Abstract
Background
Cardiac resynchronization therapy-defibrillators (CRT-D) are devices established as treatment for symptomatic heart failure patients at risk of sudden cardiac death. Battery depletion poses a significant clinical and economic burden; extended service life may reduce costs because of generator changes and associated complications.
Objective
This study estimated cost-savings associated with extended battery longevity in Medicare patients receiving CRT-D implantation.
Methods
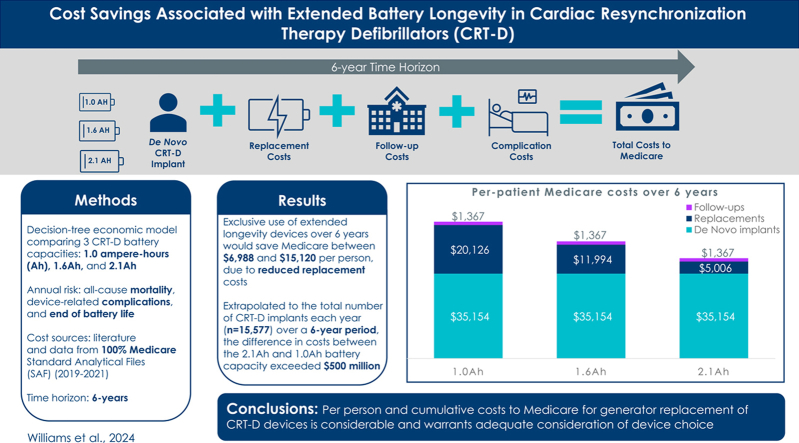
A decision tree was used to explore 3 battery capacities: 1.0 ampere-hours (Ah), 1.6Ah, and 2.1Ah. Yearly risk of all-cause mortality, device-related complications, and end of battery life were estimated. Over 6 years, estimated costs included device implantation, replacement, follow-up appointments, and complications.
Results
The average total costs to Medicare over 6 years were $41,527, $48,515, and $56,647 per person (USD 2023) for the 2.1 Ah, 1.6 Ah, and 1.0 Ah, respectively. The total per-person replacement cost for the 1.0-Ah devices was more than 4 times that of the 2.1-Ah devices ($20,126 vs $5,006). When extrapolated to the total number of CRT-D implants over a 6-year period, the difference in costs between 2.1-Ah and 1.0-Ah battery capacity exceeded $500 million.
Conclusion
Extended longevity CRT-D batteries demonstrate significant cost savings to Medicare over 6 years. These data indicate long-term economic considerations should be included in device selection.
Keywords: Implantable cardiac device, Cardiac resynchronization, Cardioverter defibrillator, Battery life, Economic cost
Graphical abstract
Key Findings.
-
▪
The average total costs to Medicare associated with the implantation of a cardiac resynchronization therapy-defibrillator (CRT-D) with 2.1-Ah battery capacity over the study period of 6 years was $41,527 per person, whereas it was $56,647 with 1.0-Ah battery capacity.
-
▪
The total per-person replacement cost for a CRT-D device that used 1.0 Ah was more than 4 times higher than that of a 2.1-Ah device ($5006 vs $20,126).
-
▪
When extrapolated to the total number of annual CRT-D implants in the Medicare population, the total cost savings of using 2.1-Ah battery capacity instead of 1.0 Ah over the 6-year study period exceeded $500 million.
Introduction
Cardiac resynchronization therapy defibrillators (CRT-D) are an established treatment for a subset of symptomatic heart failure patients at risk for sudden cardiac death.1 The service life of CRT-D poses a significant clinical and economic burden,2 and prolonged device service life is much more important than smaller generator size.3 Extended defibrillator battery longevity is preferred by patients4 and is more cost-effective for health systems.5,6 Battery capacity as measured in ampere-hours (Ah) is the strongest predictor of CRT-D battery longevity. Prior research has reported that CRT-D extended battery life exceeded patient survival in a typical heart failure cohort with reduced ejection fraction.7 Extended longevity CRT-D devices not only outlast average patient life expectancy, they also avoid costs of generator changes and associated complications.
CRT-D generator replacement procedures have elevated risks compared with initial implantation; therefore, avoiding additional procedures is a reasonable goal.8,9 Implantable cardioverter defibrillator (ICD) replacements are associated with an increased risk for pocket-related surgical reinterventions, and the need for surgical reintervention increases with every consecutive device replacement.10 One in 4 patients who undergo 2 or more replacements of a cardiovascular implantable electronic device develop infection.11 Additionally, further hospitalization is associated with increased incidence of adverse events. In a random sample of hospital admissions in Massachusetts in 2018, at least 1 adverse event was found in nearly 1 in 4 cases, and approximately one-fourth of such adverse events were preventable.12 Recent data reported a 244% increase in cost when 3 CRT-D generator implant/replacement procedures vs only 1 were performed among 15,002 Medicare patients who underwent CRT-D implant or replacement from 2009 to 2020.6 Given the increased risk of complications and additional costs associated with generator replacement, the objective of this study was to estimate the potential cost-savings associated with extended battery longevity in a cohort of Medicare patients receiving an initial CRT-D implantation.
Methods
Model structure and assumptions
A Microsoft Excel–based economic model using modeling good research practices13 was developed in the form of a decision tree to explore the potential cost-savings associated with increased battery longevity. The model was used to estimate the average costs associated with an initial CRT-D implantation and replacements per person over a 6-year follow-up from a Medicare perspective, using the model structure developed by Gadler et al.14 This model explored different battery longevities corresponding to 3 capacities to represent the leading device manufacturers available: 1.0 Ah, 1.6 Ah, and 2.1 Ah. The annual risk of all-cause death,15,16 device-related complications,17 and the end of battery life18 (based on the manufacturer’s longevity estimate) were applied. The 6-year follow-up was based on the real-world experience from a high-volume implanting institution.18 Costs for CRT-D implantation, replacement, and follow-up appointments were calculated using the 100% Medicare Standard Analytical Files (SAF) and the national 2023 Medicare payment level for specific diagnosis-related groups (DRG), Ambulatory Payment Classifications (APC), and Current Procedural Terminology (CPT®) codes. Given the Medicare payer perspective, direct cost to the facility of the device was not included. The frequency of follow-up visits was based on the recommendation from Heart Rhythm Society.19 The costs related to CRT-D–associated complications were obtained from Schmier et al,17 who used Medicare claims to calculate costs. The conditions and procedures used by Schmier et al to identify complications are described in the Supplemental Material, and costs were converted to 2023 United States Dollars (USD) using the consumer price index.20 The costs over the 6-year follow-up period were discounted at a rate of 3%, and total cost was calculated as the sum of these costs.
The assumptions applied to patients and procedures are presented in Table 1. In the base case, a patient entering the model is indicated for and undergoes implantation of a CRT-D device at the start of the model (ie, in year 0) and is followed up for 6 years. Patient survival probability is the same regardless of device choice. The model assumed device survival would be 100% for the year of implant/replacement and the following year. As a result, a maximum of 3 replacements for an individual patient could be performed over the model time horizon of 6 years, and in that worst-case scenario, replacement procedures would occur in years 2, 4, and 6.
Table 1.
Model assumptions
| Component | Assumptions |
|---|---|
| Patient |
|
| Procedure |
|
APC = ambulatory payment classification; CRT-D = cardiac resynchronization therapy defibrillator; DRG = diagnosis-related group.
In addition to a base-case analysis, a univariate deterministic sensitivity analysis was performed varying patient survival, battery survival, incidence and costs of complications, procedure costs, and the time horizon.
Base-case analysis and inputs
The input data used for the base case analysis are shown in Table 2 and include patient survival, battery survival, incidence and costs of complications, Medicare costs of CRT-D implantation and replacement, and the number and cost of follow-up visits. Annual patient survival was obtained from Yao et al,15 who performed a Markov-based Monte Carlo simulation to estimate costs associated with CRT-D therapy from a United Kingdom (UK) health care perspective. Event-free battery survival rates were obtained from Alam et al,18 who examined battery longevity of 621 CRT-D recipients at their institution. The source of incidence rates and costs of complications was the simulation by Schmier et al,17 in which both upper and lower bounds were reported (lower bounds were used for the base case). The 100% Medicare SAFs from 2019 to 2021 were used to calculate cost inputs for CRT-D procedures and follow-up visits, using appropriate DRG/APC/CPT codes and the Medicare 2023 reimbursement amount for each code. Final model inputs represent a weighted average reimbursement for that procedure or visit, in which weights reflect the relative volume of claims during 2019 to 2021 for each DRG, APC, or CPT code. Details of these calculations can be found in the Supplementalal Material.
Table 2.
Base-Case Input Parameters
| Parameter | 2.1 Ah | 1.0 Ah | 1.6 Ah | Notes |
|---|---|---|---|---|
| Patient survival | Source: Yao et al (2007)15 | |||
| Year 0 | 100% | 100% | 100% | |
| Year 1 | 95% | 95% | 95% | |
| Year 2 | 90% | 90% | 90% | |
| Year 3 | 85% | 85% | 85% | |
| Year 4 | 81% | 81% | 81% | |
| Year 5 | 77% | 77% | 77% | |
| Year 6 | 72% | 72% | 72% | |
| Event-free battery survival | Source: Alam et al (2017)18 | |||
| Year 0 | 100% | 100% | 100% | |
| Year 1 | 100% | 100% | 100% | |
| Year 2 | 98% | 99% | 100% | |
| Year 3 | 98% | 92% | 100% | |
| Year 4 | 95% | 74% | 90% | |
| Year 5 | 90% | 36% | 69% | |
| Year 6 | 77% | 10% | 44% | |
| Incidence of complication | Source: Schmier et al (2017)17 lower bound | |||
| Complication first year after primary implant | 4% | 4% | 4% | |
| Complication first year after replacement | 2% | 2% | 2% | |
| Infection first year after primary implant | 2% | 2% | 2% | |
| Infection first year after replacement | 3% | 3% | 3% | |
| Complication cost to Medicare | Source: Schmier et al. (2017)17 lower bound; 2023 USD | |||
| Complication | $1112 | $1112 | $1112 | |
| Infection | $29,550 | $29,550 | $29,550 | |
| Medicare costs of CRT-D | Sources: implantation and replacement costs: claims data (see Appendix), reflect weighted average of IP and OP costs using 2023 Medicare reimbursement amounts; visits: frequency from Wilkoff et al (2008), cost reflects weighted average of follow-up visit at facility using 2023 Medicare reimbursement amount |
|||
| Initial implantation | $34,436 | $34,436 | $34,436 | |
| Replacement | $32,123 | $32,123 | $32,123 | |
| Follow-up visit (per visit; 4 visits per year) | $82 | $82 | $82 |
CRT-D = cardiac resynchronization therapy defibrillator; USD = US dollars; IP = inpatient; OP = outpatient.
These inputs were used to calculate the average cost per patient over a 6-year period for initial implantation, replacements, and follow-up visits. These costs were summed to arrive at the total cost per patient to Medicare. To calculate the total cumulative cost of replacements among the CRT-D Medicare population, costs were summed for annual cohorts of patients who were assumed to have received initial implantations during years 0 through 6. Specifically, it was assumed that each year there were 15,577 initial implantations (which represents the average number of annual CRT-D implantations observed in the 100% Medicare SAF claims files during 2019–2021), and each annual cohort had between 0 and 6 years of follow-up costs, depending on the year they entered the model. That is, those who entered at year 0 had 6 years of follow-up costs, those who entered at year 1 had 5 years of follow-up costs, and so forth. Therefore, the total cumulative cost reflects 109,039 patients who received initial implantations between years 0 and 6 and had between 0 and 6 years of follow-up costs.
Sensitivity analysis and inputs
To perform univariate deterministic sensitivity analysis, individual inputs were varied, and the model was re-run to produce alternative cases. For 1 scenario, patient survival inputs were based on a prospective study (ALTITUDE) of patients who received ICD or CRT-D devices (Saxon et al, 2010).16 These data provide estimates for up to 5 years of follow-up; the survival probability for year 6 was derived using the trend from these estimates along with physician input. In a second scenario, the follow-up time was extended to 15 years, approximating the lifetime of the patient’s survival, using similar methods: deriving years 6 through 15 based on base-case battery and patient survival probabilities during years 0 through 5 and physician guidance. A third scenario explored the effect of using the upper bounds of complication incidence and cost estimates from Schmier et al.17 Other scenarios varied the cost to Medicare for CRT-D implantation and replacement to 20% more or 20% less of the base-case amount. The inputs used for these scenarios are shown in Appendix 2.
Results
Base case
The average total costs to Medicare associated with a 2.1-Ah CRT-D device implantation over 6 years was $41,527 per person (Table 3) in the base-case. The corresponding costs for the 1.6-Ah and 1.0-Ah devices were $48,515 and $56,647 per person, respectively. The use of a 2.1-Ah CRT-D device saved Medicare an average of $15,120 per person compared with the use of a 1.0-Ah CRT-D device, and an average of $6988 per person compared with a 1.6-Ah CRT-D device. The costs of the initial implantation, related complications, and routine follow-up visits were the same across devices; thus, the differences in total average per person costs were driven by costs associated with replacements. The total replacement cost for 1.0-Ah CRT-D devices ($20,126 per person) was more than 4 times that of 2.1-Ah devices ($5006); the total replacement cost for 1.6-Ah CRT-D devices ($11,994) was more than double that of 2.1-Ah devices.
Table 3.
Base-case analysis results
|
Cost category |
Average per-patient Medicare costs over 6-year follow-up |
Difference between 1.0 Ah and 2.1 Ah, USD (%) | Difference between 1.6Ah and 2.1Ah, USD (%) | ||
|---|---|---|---|---|---|
| 2.1 Ah | 1.0 Ah | 1.6 Ah | |||
| Initial implant | No difference by definition | No difference by definition | |||
| Procedure | $34,436 | $34,436 | $34,436 | ||
| Complications | $635 | $635 | $635 | ||
| Postprocedure follow-ups | $82 | $82 | $82 | ||
| Total | $35,154 | $35,154 | $35,154 | ||
| Replacements | |||||
| Procedures | $4856 | $19,524 | $11,635 | $14,668 (302%) | $6779 (140%) |
| Complications | $137 | $552 | $329 | $415 (302%) | $192 (140%) |
| Postprocedure follow-ups | $12 | $50 | $30 | $38 (302%) | $17 (140%) |
| Total | $5006 | $20,126 | $11,994 | $15,120 (302%) | $6988 (140%) |
| Routine follow-ups | $1367 | $1367 | $1367 | No difference by definition | No difference by definition |
| Total | $41,527 | $56,647 | $48,515 | $15,120 (36%) | $6988 (17%) |
| Cumulative cost of replacement in full Medicare population over 6 years if 15,577 CRT-D implants per year | $152,681,105 | $679,635,453 | $333,012,260 | $526,954,348 (345%) | $180,331,156 (118%) |
All values are in 2023 USD.
CRT-D = cardiac resynchronization therapy defibrillator; USD = US dollars.
Using these values and assuming 15,577 CRT-D implants annually over 6 years, the cumulative replacement cost to Medicare would be $152,681,105 for 2.1-Ah devices, $679,635,453 for 1.0-Ah devices, and $333,012,260 for 1.6-Ah devices (Table 3). The difference in cumulative replacement costs between the 2.1-Ah and 1.0-Ah devices was $526,954,348, and $180,331,156 between the 2.1-Ah and 1.6-Ah devices.
Sensitivity analysis
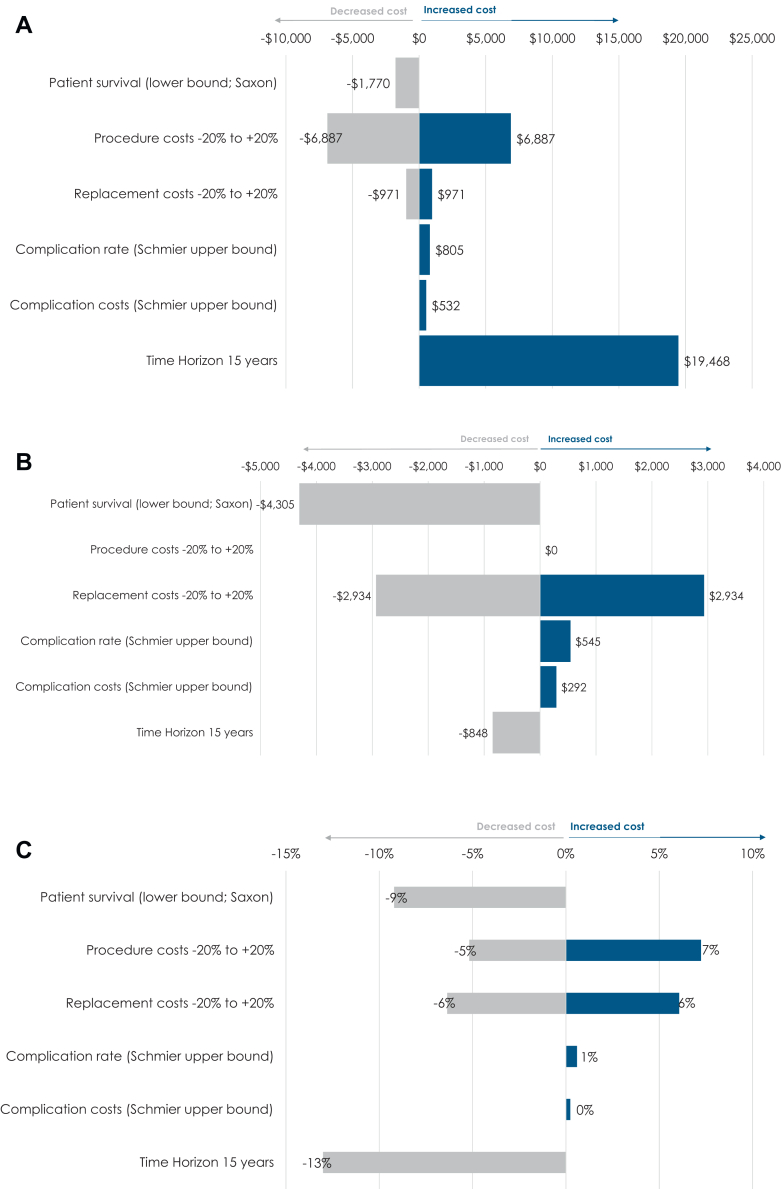
The results of the univariate deterministic sensitivity analyses produced average total costs to Medicare associated with 2.1-Ah CRT-D device implantation over 6 years per patient that ranged from $34,640 to $48,414 (Appendix 3 and Figure 1). The highest and lowest total cost estimates occurred when the procedure cost inputs were varied to 20% higher and 20% lower than the base-case values. The per-person savings to Medicare for using the 2.1-Ah CRT-D device ranged from $10,815 to $18,054 compared with the 1.0-Ah device, and $4,727 to $8,344 compared with the 1.6-Ah device.
Figure 1.
Tornado diagrams (all values in 2023 USD) for A: Average cost per patient over 6 years with 2.1-Ah battery (base: 2.1-Ah CRT-D costs $41,523). B: Dollar savings associated with 2.1-Ah battery vs 1.0-Ah battery (base: cost-saving $15,118). C: Percent savings associated with 2.1-Ah battery vs 1.0 Ah battery (base: 36%).
The average total cost per person to Medicare associated with CRT-D implantation and replacement over a 15-year time horizon was $60,994 (2.1 Ah), $67,491 (1.6 Ah), and $75,266 (1.0 Ah). That value was 11% higher for 1.6-Ah devices and 23% higher for 1.0-Ah devices compared with 2.1-Ah devices (Appendix 3).
Discussion
The data presented here demonstrate significant cost savings when extended-longevity CRT-D devices are used in Medicare patients. The cumulative cost of replacement in the Medicare cohort over 6 years was $152.7 million for the 2.1-Ah device vs $333.0 million and $679.6 million for devices with 1.6-Ah and 1.0-Ah batteries, respectively. The difference in costs between the 2.1-Ah and 1.0-Ah devices was over $527.0 million (345% higher).
CRT-D replacement because of battery depletion is a significant cost driver for payers14,21 and a significant complication driver for patients.22,23 Landolina et al21 found the need for device replacements at 6 years was reduced from 83% to 68% with the use of devices with improved battery longevity.21 Modeling has shown that increased utilization of extended-longevity CRT-D led to a 39% annual reduction in major complications and a 12.8% reduction in total annual costs ($496 million) for Medicare.24 A prior study examining 15,002 Medicare patients who underwent CRT-D implant or replacement from 2009 through 2020 reported a total cumulative cost to Medicare for a patient undergoing 1, 2, and 3 generator implant or replacement procedures to be $52,795, $88,976, and $128,846, respectively.6 These data demonstrate the substantial increased costs to Medicare when patients are subjected to repeat CRT-D generator changes that extended longevity devices may help to reduce. More importantly, longevity seems to be more important to patients than the size of the device; most prefer a larger device when it is accompanied by greater longevity.4 Guidelines should consistently emphasize the importance of patient preferences in all clinical decisions.25 The value offered by extended-longevity CRT-D led the National Institute for Health and Care Excellence, which provides guidance to the National Health Service of the United Kingdom, to conclude extended-longevity CRT-D benefits patients, are associated with fewer procedures, and save the National Health Service approximately £6 million within first 5 years of utilization.5 The Board of Medicare Trustees determined in 202326 that the Hospital Insurance trust fund is not adequately financed over the next 10 years; the program can only guarantee 8 years of paying 100% of scheduled benefits to over 65 million Americans. Incremental improvements to clinical practice that reduce complications and improve cost-effectiveness may help the financial stability of the Medicare program.
Although this study has several strengths, the results should be viewed in light of its limitations. First, the available data on input parameters such as patient survival, battery survival, complication rate, and infection rate are limited. Some of these data are from older studies, from countries other than the United States (the setting for this study), and conditional on other factors such as co-morbidity. Second, the model does not include societal costs among patients, which include transportation and opportunity costs. Third, the primary factor of interest for device replacement in this model is battery survival, and we did not consider other factors related to replacement, such as electronic component failure or device programming. Finally, the model also does not consider patient preference, although previous research suggests patients would prefer options with extended battery longevity.
Conclusions
The per-person and estimated cumulative cost to Medicare for generator replacement of CRT-D devices is substantial. The exclusive use of extended-longevity devices over 6 years would save Medicare between $15,120 and $6988 per person, and between $180 million and $527 million cumulatively. Adequate consideration of the multiple factors affecting device choice, including the economic impact of generator replacement because of battery longevity, should be a consideration in physician decision-making at the time of initial implant.
Disclosures
Ryoko Sato and Caroline M. Jacobsen are full-time employees of Boston Scientific. Jeffrey L. Williams is a Clinical Cardiac Electrophysiologist at the James A. Haley Veterans Affairs Medical Center in Tampa, FL. Jeffrey L. Williams was not compensated for his participation in this study.
Acknowledgements
The authors thank Craig Solid and Ally Pachelli for assistance in preparing this manuscript, as well as Claire Duxbury for her contributions to the model.
Funding Sources
This study was supported by Boston Scientific.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2024.09.008.
Appendix. Supplementary Data
References
- 1.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/cir.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 2.Hauser R.G. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;45:2022–2025. doi: 10.1016/j.jacc.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 3.Neuzner J. The mismatch between patient life expectancy and the service life of implantable devices in current cardioverter-defibrillator therapy: a call for larger device batteries. Clin Res Cardiol. 2015;104:456–460. doi: 10.1007/s00392-014-0807-y. [DOI] [PubMed] [Google Scholar]
- 4.Wild D.M., Fisher J.D., Kim S.G., Ferrick K.J., Gross J.N., Palma E.C. Pacemakers and implantable cardioverter defibrillators: device longevity is more important than smaller size: the patient's viewpoint. Pacing Clin Electrophysiol. 2004;27:1526–1529. doi: 10.1111/j.1540-8159.2004.00671.x. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence (NICE) Committee EnduraLife powered CRT-D devices for treating heart failure. 2021. https://www.nice.org.uk/guidance/mtg33
- 6.Williams J.L., Liu Y., Haley J.A. Costs of care in biventricular defibrillator medicare patients undergoing implant or replacement procedures. J Am Coll Cardiol. 2023;81:75. doi: 10.1016/S0735-1097(23)00519-3. 75. [DOI] [Google Scholar]
- 7.Williams J.L., Harley B., Williams G. First demonstration of cardiac resynchronization therapy defibrillator service life exceeding patient survival in a heart failure with reduced ejection fraction cohort. J Innov Card Rhythm Manag. 2020;11:4325–4332. doi: 10.19102/icrm.2020.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould P.A., Gula L.J., Champagne J., et al. Outcome of advisory implantable cardioverter-defibrillator replacement: one-year follow-up. Heart Rhythm. 2008;5:1675–1681. doi: 10.1016/j.hrthm.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds M.R., Cohen D.J., Kugelmass A.D., et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493–2497. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borleffs C.J., Thijssen J., de Bie M.K., et al. Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. Pacing Clin Electrophysiol. 2010;33:1013–1019. doi: 10.1111/j.1540-8159.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 11.Dai M., Cai C., Vaibhav V., et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol. 2019;5:1071–1080. doi: 10.1016/j.jacep.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Bates D.W., Levine D.M., Salmasian H., et al. The safety of inpatient health care. N Engl J Med. 2023;388:142–153. doi: 10.1056/NEJMsa2206117. [DOI] [PubMed] [Google Scholar]
- 13.Caro J.J., Briggs A.H., Siebert U., Kuntz K.M. ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—1. Value Health. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Gadler F., Ding Y., Verin N., et al. Economic impact of longer battery life of cardiac resynchronization therapy defibrillators in Sweden. Clinicoecon Outcomes Res. 2016;8:657–666. doi: 10.2147/CEOR.S114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao G., Freemantle N., Calvert M.J., Bryan S., Daubert J.C., Cleland J.G. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28:42–51. doi: 10.1093/eurheartj/ehl382. [DOI] [PubMed] [Google Scholar]
- 16.Saxon L.A., Hayes D.L., Gilliam F.R., et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–2367. doi: 10.1161/circulationaha.110.960633. [DOI] [PubMed] [Google Scholar]
- 17.Schmier J.K., Lau E.C., Patel J.D., Klenk J.A., Greenspon A.J. Effect of battery longevity on costs and health outcomes associated with cardiac implantable electronic devices: a Markov model-based Monte Carlo simulation. J Interv Card Electrophysiol. 2017;50:149–158. doi: 10.1007/s10840-017-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam M.B., Munir M.B., Rattan R., Adelstein E., Jain S., Saba S. Battery longevity from cardiac resynchronization therapy defibrillators: differences between manufacturers and discrepancies with published product performance reports. Europace. 2017;19:421–424. doi: 10.1093/europace/euw044. [DOI] [PubMed] [Google Scholar]
- 19.Wilkoff B.L., Auricchio A., Brugada J., et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–925. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 20.US Bureau of Labor Statistics Consumer Price Index. 2023. https://www.bls.gov/cpi/
- 21.Landolina M., Morani G., Curnis A., et al. The economic impact of battery longevity in implantable cardioverter-defibrillators for cardiac resynchronization therapy: the hospital and healthcare system perspectives. Europace. 2017;19:1349–1356. doi: 10.1093/europace/euw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole J.E., Gleva M.J., Mela T., et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/circulationaha.110.976076. [DOI] [PubMed] [Google Scholar]
- 23.Prutkin J.M., Reynolds M.R., Bao H., et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation. 2014;130:1037–1043. doi: 10.1161/CIRCULATIONAHA.114.009081. [DOI] [PubMed] [Google Scholar]
- 24.Williams J.L., Williams G.M. B-PO05-062 Modeling long-term effect of biventricular defibrillator battery capacity on major complications and costs associated with replacement procedures. Heart Rhythm. 2021;18:S396–S397. doi: 10.1016/j.hrthm.2021.06.982. [DOI] [Google Scholar]
- 25.Caverly T.J., Al-Khatib S.M., Kutner J.S., Masoudi F.A., Matlock D.D. Patient preference in the decision to place implantable cardioverter-defibrillators. Arch Intern Med. 2012;172:1104–1105. doi: 10.1001/archinternmed.2012.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.2023 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. CMS. 2023 https://www.cms.gov/oact/tr/2023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.