP, by farm is presented in Figure 3A and 3B, respectively. When categorized using uTP, 22 (3.1%) calves had poor TPI, 113 (16.2%) calves had fair TPI, 164 (23.6%) calves had good TPI, and 397 (57.1%) calves had excellent TPI. The mean (range) proportion of calves in each TPI category, determined with uTP, by farm was 3.9% (0% to 16%) for poor, 19.0% (2% to 36%) for fair, 25.3% (10% to 42%) for good, and 51.8% (26% to 83%) for excellent. Adjusting TP with the mean PP affected categorization of TPI (P < 0.01) such that TPI determined using aTP resulted in 52 (7.5%, +4.4 percentage points) calves in poor, 137 (19.7%, +3.5 percentage points) calves in fair, 122 (17.5%, −6.1 percentage points) calves in good, and 385 (55.3%, −1.8 percentage points) calves in excellent. The mean (range) proportion of calves in each TPI category, determined with aTP, by farm was 8.1% (0% to 21%) for poor, 20.5% (8% to 42%) for fair, 19.1% (6% to 33%) for good, and 52.4% (27% to 83%) for excellent.
Figure 3.
Transfer of passive immunity classification for 696 Holstein calves aged 1 to 9 d old from 19 commercial dairy farms. Calves were categorized by their unadjusted total protein concentration (A) or total protein concentration adjusted to the sample mean plasma proportion to account for hemoconcentration (B; excellent = ≥6.2, good = 5.8–6.1, fair = 5.1–5.7, poor <5.1 g/dL; Lombard et al., 2020).
The first objective of this study was to describe the variability in PP and TPI in Holstein calves in New York State. Hematologic parameters from 1- to 9-d-old Holstein calves showed a mean ± SD (range) PP of 72.8% ± 5.6% (57.2%–87.6%) and uTP of 6.3 ± 0.9 (4.0–9.0) g/dL, and calves demonstrated a greater variability in PP compared with mature cows (Panousis et al., 2018). Previous authors have suggested that several factors such as the breed, sex, health status, and age as well as feeding volume and transportation can, in part, explain the variability in hemoconcentration. Compared with Jersey and female calves, plasma volume or PP were greater in Holstein (Quigley et al., 1998) and male calves (Raleigh and Wallace, 1962; Dillane et al., 2018; Panousis et al., 2018), respectively. Furthermore, PP was lower in diarrheic calves (56.2% ± 0.5%) compared with healthy control calves (65.9% ± 0.4%; Singh et al., 2014), likely due to dehydration. In agreement with the current study, PP was affected by age of the calf such that PP was higher at 24 and 48 h compared with at birth in beef calves raised on their dams (Adams et al., 1992) and median PP decreased from 1 to 2 d, increased from 2 to 4 d, and then decreased until 8 d in Holstein calves (Panousis et al., 2018). Moreover, variables that could affect hydration such as feeding volume and frequency as well as transportation were shown to affect average PP (Knowles et al., 1997; Bernardini et al., 2012; Jongman et al., 2020).
In recognition of the variability in PP during the time of TPI assessment, our second objective was to describe the influence of adjusting TP to the average sample PP on TPI assessment. Under the assumptions that the number and volume of red blood cells is constant, PP is a reliable method to estimate plasma volume during this dynamic period (Miki et al., 1987; Austin et al., 2011). Thus, a lower and higher PP compared with the sample mean can be interpreted as a decrease and increase in plasma volume, respectively. Because the variability in PP affects uTP concentration, misclassification bias occurred when calves were classified into TPI categories using uTP. Dehydration is a plausible explanation for a lower plasma volume in neonatal calves. In work by Constable et al. (1998), Walker et al. (1998), and Slanina et al. (1984b), calves with dehydration, induced by diuretic agents, had a lower PP and increased uTP. Moreover, uTP was greater in diarrheic calves (7.74 ± 0.08 g/dL) compared with healthy calves (7.16 ± 0.07 g/dL; Singh et al., 2014). In contrast, a relative increase in PP might be explained by anemia, a condition associated with nutrient or trace mineral deficiency during pregnancy (Abu-Ouf and Jan, 2015), or excessive blood loss during or after birth. For example, serum iron (54.1 ± 40.9 µg/100 mL) was lower in ≤3-d-old anemic calves with PCV ≤25.0% compared with calves with a PCV >25.0% (129.2 ± 62.9 µg/100 mL; Tennant et al., 1975).
The concept of adjusting hematological parameters using an indicator of hemoconcentration has been previously reported (Slanina et al., 1984a; Zhang et al., 2022), but has received little attention for the widespread assessment of TPI in neonatal calves. In Zhang et al. (2022), PCV correction in human blood samples improved the correlation between procalcitonin concentrations determined in plasma and whole blood. By adjusting TP using the mean PP in the sample of calves in the current study, we observed a reduction of calves in the excellent and good TPI categories and an increase in the proportion of calves with poor and fair TPI. Furthermore, adjustment of TP increased the number of farms (n = 7) that did not achieve the benchmark of <10% of calves with poor TPI (Lombard et al., 2020).
Our data suggest that recognition of the variability in PP during the time of TPI assessment is important for individual TPI categorization as well as herd-level monitoring. In alignment with the recommendation by Tyler et al. (1999), addressing the variability during statistical analysis by offering PCV as a covariate adjustment in multivariable models, or mathematically adjusting TP with PP, as performed here, could provide a quantitative approach to minimize the influence of hemoconcentration on TPI assessment and should be taken into consideration when assessing hematological parameters, including IgG concentration, in newborn calves. Adjustment of TPI measures by one of the aforementioned methods allows inclusion of all eligible calves in a source population and addresses the concern that exclusion of calves with obvious dehydration or low PP values likely leads to underestimation of poor TPI.
Because IgG concentration was not determined in the current study, the correlation between aTP and aIgG concentrations warrants further investigation. Furthermore, the cutpoints derived using uTP, described in Lombard et al. (2020), were used in this study to emphasize the influence of PP on TPI assessment. Comparisons of morbidity, mortality, and future performance of calves in TPI categories assessed using aTP or aIgG are needed to confirm the validity of these cutpoints for use with aTP and aIgG concentration. Since several factors affect hemoconcentration, as described above, TPI measures should be adjusted at the sample level to increase the internal validity of findings as well as improve comparison with findings from other source populations. Future research is needed to understand the variability in PP in different sample sets, regions, and management styles as well as determine whether adjustment is robust to outliers in samples with low or high mean PP. As a first step, we envision that PP variability in the study sample should be routinely reported and unadjusted and adjusted values presented to inform the reader.
Results from this study show that PP was variable during the time of TPI assessment and this variability could influence the interpretation of TPI at the individual, herd, and source population level. When categorized using uTP, 22 (3.1%) calves had poor TPI and 561 (80.7%) calves were classified as having good or excellent TPI. However, when adjusting TP for the sample mean PP, the proportion of calves with poor TPI increased by 4.4 percentage points and the proportion of calves with good or excellent TPI decreased by 7.9 percentage points. In conclusion, variability in hemoconcentration as well as serum or plasma volume should be considered when assessing individual or herd-level TPI in calves.
Notes
This work was supported in part by the New York Farm Viability Institute (Syracuse, NY).
The authors thank Erica Behling-Kelly (Cornell University, Ithaca, NY) for providing expertise in preparation of this work.
Procedures for each study were approved by the Cornell University Institutional Animal Care and Use Committee.
The authors have not stated any conflicts of interest.
Nonstandard abbreviations used: aTP = adjusted TP; PCV = packed cell volume; PP = proportion of plasma in blood; TP = total protein; TPI = transfer of passive immunity; uTP = unadjusted TP.
References
- Abu-Ouf N.M., Jan M.M. The impact of maternal iron deficiency and iron deficiency anemia on child's health. Saudi Med. J. 2015;36:146–149. doi: 10.15537/smj.2015.2.10289. 25719576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R., Garry F., Aldridge B., Holland M., Odde K. Hematologic values in newborn beef calves. Am. J. Vet. Res. 1992;53:944–950. doi: 10.2460/ajvr.1992.53.06.944. 1626785. [DOI] [PubMed] [Google Scholar]
- Austin A.W., Patterson S.M., von Känel R. Hemoconcentration and hemostasis during acute stress: Interacting and independent effects. Ann. Behav. Med. 2011;42:153–173. doi: 10.1007/s12160-011-9274-0. 21562905. [DOI] [PubMed] [Google Scholar]
- Bernardini D., Gerardi G., Peli A., Nanni Costa L., Amadori M., Segato S. The effects of different environmental conditions on thermoregulation and clinical and hematological variables in long-distance road-transported calves. J. Anim. Sci. 2012;90:1183–1191. doi: 10.2527/jas.2011-4113. 22100587. [DOI] [PubMed] [Google Scholar]
- Constable P.D., Walker P.G., Morin D.E., Foreman J.H. Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J. Am. Vet. Med. Assoc. 1998;212:991–996. doi: 10.2460/javma.1998.212.07.991. 9540870. [DOI] [PubMed] [Google Scholar]
- Crannell P., Abuelo A. Comparison of calf morbidity, mortality, and future performance across categories of passive immunity: A retrospective cohort study in a dairy herd. J. Dairy Sci. 2023;106:2729–2738. doi: 10.3168/jds.2022-22567. 36823003. [DOI] [PubMed] [Google Scholar]
- Deelen S.M., Ollivett T.L., Haines D.M., Leslie K.E. Evaluation of a Brix refractometer to estimate serum immunoglobulin G concentration in neonatal dairy calves. J. Dairy Sci. 2014;97:3838–3844. doi: 10.3168/jds.2014-7939. 24704239. [DOI] [PubMed] [Google Scholar]
- Dillane P., Krump L., Kennedy A., Sayers R.G., Sayers G.P. Establishing blood gas ranges in healthy bovine neonates differentiated by age, sex, and breed type. J. Dairy Sci. 2018;101:3205–3212. doi: 10.3168/jds.2017-13445. 29398022. [DOI] [PubMed] [Google Scholar]
- Elsohaby I., McClure J.T., Keefe G.P. Evaluation of digital and optical refractometers for assessing failure of transfer of passive immunity in dairy calves. J. Vet. Intern. Med. 2015;29:721–726. doi: 10.1111/jvim.12560. 25818225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohaby I., McClure J.T., Waite L.A., Cameron M., Heider L.C., Keefe G.P. Using serum and plasma samples to assess failure of transfer of passive immunity in dairy calves. J. Dairy Sci. 2019;102:567–577. doi: 10.3168/jds.2018-15070. 30415862. [DOI] [PubMed] [Google Scholar]
- Fischer-Tlustos A.J., Lopez A., Hare K.S., Wood K.M., Steele M.A. Invited Review: Effects of colostrum management on transfer of passive immunity and the potential role of colostral bioactive components on neonatal calf development and metabolism. Can. J. Anim. Sci. 2021;101:405–426. doi: 10.1139/cjas-2020-0149. [DOI] [Google Scholar]
- Jongman E.C., Conley M.J., Borg S., Butler K.L., Fisher A.D. The effect of milk quantity and feeding frequency on calf growth and behaviour. Anim. Prod. Sci. 2020;60:944–952. doi: 10.1071/AN19049. [DOI] [Google Scholar]
- Knowles T.G., Warriss P.D., Brown S.N., Edwards J.E., Watkins P.E., Phillips A.J. Effects on calves less than one month old of feeding or not feeding them during road transport of up to 24 hours. Vet. Rec. 1997;140:116–124. doi: 10.1136/vr.140.5.116. 9042695. [DOI] [PubMed] [Google Scholar]
- Lombard J., Urie N., Garry F., Godden S., Quigley J., Earleywine T., McGuirk S., Moore D., Branan M., Chamorro M., Smith G., Shivley C., Catherman D., Haines D., Heinrichs A.J., James R., Maas J., Sterner K. Consensus recommendations on calf-and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 2020;103:7611–7624. doi: 10.3168/jds.2019-17955. 32448583. [DOI] [PubMed] [Google Scholar]
- Lopez A.J., Heinrichs A.J. Invited review: The importance of colostrum in the newborn dairy calf. J. Dairy Sci. 2022;105:2733–2749. doi: 10.3168/jds.2020-20114. 35094859. [DOI] [PubMed] [Google Scholar]
- McCracken M.M., Morrill K.M., Fordyce A.L., Tyler H.D. Technical note: Evaluation of digital refractometers to estimate serum immunoglobulin G concentration and passive transfer in Jersey calves. J. Dairy Sci. 2017;100:8438–8442. doi: 10.3168/jds.2017-12847. 28755946. [DOI] [PubMed] [Google Scholar]
- Miki K., Itoh T., Nose H., Tanaka Y., Morimoto T. Estimation of plasma volume from hematocrit and plasma oncotic pressure during volume expansion in dogs. Jpn. J. Physiol. 1987;37:687–698. doi: 10.2170/jjphysiol.37.687. 3430873. [DOI] [PubMed] [Google Scholar]
- Molano R.A., Saito A., Luchini D.N., Van Amburgh M.E. Effects of rumen-protected methionine or methionine analogs in starter on plasma metabolites, growth, and efficiency of Holstein calves from 14 to 91 d of age. J. Dairy Sci. 2020;103:10136–10151. doi: 10.3168/jds.2020-18630. 32952015. [DOI] [PubMed] [Google Scholar]
- Panousis N., Siachos N., Kitkas G., Kalaitzakis E., Kritsepi-Konstantinou M., Valergakis G.E. Hematology reference intervals for neonatal Holstein calves. Res. Vet. Sci. 2018;118:1–10. doi: 10.1016/j.rvsc.2018.01.002. 29331737. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., 3rd, Drewry J.J., Martin K.R. Estimation of plasma volume in Holstein and Jersey calves. J. Dairy Sci. 1998;81:1308–1312. doi: 10.3168/jds.S0022-0302(98)75693-0. 9621233. [DOI] [PubMed] [Google Scholar]
- Raleigh R.J., Wallace J. The influence of iron and copper on hematologic values and on body weight of range calves. Am. J. Vet. Res. 1962;23:296–299. 14490031. [PubMed] [Google Scholar]
- Singh M., Gupta V., Mondal D., Bansal S., Sharma D., Shakya M., Gopinath D. A study on alteration in haemato-biochemical parameters in Colibacillosis affected calves. Int. J. Adv. Res. (Indore) 2014;2:746–750. [Google Scholar]
- Slanina L., Bomba A., Lehocký J., Paulík S., Batta G., Polácek M. Hemoconcentration in calves in relation to actual and corrected values of metabolic profile parameters. Vet. Med. (Praha) 1984;29:435–445. 6437048. [PubMed] [Google Scholar]
- Slanina L., Bomba A., Lehocký J., Paulík S., Polácek M., Batta G. Hemoconcentration in calves and its relation to the hematologic, protein, mineral and electrolyte profile. Vet. Med. (Praha) 1984;29:425–434. 6437047. [PubMed] [Google Scholar]
- Sutter F., Venjakob P.L., Heuwieser W., Borchardt S. Association between transfer of passive immunity, health, and performance of female dairy calves from birth to weaning. J. Dairy Sci. 2023;106:7043–7055. doi: 10.3168/jds.2022-22448. 37532624. [DOI] [PubMed] [Google Scholar]
- Tennant B., Harrold D., Reina-Guerra M., Kaneko J. Hematology of the neonatal calf. III. Frequency of congenital iron deficiency anemia. Cornell Vet. 1975;65:543–556. [PubMed] [Google Scholar]
- Tyler J.W., Parish S.M., Besser T.E., Van Metre D.C., Barrington G.M., Middleton J.R. Detection of low serum immunoglobulin concentrations in clinically ill calves. J. Vet. Intern. Med. 1999;13:40–43. doi: 10.1111/j.1939-1676.1999.tb02163.x. 10052062. [DOI] [PubMed] [Google Scholar]
- Urie N.J., Lombard J.E., Shivley C.B., Kopral C.A., Adams A.E., Earleywine T.J., Olson J.D., Garry F.B. Preweaned heifer management on US dairy operations: Part I. Descriptive characteristics of preweaned heifer raising practices. J. Dairy Sci. 2018;101:9168–9184. doi: 10.3168/jds.2017-14010. 29908815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA . USDA-Animal and Plant Health Inspection Service (APHIS), Veterinary Services, Centers for Epidemiology and Animal Health; Fort Collins, CO: 1993. Transfer of maternal immunity to calves. [Google Scholar]
- Walker P.G., Constable P.D., Morin D.E., Foreman J.H., Drackley J.K., Thurmon J.C. Comparison of hypertonic saline-dextran solution and lactated Ringer's solution for resuscitating severely dehydrated calves with diarrhea. J. Am. Vet. Med. Assoc. 1998;213:113–121. doi: 10.2460/javma.1998.213.01.113. 9656036. [DOI] [PubMed] [Google Scholar]
- Westhoff T.A., Womack S.J., Overton T.R., Ryan C.M., Mann S. Epidemiology of bovine colostrum production in New York Holstein herds: Cow, management, and environmental factors. J. Dairy Sci. 2023;106:4874–4895. doi: 10.3168/jds.2022-22447. 36567249. [DOI] [PubMed] [Google Scholar]
- Wilm J., Costa J.H.C., Neave H.W., Weary D.M., von Keyserlingk M.A.G. Technical note: Serum total protein and immunoglobulin G concentrations in neonatal dairy calves over the first 10 days of age. J. Dairy Sci. 2018;101:6430–6436. doi: 10.3168/jds.2017-13553. 29680639. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhuo H., Yang L., Wang J., Rui Z., Huang S., Wang Y., Zhang X., Huang S., Li Y. A corrective method for different hematocrit values of whole blood samples on the detection of procalcitonin. Clin. Lab. 2022;68:1702–1709. doi: 10.7754/Clin.Lab.2021.211119. 35975509. [DOI] [PubMed] [Google Scholar]