Abstract
Preparations of acetyl-CoA carboxylase [acetyl-CoA-carbon-dioxide ligase (ADP-forming), EC 6.4.1.2] have been obtained from the plastids of avocado (Persea americana) fruit mesocarp and from spinach (Spinacia oleracea) chloroplasts. Both preparations required bovine serum albumin, HCO3-, citrate and glycerol for stabilization. The molecular weight of the avocado enzyme was about 6.5 X 10(5) on the basis of 1 mol of biotin/mol of enzyme, the behaviour of both enzymes on gel filtration being in accord with such a value. Removal of the stabilizing bovine serum albumin resulted in the loss of a biotin-containing fragment from the avocado enzyme. Citrate stabilized the enzyme at 10 mM and activated it optimally at 3.0 mM, effecting an approx. 2-fold increase in Vmax. It is suggested that in vivo the enzyme may be located within the chloroplast lamellae.
Full text
PDF






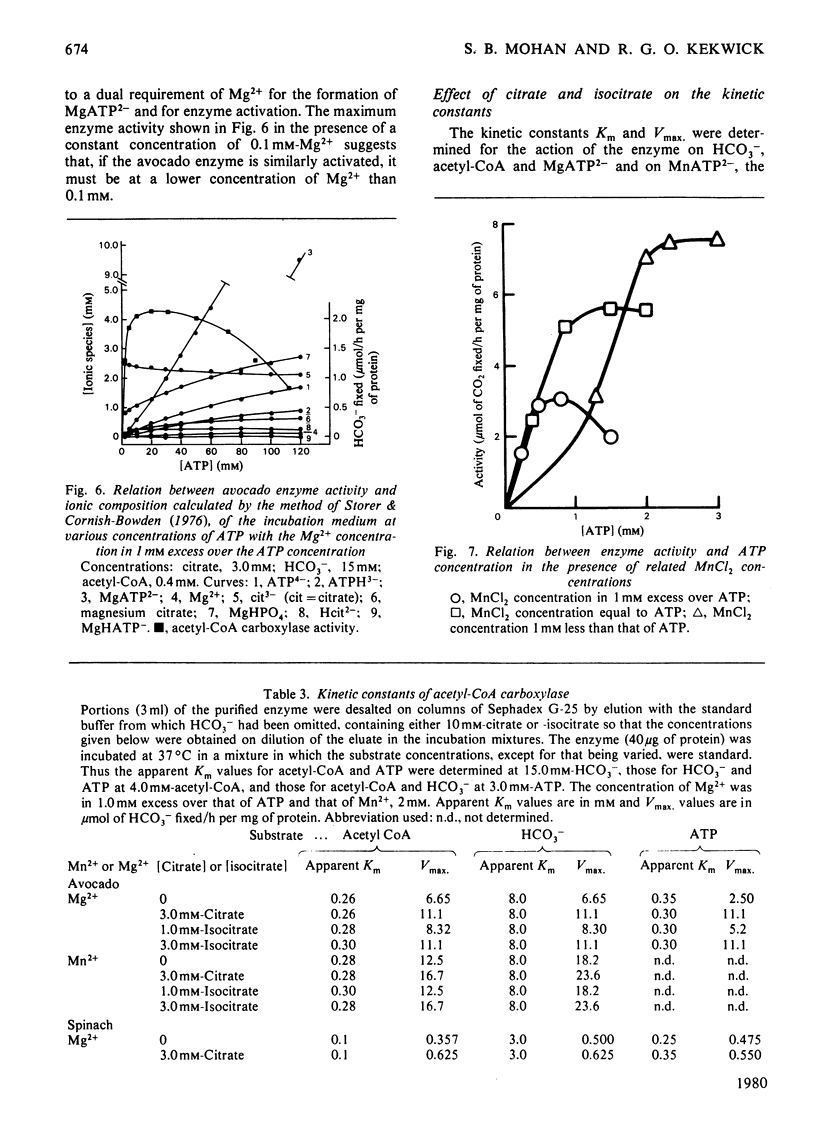


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Hsu M. P. The effects of palmitylcarnitine on hepatic fatty acid synthesis and on acetyl CoA carboxylase activity. Biochem Biophys Res Commun. 1966 Mar 22;22(6):737–743. doi: 10.1016/0006-291x(66)90210-5. [DOI] [PubMed] [Google Scholar]
- GOULD N. R., LIENER I. E. REACTION OF FICIN WITH DIISOPROPYLPHOSPHOROFLUORIDATE. EVIDENCE FOR A CONTAMINATING INHIBITOR. Biochemistry. 1965 Jan;4:90–98. doi: 10.1021/bi00877a016. [DOI] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- HATCH M. D., STUMPF P. K. Fat metabolism in higher plants. XVI. Acetyl coenzyme A carboxylase and acyl coenzyme A-malonyl coenzyme A transcarboxylase from wheat germ. J Biol Chem. 1961 Nov;236:2879–2885. [PubMed] [Google Scholar]
- Heinstein P. F., Stumpf P. K. Fat metabolism in higher plants. 38. Properties of wheat germ acetyl coenzyme A carboxylase. J Biol Chem. 1969 Oct 10;244(19):5374–5381. [PubMed] [Google Scholar]
- Kannangara C. G., Jensen C. J. Biotin carboxyl carrier protein in barley chloroplast membranes. Eur J Biochem. 1975 May;54(1):25–30. doi: 10.1111/j.1432-1033.1975.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Levy H. R. Rat mammary acetyl coenzyme A carboxylase. I. Isolation and characterization. J Biol Chem. 1969 May 10;244(9):2334–2342. [PubMed] [Google Scholar]
- Moss J., Yamagishi M., Kleinschmidt A. K., Lane M. D. Acetyl coenzyme A carboxylase. Purification and properties of the bovine adipose tissue enzyme. Biochemistry. 1972 Sep 26;11(20):3779–3786. doi: 10.1021/bi00770a017. [DOI] [PubMed] [Google Scholar]
- Nielsen N. C., Adee A., Stumpf P. K. Fat metabolism in higher plants. Further characterization of wheat germ acetyl coenzyme A carboxylase. Arch Biochem Biophys. 1979 Feb;192(2):446–456. doi: 10.1016/0003-9861(79)90114-0. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- RABIN B. R., TROWN P. W. INHIBITION OF CARBOXYDISMUTASE BY IODOACETAMIDE. Proc Natl Acad Sci U S A. 1964 Mar;51:497–501. doi: 10.1073/pnas.51.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem J. 1976 Oct 1;159(1):7–14. doi: 10.1042/bj1590007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The fractionation of the fatty acid synthetase activities of avocado mesocarp plastids. Biochem J. 1975 Feb;146(2):439–445. doi: 10.1042/bj1460439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]