Abstract
Objective:
The main objective was to assess the therapeutic efficacy of selenium alone versus a combination of myo-inositol and selenium (MI + Se) in treating patients with autoimmune thyroiditis (AIT). The study aims to determine which treatment option is more effective in restoring euthyroid state, as indicated by changes in thyroid-stimulating hormone (TSH), T3, T4, thyroid peroxidase antibodies (TPOAb), and thyroglobulin antibodies (TgAb)
Methods:
Google Scholar and PubMed databases were searched for randomized controlled trials (RCTs) and observational studies that reported outcomes of combined treatment (MI + Se) in restoring a euthyroid state, specifically comparing it with selenium-only (Se-only) treatment. Changes in TSH, T3, T4, TPOAb, and TgAb levels from baseline were defined as indicators to compare the effect of combined versus selenium-only treatment in restoring euthyroid levels. The Cochrane risk of bias tool and Newcastle Ottawa Scale were used to assess the quality of the randomized control trials included in the study. Review Manager (version 5.4, Nordic Cochrane Centre, Copenhagen, Denmark) was used for statistical analysis.
Result:
We pooled three studies, enrolling 151 participants in the MI + Se group and 137 participants in the Se group. Supplementation of Se with MI demonstrated a significant reduction in TSH levels compared to Se alone (SMD = −1.15, 95% CI: −1.60 to −0.69, P < .00001). MI + Se treatment also significantly reduced TgAb levels compared to Se (SMD = −0.51, 95% CI: −0.78 to −0.24, P = .0002). In contrast, TPOAB, T3 and T4 levels were non-significantly reduced from baseline in patients treated with MI + Se when compared to Se alone (SMD = −0.81, 95% CI: −0.44 to 0.09, P = .20), (SMD = 0.16, 95% CI: −0.09 to 0.42, P = .22), and (SMD = 0.30, 95% CI: −0.23 to 0.83, P = .26) respectively.
Conclusion:
Supplementation of Se with MI showed a significant reduction in TSH and TgAb levels compared to selenium-only treatment, with a non-significant reduction in TPOAB, T3, and T4 levels. This entails the need for powered clinical trials and observational studies with longer follow-ups to critically assess the role of combined therapy in restoring euthyroid state in patients with AIT.
Keywords: Autoimmune thyroiditis (AIT), selenium, myo-inositol, myo-inositol and selenium, randomized controlled trials (RCTs), observational study, meta-analysis
Plain Language Summary
Summary of Role of Supplementation with Selenium and Myo-inositol vs. Selenium alone in patients of Autoimmune Thyroiditis
The study aimed to determine whether taking the supplementation therapy of myo-inositol and selenium (MI+Se) together is more effective than taking selenium alone for people suffering from autoimmune thyroiditis, a condition in which the immune system attacks the thyroid gland and often leads to hypothyroidism. Researchers reviewed relevant studies from Google Scholar and PubMed, focusing on randomized control trials (RCTs) and observational studies that compared the effects of selenium alone versus selenium plus myo-inositol. They analyzed changes in thyroid-related blood markers TSH, T3, T4, TPOAb, and TgAb levels) to assess effectiveness of the given therapy. The study included three RCTs with 288 participants that were assessed through the supplementation therapy of selenium alone and myo-inositol plus selenium (MI+Se). Findings suggest that the combination of selenium and myo-inositol significantly reduced TSH levels, which indicates thyroid activity and improvement in the condition, more than selenium alone. It also lowered TgAb levels, antibodies that attack the thyroid, more effectively than selenium alone. However, the two groups had no significant changes in other markers like TPOAb, T3, and T4. The results suggest that the combination treatment might be more effective in reducing harmful antibodies and improving thyroid function compared to selenium alone. This finding is particularly relevant for managing AIT and improving thyroid health, pointing to potentially better treatment strategies. Nevertheless, the study emphasizes the need for more extensive and longer-term research to confirm these benefits and fully understand the implications. This research highlights the promise of combining supplementation with selenium and myo-inositol as a more effective approach for people with autoimmune thyroiditis (AIT). This could lead to improved management and outcomes for this common thyroid condition.
Introduction
Autoimmune thyroiditis (AIT) implies a group of conditions in which the thyroid gland is inflamed secondary to thyroid autoantibodies, resulting in either hypothyroidism or hyperthyroidism. Among these conditions, Hashimoto’s thyroiditis (HT), also called chronic AIT and lymphocytic thyroiditis, is one of the most prevalent types. It is characterized by the gradual destruction of the thyroid gland via the body’s own immune response. With a global prevalence of approximately 10%-12% and a higher impact on females compared to males, this disorder is widely recognized as the leading factor behind hypothyroidism.1,2 Hashimoto’s thyroiditis is associated with many complications, including thyroid carcinoma. 3 Therefore, prompt treatment is very crucial. Currently, thyroid hormone replacement therapy, specifically levothyroxine, also known as L-thyroxine, is considered the standard treatment for AIT. 4 This medication is a synthetic form of the thyroid hormone thyroxine, typically administered orally for lifelong treatment. In addition to this, a randomized, placebo-controlled prospective study indicated that simultaneous administration of selenium alongside L-thyroxine can serve as a potentially effective complementary treatment for AIT. 5
Selenium is an essential trace element that plays a critical role in supporting the activity of selenium-dependent enzymes with antioxidant and anti-inflammatory properties. It also helps to potentially enhance immune response and provide additional support for thyroid health. 6 Therefore, even mild selenium deficiency may contribute to the development of autoimmune thyroid diseases. Moreover, Myo-inositol supplemented with selenium is fruitful in curing HT. Myo-inositol acts via second messenger pathways and regulates H2O2-mediated iodination of thyroglobulin. 7 Various studies suggest that selenium in combination with Myo-inositol, can restore the euthyroid state in Hashimoto’s patients. 8 However, no prior meta-analysis has been conducted to evaluate the role of combination treatment in the restoration of euthyroid levels, particularly for treatment for AIT. Therefore, we aim to assess the efficacy of Myo-Inositol in conjunction with Selenium for the treatment of AIT by pooling evidence from three distinct studies.
Methodology
This meta-analysis was carried out following the guidelines set by the Cochrane Collaboration and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 9 and AMSTAR guidelines. 10 This study did not require an institutional board review (IRB) because the data was publicly available. This meta-analysis has been registered with Prospero ID: CRD42024500545
Search strategy
Two independent authors (A.T.S and N.S) conducted the literature search using PubMed and Google Scholar from inception till 2020 for randomized controlled trials (RCT) and observational studies reporting the outcome with Myo inositol plus selenium supplements treatment in restoring euthyroid state compared with selenium only. We used a snowballing strategy and checked the reference lists of eligible articles to ensure no relevant articles were missed. The search strategy for each database is presented in Supplementary Table 1. The keywords used for creating the search string were autoimmune thyroiditis (AIT), autoimmune thyroidopathy, Hashimoto thyroiditis, subclinical hypothyroidism cases and treatment, selenium therapy, and myo-inositol supplementation.
Additionally, the reference lists of all included studies were manually examined to identify any supplementary studies. Two authors (A.B. and V.Z) independently reviewed and extracted the data according to pre-established search criteria and conducted quality assessments. A third review author (A.A.) was involved in resolving any discrepancies.
Eligibility criteria
The following criteria were used to determine which studies would be included in this meta-analysis:
(1) Both RCTs and observational studies were considered.
(2) Studies that compared MI + Se and Se only groups.
(3) Participants had to have AIT.
(4) The studies had to evaluate any of the predetermined outcomes.
(5) The articles had to be written in English.
Excluded from consideration were studies that:
(1) Lacked a control group.
(2) Had overlapping study populations.
(3) Involved non-human subjects.
(4) Which were not available in full texts.
Data extraction and quality assessment
Two independent reviewers (A.T.S and V.Z) thoroughly reviewed the publications, and selected the trials that satisfied the inclusion criteria. The decision to include/exclude an article was solely hierarchical, with an initial inquiry of databases for title and abstract, followed by a review of the full-text search strategy for each database, presented in Supplementary Table 1. Additionally, the reference lists of all included studies were manually examined to identify any supplementary studies. Two authors (A.S and M.H) independently reviewed and extracted the data according to pre-established search criteria and conducted quality assessments. A third review author (A.K) was involved in resolving any discrepancies.
A review of each article was done to determine relevance. Study characteristics and outcomes were abstracted from the finalized trials. Because this analysis includes randomized controlled trials (RCTs), the Cochrane risk of bias tool 11 was utilized to evaluate the studies’ quality. Several aspects of bias were assessed, including selection bias, performance bias, attrition bias, and reporting bias. The New Ottawa Scale 12 (NOS) focuses on the choice of study subjects (four items), comparability between groups (two items), and outcome (three items applicable to cohort studies). The NOS score of each study varied from 0 to 9 points (1 point for each item, points 9 in total). An original study with a NOS score ⩾6 points was considered “high quality.” A score of less than six was considered “high risk.” The quality assessment is summarized in Supplementary Tables 2a and 2b.
Statistical analysis
All statistical analyses were performed using Review Manager (Version 5.4, Nordic Cochrane Centre, Copenhagen, Denmark). The evidence from trials was pooled using a random effect model and recorded as standard mean difference (SMD) with 95% confidence intervals (CIs). We pooled outcomes with ⩾2 studies and visualized them using forest plots. Higgins I2 was used to assess study heterogeneity, and I2 values less than 50% were considered acceptable.12,13 A P value of less than .05 was considered significant in all cases.
Results
Literature search results
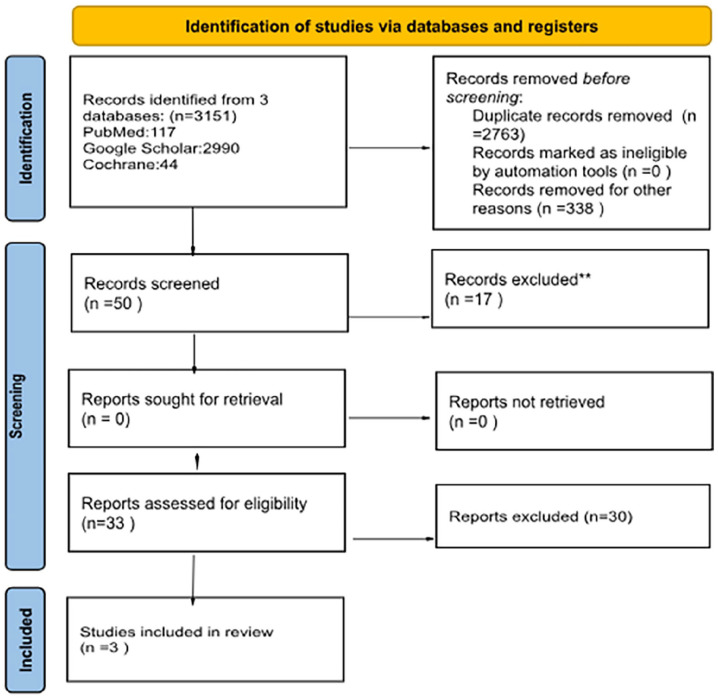
After a thorough search, 50 articles were identified, of which 17 were excluded after reading their title and abstracts. A full-text review was conducted, after which 30 articles were excluded. A total of 3 articles met our inclusion criteria.8,14,15 Thus, the selection process is summarized in the PRISMA flow diagram (Figure 1).
Figure 1.
PRISMA flow diagram.
Study characteristics and quality assessment
The included trials randomly assigned 288 patients of AIT, with 151 people in the intervention group given Myo-inositol plus Selenium (MI + Se) and 137 in the control group given Selenium (Se) only. Overall, the studies continued for six months, during which the patients were carefully followed up for T3, T4, TSH, TPOAb, and TgAb levels after receiving the combination therapy or monotherapy. The characteristics of the trials are highlighted in Table 1 evaluating the effects of myo-inositol (MI) and selenium (Se) on individuals with autoimmune thyroiditis (AIT) using different formulations and dosages. A trial as well as retrospective study was conducted by Nordio (2013) and Pace (2020) in which 600 mg of myo-inositol and 83 μg of selenium were given to intervention group (MI + Se) whereas 83 μg of selenium were given to the control group (Se-only). Another trial was conducted by Nordio (2017) in which 600 mg of myo-inositol plus 16.6 mg of selenium were given to the intervention group (MI + Se) whereas 16.6 mg of selenium was given to the control group (Se-only). The objective of this formulation was to utilize the immune-boosting and antioxidant characteristics of selenium in conjunction with the myo-inositol (MI + Se). The studies’ consistent dosages and formulations support the validity of the combination therapy’s reported effects on TSH and TgAb levels and help to ensure the comparability of the interventions, thereby highlighting the potential clinical benefits of this combined approach in addressing AIT. No comorbidities were reported in any of the above mentioned studies. However, the Cochrane risk of bias assessment tool was used to assess the risk of bias in each study. The bias for one study was considered to be “low risk” for all the components on a scale. 7 The other study only mentioned the word “randomization” but did not explain it; thus, its bias was considered “some concerns.” 14 The quality of the observational study 15 was assessed through the Newcastle-Ottawa-scale tool. The study received eight scores, which is considered “high quality.”
Table 1.
Study and patient characteristics.
| Study (year) | Location | Study Type | Intervention | Control | Sample size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sample size | Intervention (Group B) | Control (Group A) | Mean Age | Comorbidities | |||||||||
| Total | No. of males | No. of females | Total | No. of males | No. of females | ||||||||
| Maurizio Nordio and Raffaella Pajalich (2013) | Italy | RCT | 600 mg Myoinositol plus 83 μg selenomethionine | 83 μg Selenomethionine | 48 | 24 | 0 | 24 | 24 | 0 | 24 | 38 | None |
| M Nordio and S Basciani (2017) | Italy | RCT | 600 mg Myoinositol plus 16.6 mg L-selenomethionine | 16.6 mg L-selenomethionine | 168 | 84 | 9 | 75 | 84 | 10 | 74 | 40.85 | None |
| Cinzia Pace, Dario Tumino (2020) | Italy | Observational (Retrospective Study) | 600 mg Myoinositol plus 83 μg L-selenomethionine | 83 μg L-Selenomethionine | 72 | 43 | 7 | 36 | 29 | 5 | 24 | 44.05 | None |
TSH levels
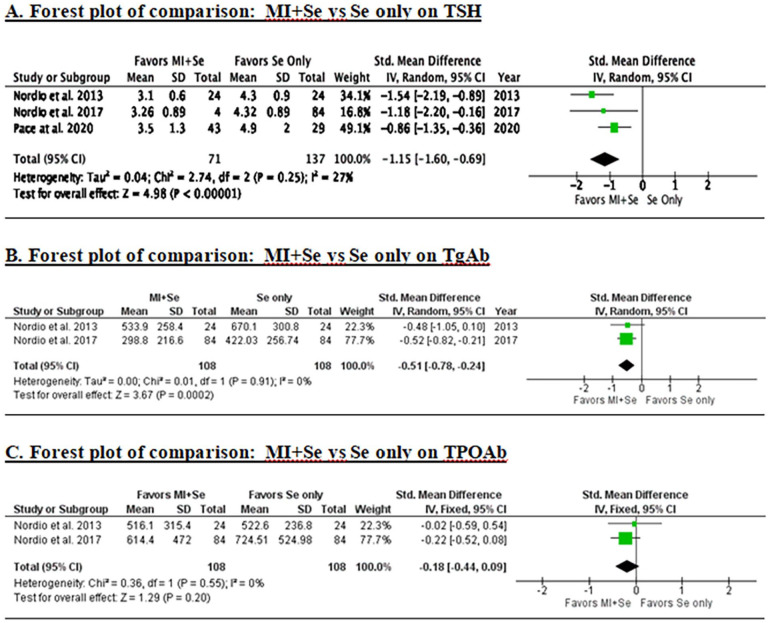
Changes in TSH levels from baseline were assessed by three studies enrolling 151 participants in the MI + Se group and 137 participants in the Se alone group. The pooled analysis showed that participants who received combination treatment with MI + Se had a significant reduction in TSH levels when compared to those who received monotherapy with Se (SMD = −1.15, 95% CI: −1.60 to −0.69, P < .00001; Figure 2A).
Figure 2.
Changes in TSH, TgAb, TPOAb, T3 and T4 levels following MI + Se therapy versus Se alone.
TgAb levels
Changes in TgAb levels from baseline were assessed in two out of the three studies, enrolling 108 participants in the MI + Se group and 108 participants in the Se group only. The pooled analysis showed that the participants who received combination treatment with MI + Se had significantly reduced TgAb levels compared to monotherapy with Se (SMD = −0.51, 95% CI: −0.78 to −0.24, P = .0002; Figure 2B).
TPOAb
Changes in TPOAb levels from baseline were assessed in two out of three studies, enrolling 108 participants in the MI + Se group and 108 participants in the Se group only. The pooled analysis showed that the participants who received combination treatment with MI + Se did not significantly reduce TPOAb levels compared to monotherapy with Se only (SMD = −0.81, 95% CI: −0.44 to 0.09, P = .20; Figure 2C).
T3 levels
Changes in T3 levels from baseline were assessed in two out of three studies, enrolling 127 participants in the MI + Se group and 113 participants in the Se group only. The pooled analysis showed that the participants who received combination therapy with MI + Se had no significant effect on T3 levels compared to monotherapy (Se only). (SMD = 0.16, 95% CI: −0.09 to 0.42, P = .22; Figure 2D).
T4 levels
Changes in T4 levels from baseline were assessed in two out of three studies, enrolling 127 participants in the MI + Se group and 113 participants in the Se group only. The pooled analysis showed that the participants who received combination therapy with MI + Se had no significant effect on T3 levels compared to monotherapy Se only. (SMD = 0.30, 95% CI: −0.23 to 0.83, P = .26; Figure 2E).
We observed clinically significant changes in TSH and TgAb levels in patients with autoimmune thyroiditis (AIT) who were supplemented with myo-inositol plus selenium (MI + Se) as opposed to selenium (Se) alone. When compared to Se alone, the combination of MI and Se significantly lowered TSH levels, suggesting either better thyroid function or a precise restoration of a euthyroid condition. This decrease is important as high TSH levels are a sign of hypothyroidism whereas low TSH can improve quality of life by reducing symptoms including depression, weight gain, and fatigue. Furthermore, the combination therapy (MI + Se) markedly decreased TgAb levels, indicating a reduction in autoimmune activity directed against the thyroid gland, which may eventually stabilize or enhance thyroid function. Less thyroid-related symptoms and improved general health may result from these adjustments. Therefore, the TSH and TgAb levels that have been reported to have improved are probably going to have a positive impact on patients’ everyday functioning, energy levels, mood, and general physical health. However, further long-term research is required to completely comprehend the influence on diverse thyroid-related results and validate the durability of these advantages.
Discussion
In this systematic review and meta-analysis, we found significantly reduced levels of TSH and TgAb in response to therapy with MI + Se as compared to exclusive Se therapy in patients suffering from subclinical hypothyroidism due to AIT. Meanwhile, neither group observed significant changes in the TPOAb, fT3, and fT4 levels. To the best of our knowledge, our study is the first meta-analysis of its kind and it provides compelling evidence regarding the efficacy of MI + Se therapy in achieving a euthyroid state in patients suffering from AIT, which is consistent with the findings reported by other RCTs.8,14
Our meta-analysis found that TSH levels were significantly reduced in patients who were given oral MI + Se versus those who received only Se to enable restoring the euthyroid state. One possible explanation for these findings is that MI regulates TSH levels by the second messenger pathway which controls its release and increases TSH sensitivity helping in the treatment of hypothyroidism. 16 MI is the most abundant stereoisomer of the inositol family, existing in 9 different forms, out of which MI is the most prevalent type in eukaryotic cells. Dietary items rich in Phytates (IP6), such as fresh fruits, vegetables, dried nuts, beans, and cereals, are some of the best sources of MI. 17 It is a precursor of Phosphatidylinositol 4, 5-bisphosphate (PIP2), which is a precursor of Inositol triphosphate (IP3). Apart from that, IP3 is a second messenger of several hormones, including TSH. Through its second messenger pathway, MI leads to the production of H2O2, which is required to synthesize thyroid hormones. 18 Therefore, depletion of MI or abnormalities in the Inositol-dependent TSH signaling cascade can lead to the development of TSH resistance and hypothyroidism, and treatment with MI may raise the levels of the second messenger and improve TSH sensitivity, thus treating the hypothyroidism. 19
We also observed a significant reduction in TgAb levels of those who received MI + Se compared to Se-only. This finding may be explained by the immune-modulatory role of MI and Se by reducing the activity of regulatory T cells and preventing apoptosis. 16 Clinical studies have exhibited that after treatment with MI + Se, there was a decline in anti-thyroid autoantibodies as well as CXCL10 (an inflammatory chemokine), supporting the role of MI in immune modulation. 19
The role of Se in controlling thyroid activity can be estimated by the fact that out of all human organs, the thyroid gland contains the most Se g/tissue 17 in the form of Selenomethionine, Se-methylselenocysteine (MSC), Methylselenol, Selenite, or is assimilated as Selenocysteine into Selenoproteins. 16 The levels of Se (a micronutrient) depend on population characteristics, diet, and soil composition. Se is necessary for the synthesis of deiodinases (DIOs) which result in the deiodination of thyroxine (T4), converting it into its active form, T3. 17 Sufficient intake of Se also helps in the breakdown of excess H2O2, by enhancing plasma glutathione peroxidase (GPX) and thioredoxin reductase (TR) levels, which give thyrocytes protection from peroxidases. 16 Selenium plays an essential role in reducing inflammation by decreasing cytokine secretion. It also prevents follicular cell apoptosis by increasing CD4+/CD25 FOXP3 as well as the activity of regulatory T cells. 16 However, we found no significant changes in either group in our analysis of TPOAb, fT3, and fT4 levels. This may be due to inadequate data, so more studies that analyze various outcomes, such as TPOAb, fT3, and fT4 levels, are required to confirm our findings. Nonetheless, in subclinical hypothyroidism, the levels of T3 and T4 hormones still remain unaltered.
| Pre-treatment lab values | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TSH levels | Free T4 | Free T3 | TgAb | TPOAb | |||||
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| 4.43 ± 0.89 | 4.33 ± 0.91 | 18.87 ± 4.02 | 18.75 ± 3.89 | 6.49 ± 2.36 | 6.60 ± 1.66 | 1019.74 ± 374.21 | 1080.80 ± 485.1 | 913.9 ± 543.9 | 905.6 ± 401.6 |
| 4.22 ± 0.6 | 4.32 ± 0.86 | 0.93 ± 0.15 | 0.9 ± 0.25 | 2.71 ± 0.44 | 2.73 ± 0.35 | 355 ± 220.5 | 415.43 ± 275.89 | 733.7 ± 485.8 | 820.13 ± 513.99 |
| 4.7 ± 1.2 | 4.5 ± 1.6 | 1.0 ± 0.2 | 1.0 ± 0.2 | 2.8 ± 0.6 | 2.6 ± 0.7 | 110 (51-394) | 152 (54-490) | 228 (68-645) | 172 (80-600) |
Limitations
One notable limitation of this study is the unavailability of individual patient data, which renders this meta-analysis reliant upon the data considered pertinent by the respective authors of the incorporated studies. The consequence of this constraint is a lack of precision in negating the potential influence of other confounding factors on the observed significance of results, which may explain the heightened heterogeneity (I2 = 72%) in one of our analyzed outcomes (fT4 levels). Included studies have different study durations and treatment dosages along with difference in study design; retrospective cohort versus randomized control trials (RCTs) which contributes to heterogeneity. Moreover, the observational study by pace et al. being a retrospective cohort may have had recall bias which could be a cause of heterogeneity. The RCTs were not controlled for confounding bias such as history of medications, radiation therapy, congenital thyroid disorders, pregnancy or iodine deficiency which could have led to variation in results. Furthermore, the restricted inclusion of only two studies for assessing fT4 levels precluded the execution of a sensitivity analysis, exacerbating the limitations imposed by data scarcity.
A key avenue for future RCTs lies in the imperative evaluation of the efficacy of combining MI with Se and in assessing the impact of this therapeutic approach on fT3, fT4, and TPOAb. Presently, the existing literature remains insufficient to reach any decisive conclusions, and additional RCTs, encompassing a larger and more diverse cohort, are indispensable to systematically evaluate and corroborate the outcomes observed in this study, thereby advancing the understanding of the potential benefits and limitations associated with the combined use of MI and Se in thyroid disorders.
Conclusion
This systematic review and meta-analysis provide strong evidence that combined therapy with MI and Se significantly reduces TSH and TgAb levels in patients with subclinical hypothyroidism due to autoimmune thyroiditis, compared to Se-only therapy. These findings suggest that MI, through its role in the second messenger pathway and TSH regulation, enhances TSH sensitivity and thereby aids in restoring the euthyroid state. Additionally, the immune-modulatory properties of MI and Se contribute to the observed reduction in TgAb levels. While our study did not find significant changes in TPOAb, fT3, and fT4 levels, this may be attributed to insufficient data, indicating a need for further research. Overall, this study underscores the potential of MI + Se therapy as an effective treatment strategy for AIT-related subclinical hypothyroidism, aligning with outcomes from other RCTs.
Supplemental Material
Supplemental material, sj-docx-1-end-10.1177_11795514241300998 for Role of Supplementation with Selenium and Myo-Inositol Versus Selenium Alone in Patients of Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis by Varisha Zuhair, Areeba Tufail Sheikh, Nimra Shafi, Areesha Babar, Areeb Khan, Arooba Sadiq, Muhammad Afnan Ashraf, Khuld Nihan, Muhammad Hamza, Burhan Khalid, Syeda Haya Fatima, Mirza Ammar Arshad and Eman Ali in Clinical Medicine Insights: Endocrinology and Diabetes
Acknowledgments
Not applicable.
Abbreviations: Varisha Zuhair (V.Z); Areeba Tufail Shaikh (A.T.S); Nimra Shafi (N.S); Areesha Babar (A.B); Areeb Khan (A.K); Arooba Sadiq (A.S); Afnan Ashraf (A.A); Khuld Nihan (K.N); Muhammad Hamza (M.H); Burhan Khalid (B.K); Haya Fatima (H.F); Mirza Ammar Arshad (M.A.A); Eman Ali (EA).
ORCID iD: Varisha Zuhair  https://orcid.org/0009-0009-8640-6872
https://orcid.org/0009-0009-8640-6872
Supplemental Material: Supplemental material for this article is available online.
Declarations
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Yes.
Author Contributions: VZ and ATS did conceptualization, VZ, NS, AB did screening and data extraction, ATS, BK, NS and KN did analysis, MH did quality assessment, AA and MH compiled results, AS and AK wrote discussion, AA, SHF and AB compiled the manuscript and created tables, MAA compiled manuscript and reviewed the article, EA reviewed the article and gave final approval for manuscript submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Availability of Data and Materials: Supplementary file.
Preprint: Submitted at research square prhttps://doi.org/10.21203/rs.3.rs-4086168/v1
References
- 1. Wiersinga WM. Hashimoto’s thyroiditis. In: Vitti P, Hegedüs L, eds. Thyroid Diseases: Endocrinology. Cham: Springer, 2018:205-247. [Google Scholar]
- 2. Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: a systematic review and meta-analysis. Front Public Health. 2022;10:1020709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noureldine SI, Tufano RP. Association of Hashimoto’s thyroiditis and thyroid cancer. Curr Opin Oncol. 2015;27:21-25. [DOI] [PubMed] [Google Scholar]
- 4. Liu J, Chen Z, Liu M, Jia Y, Yao Z, Wang G. Levothyroxine replacement alleviates thyroid destruction in hypothyroid patients with autoimmune thyroiditis: evidence from a thyroid MRI study. Front Endocrinol (Lausanne). 2019;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duntas LH, Mantzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003;148:389-393. [DOI] [PubMed] [Google Scholar]
- 6. Fan Y, Xu S, Zhang H, et al. Selenium supplementation for autoimmune thyroiditis: a systematic review and meta-analysis. Int J Endocrinol. 2014;1:904573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordio M, Basciani S. Treatment with myo-inositol and selenium ensures euthyroidism in patients with autoimmune thyroiditis. Int J Endocrinol. 2017;1:2549491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017;21:51-59. [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [DOI] [PubMed] [Google Scholar]
- 10. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br Med J. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:14898. [DOI] [PubMed] [Google Scholar]
- 12. The Ottawa Hospital Research Institute. Accessed October 18, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 13. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J (Clin Res Ed). 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordio M, Pajalich R. Combined treatment with myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J Thyroid Res. 2013;1:424163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pace C, Tumino D, Russo M, et al. Role of selenium and myo-inositol supplementation on autoimmune thyroiditis progression. Endocrine J. 2020;67:1093-1098. [DOI] [PubMed] [Google Scholar]
- 16. Duntas LH. The role of iodine and selenium in autoimmune thyroiditis. Horm Metab Res. 2015;47:721-726. [DOI] [PubMed] [Google Scholar]
- 17. Ventura M, Melo M, Carrilho F. Selenium and thyroid disease: from pathophysiology to treatment. Int J Endocr. 2017;1:1297658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benvenga S, Nordio M, Laganà AS, Unfer V. The role of inositol in thyroid physiology and in subclinical hypothyroidism management. Front Endocrinol (Lausanne). 2021;12:662582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fallahi P, Ferrari SM, Elia G, et al. Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev Endocr Metab Disord. 2018;19:349-354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-end-10.1177_11795514241300998 for Role of Supplementation with Selenium and Myo-Inositol Versus Selenium Alone in Patients of Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis by Varisha Zuhair, Areeba Tufail Sheikh, Nimra Shafi, Areesha Babar, Areeb Khan, Arooba Sadiq, Muhammad Afnan Ashraf, Khuld Nihan, Muhammad Hamza, Burhan Khalid, Syeda Haya Fatima, Mirza Ammar Arshad and Eman Ali in Clinical Medicine Insights: Endocrinology and Diabetes