Abstract
This study aims to investigate the diagnostic and prognostic relevance of MMP-2 and MMP-9 as biomarkers for breast cancer, as well as their association with clinicopathological factors. Breast cancer is a leading contributor to cancer-related deaths among women worldwide. The discovery of biomarkers is crucial for early diagnosis, outcome prediction, and effective treatment. Matrix metalloproteinases (MMPs) play a significant role in various physiological and pathological activities, including development, tissue repair, inflammation, cancer spread, and metastasis. While the prognostic significance of MMP-2 and MMP-9 levels in breast cancer has been studied, the findings remain inconclusive. Participants were divided into three groups, with each group consisting of 62 individuals: Group I comprised healthy controls, Group II consisted of newly diagnosed breast cancer patients (stage I-III), and Group III included patients with metastatic breast cancer. Levels of MMP-2 and MMP-9 were evaluated in these groups using the ELISA method. An evident increase in MMP-2 and MMP-9 levels was noted when comparing the control group with both the breast cancer and metastatic groups. Furthermore, a notable correlation was identified between serum MMP-9 levels and the pathological diagnosis of breast cancer (P < 0.001) as well as tumor size (P < 0.01). MMP-2 and MMP-9 have emerged as promising biomarkers for breast cancer, with MMP-9 specifically associated with disease prognosis. Continued investigation into the anti-tumor mechanisms of MMPs may yield significant advancements in the development of targeted therapeutic strategies for the management of breast cancer.
Keywords: biomarker, breast cancer, Egyptian, MMP-2 and MMP-9
Introduction
Breast cancer is the most common cancer worldwide, with a rising incidence in recent years. In 2020, there were over 2.3 million new cases and 685,000 deaths attributed to breast cancer. Incidence rates differ by region, with certain Asian and African nations reporting less than 40 cases per 100,000 women. 1 In Egypt, breast cancer accounts for over 32% of cancer cases in women, with projections indicating a further increase by 2050. 2 Additionally, breast cancer contributes to 29.1% of cancer-related deaths in the country. 3
Despite progress in treatment and the introduction of screening initiatives for early detection, breast cancer continues to be a significant factor in female mortality. Ongoing endeavors are focused on discovering biomarkers that can improve the diagnosis and prognosis of breast cancer. 4 The advancement of cancer and its invasive nature, which are primary reasons for treatment challenges, are associated with a sequence of molecular alterations in cancer cells. 5
Matrix Metalloproteinases (MMPs) are a group of enzymes essential for breaking down the extracellular matrix (ECM), which affects tissue restructuring. Among these enzymes, MMP-9 is significant for breaking down ECM proteins such as collagen, which influences tissue organization and affects the invasion, movement, and advancement of cancer cells.6,7 The activity of MMP-9 is controlled by different biochemical substances, and its presence in breast cancer has been examined concerning patient prognosis.8,9
MMP-2, a member of the gelatinase subgroup, is another important matrix metalloproteinase to consider. It has the ability to break down type IV collagen found in the basement membrane. The activities of both MMP-2 and MMP-9 play a role in promoting cancer metastasis and angiogenesis by breaking down the extracellular matrix and activating pro-angiogenic factors. This association with unfavorable prognosis has been documented. 10
Prior research on MMP-2 and MMP-9 in breast cancer shows divergent findings, with certain studies indicating a link between increased expression and unfavorable outcomes, while others do not observe such a connection. 11 In light of these inconsistencies, the objective of this study is to evaluate the diagnostic and prognostic significance of MMP-2 and MMP-9 in Egyptian breast cancer patients, investigating their relationship with clinicopathological characteristics.
Subjects and methods
Characteristics of subjects
The study was conducted at the Baheya Foundation for Early Detection and Treatment of Breast Cancer in Giza, Egypt, and included 186 adult women recruited between March 2022 and December 2022. Participants were categorized into three groups: Group I (62 healthy women as controls), Group II (62 women with non-metastatic breast cancer), and Group III (62 women with metastatic breast cancer), with all groups carefully matched by age to ensure comparability. Ethical approval was granted by the Ethical Committee of the Baheya Research Center (IRB202204260015, 16, 19, 20), and the study adhered to the ethical principles outlined in the Declaration of Helsinki. Informed written consent was obtained from all participants prior to enrollment.
Breast cancer patients (metastatic or non-metastatic) included in the study were newly diagnosed and their diagnosis was based on physical examinations, radiological evaluations, and histopathological analysis, with the exclusion of any individuals with a prior history of chemotherapy or radiotherapy, as well as those with other malignancies. Collected socio-demographic data included age, menopausal status, number of children, breastfeeding history, marital status, hormonal contraception use, and family history of breast cancer. Tumor characteristics, such as size, histological grade, subtypes, and TNM stage, were evaluated following biopsy, based on the American Joint Committee on Cancer Classification. Hormone receptor (estrogen/progesterone) and HER2 statuses were determined via immunohistochemical analysis. Healthy controls were matched for demographic factors and excluded if they had any history of breast disease.
Measurement of serum MMP-2 and MMP-9
We used Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) kits (SG10407 & SG10412, respectively; Sino Gene Clone Biotech Co., Ltd.) to measure human MMP-2 and MMP-9 serum levels as per the manufacturer’s instructions. Absorbance at 450 nm was determined with an automatic microplate spectrophotometer (Multiskan FC Microplate Photometer, Thermo Fisher Scientific). The average absorbance of the reference standards was used to generate a standard curve, and the corresponding concentration was calculated for each sample based on this curve.
Statistical analysis
Statistical analysis was conducted utilizing the SPSS software (version 20). The sample size for this study was calculated using Epi Info software, 12 based on the comparison of mean serum MMP-2 levels between the breast cancer group and the healthy control group. The mean serum MMP-2 level in the breast cancer group was estimated at 806.50, while in the healthy control group, it was 771.17, with a pooled standard deviation of 59.94. 13 To detect a statistically significant difference at a significance level of 0.05 and with 80% power, 45 participants per group are required. Additionally, this sample size is sufficient to detect a significant difference in serum MMP-9 levels, with a mean of 371.83 ± 47.10 in the breast cancer group and 272.50 ± 41.56 in the healthy control group. 13 This ensures that the study has adequate power to identify meaningful differences in both MMP-2 and MMP-9 levels between the groups.
Quantitative data estimates were presented as mean and standard deviation, while median and range were utilized when appropriate. Qualitative data was displayed in terms of frequency and percentage. The Mann-Whitney test was employed to compare non-normally distributed quantitative data between two independent groups, whereas the Kruskal-Wallis test was used for comparisons involving more than two groups. The Chi-square test was utilized to assess the association between qualitative variables, with Fisher’s exact test applied for 2 × 2 qualitative variables when more than 25% of cells had an expected count below 5. Correlation analysis was performed to determine the strength of association between numerical variables. Receiver Operator Characteristic (ROC) analysis was used to establish cut-off levels, and sensitivity, specificity, and predictive values were calculated. A P-value of less than 0.05 was regarded as statistically significant.
Results
Patient’s characteristics
The research involved 186 women aged between 22 and 83 years. The prevalence of hypertension, diabetes, and the use of hormonal contraception was notably higher in the groups of breast cancer patients compared to the healthy control group (P < 0.001, P = 0.02, P = 0.005, respectively). There were no significant differences in clinicopathological data between the metastatic and non-metastatic groups, except for a higher incidence of regional lymph node involvement in metastatic breast cancer (see Supplemental Table 1).
Levels of MMP-2 and MMP-9 in serum
The levels of both MMP-2 and MMP-9 were higher in the serum of both non-metastatic and metastatic breast cancer patients compared to that of healthy controls (P < 0.001). Further analysis indicated significant variances in the concentrations of MMP-2 and MMP-9 between the healthy control group and each of the non-metastatic breast cancer group (P1 ⩽ 0.001) and the metastatic breast cancer group (P2 ⩽ 0.001). Nevertheless, there was no notable distinction between non-metastatic breast cancer patients and metastatic breast cancer patients (Tables 1 and 2).
Table 1.
Patients’ general characteristics and clinicopathological features of the disease.
| Studied variable | Healthy controls N = 62 No (%) |
Malignant group N = 62 No (%) |
Metastatic group N = 62 No (%) |
P-value |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 53.9 ± 10.25 | 54.37 ± 12.8 | 55.74 ± 11.33 | 0.65 |
| Median | 53.00 | 54.0 | 55.00 | |
| Min–max | 22–77 | 28–81 | 31–83 | |
| Hypertension | 7 (11.3) | 23 (37.1) | 27 (43.5) | <0.001 |
| Diabetes | 6 (9.7) | 16 (25.8) | 18 (29.0) | 0.02 |
| BMI | 0.37 | |||
| Mean ± SD | 31.26 ± 6.14 | 32.88 ± 5.97 | 31.93 ± 6.86 | |
| Median | 30.0 | 33.50 | 32.00 | |
| Min–max | 22–51 | 22–55 | 2–45 | |
| Family history | ||||
| Positive | 21 (33.9) | 14 (22.6) | 15 (22.4) | 0.20 |
| Menopausal status | ||||
| Pre | 21 (33.9) | 27 (43.5) | 28 (45.2) | 0.8 |
| Post | 41 (66.1) | 35 (56.5) | 33 (53.2) | |
| Usage of hormonal contraception | 13 (21.0) | 25 (40.3) | 30 (48.4) | 0.005 |
Table 2.
Comparison of MMP-2 and MMP-9 concentrations among the study groups.
| Variable | Healthy controls N = 62 |
Non metastatic group N = 62 |
Metastatic group N = 62 |
P-value | Post hoc test |
|---|---|---|---|---|---|
| MMP-2 | |||||
| Mean ± SD | 1.75 ± 1.35 | 3.61 ± 0.79 | 3.60 ± 0.71 | <0.001 | P1 ≤ 0.001 |
| Median | 1.750 | 3.61 | 3.61 | P2 ≤ 0.001 | |
| Min–max | 1.08–2.69 | 1.93–4.95 | 1.99–5.39 | P3 = 0.91 | |
| MMP-9 | |||||
| Mean ± SD | 185.77 ± 66.72 | 347.71 ± 77.315 | 322.32 ± 69.79 | <0.001 | P1 ≤ 0.001 |
| Median | 157.50 | 339.00 | 331.0 | P2 ≤ 0.001 | |
| Min–max | 109.0–380.00 | 166.0–549.0 | 192.0–478.00 | P3 = 0.10 | |
Concentration was expressed in ng/ml. P1 = comparison between healthy controls & non-metastatic groups. P2 = comparison between healthy controls & metastatic groups. P3 = comparison between non-metastatic & metastatic group.
ROC curve analysis
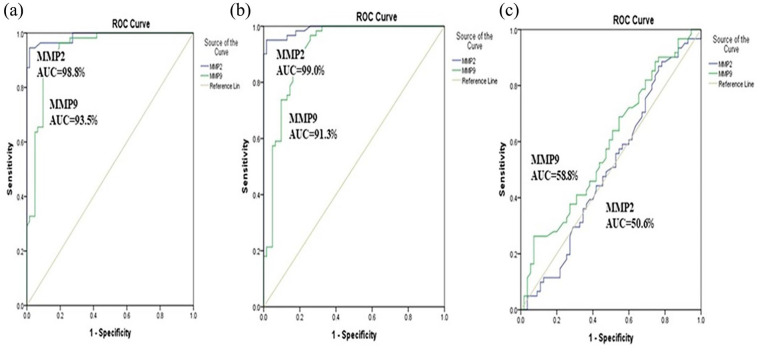
In the current study, the analysis using ROC curves demonstrated that both MMP-9 and MMP-2 can serve as significant parameters for distinguishing between healthy females and those with non-metastatic breast cancer. The area under the curve for MMP-9 was found to be 0.935, and for MMP-2, it was 0.988. Using a cut-off value of 259.0 ng/mL, MMP-9 exhibited a sensitivity of 92.7% and a specificity of 87.1%, while MMP-2 at cut-off value 2.28 ng/mL, showed a sensitivity of 96.4% and a specificity of 91.1% (Figure 1(a)). Moreover, the AUC values for predicting metastatic breast cancer were 0.913 for MMP-9 and 0.99 for MMP-2, with a sensitivity of 90.2% and a specificity of 80.6% at a cut-off point of 229.0 ng/mL for MMP-9. Conversely, MMP-2 achieved a sensitivity of 95.1% and a specificity of 90.3% at a cut-off point of 2.25 ng/mL (Figure 1(b)). Additionally, both serum levels of MMP-9 and MMP-2 demonstrated limited predictive value in distinguishing between patients with metastatic and non-metastatic breast cancer, as indicated by the AUC values of 0.588 (95% CI, 0.484–0.691) and 0.506 (95% CI, 0.400–0.613), respectively (Figure 1(c)).
Figure 1.
Receiver operating characteristic curves for prediction capacity of MMP-2 and MMP-9: (a) non-metastatic versus healthy controls, (b) metastatic versus healthy controls, (c) non-metastatic versus metastatic.
MMP: matrix metalloproteinase; AUC: area under curve.
Association of MMP-9 and MMP-2 expression with clinicopathological characteristics
Analysis of MMP-2 and MMP-9 levels in relation to clinicopathological factors of breast cancer patients indicated a notable correlation between serum MMP-9 and both the pathological diagnosis of breast cancer (P < 0.001) and tumor size (P < 0.01) (Table 3).
Table 3.
Association of serum MMP-2 and MMP-9 and clinicopathological features of the study groups.
| Studied variable | MMP-2 Mean ± SD |
P-value | MMP-9 Mean ± SD |
P-value |
|---|---|---|---|---|
| Pathological diagnosis | ||||
| IDC | 3.56 ± 0.69 | 0.15 | 327.35 ± 68.18 | <0.001 |
| ILC | 3.48 ± 0.80 | 256.37 ± 47.74 | ||
| others | 4.11 ± 0.47 | 388.83 ± 39.82 | ||
| T stage | ||||
| T1 | 3.70 ± 0.96 | 0.61 | 363.11 ± 95.52 | 0.58 |
| T2 | 3.75 ± 0.82 | 329.74 ± 59.33 | ||
| T3 | 4.01 ± 0.69 | 341.22 ± 88.49 | ||
| T4 | 3.55 ± 0.81 | 357.33 ± 94.83 | ||
| Grad | ||||
| 1 | 4.00 ± 0.37 | 0.11 | 323.50 ± 129.78 | 0.82 |
| 2 | 3.54 ± 0.72 | 325.55 ± 66.35 | ||
| 3 | 4.07 ± 0.52 | 305.75 ± 80.47 | ||
| ER | ||||
| Negative | 4.11 ± 0.44 | 0.14 | 391.66 ± 66.19 | 0.09 |
| Positive | 3.58 ± 0.71 | 320.71 ± 69.68 | ||
| PR | ||||
| Negative | 4.00 ± 0.42 | 0.19 | 357.00 ± 87.91 | 0.34 |
| Positive | 3.57 ± 0.71 | 321.87 ± 69.70 | ||
| HER2 | ||||
| Negative | 3.60 ± 0.72 | 1.0 | 321.82 ± 71.67 | 0.22 |
| Positive | 3.63 ± 0.52 | 350.60 ± 57.57 | ||
| LN | ||||
| Negative | 3.54 ± 0.85 | 0.65 | 351.90 ± 90.90 | 0.31 |
| Positive | 3.62 ± 0.72 | 330.48 ± 69.99 | ||
| Tumor size | r = −0.03 | 0.81 | r = −0.3 | 0.01 |
| Site of metastasis | ||||
| Bone | 3.65 ± 0.72 | 0.406 | 332.02 ± 71.8 | 0.406 |
| Lung | 3.68 ± 0.693 | 316.28 ± 65.78 | ||
| Liver | 3.68 ± 0.766 | 322.8 ± 55.75 | ||
| LNs | 3.68 ± 0.601 | 329.76 ± 76.30 | ||
| Brain | 3.88 ± 0.382 | 287 ± 98.99 | ||
r = correlation coefficient.
Discussion
Breast cancer comprises a varied range of tumors characterized by different morphological and molecular subtypes, which makes predicting disease progression and patient outcomes challenging. Discovering novel biomarkers is essential for customizing the most effective treatments for each patient. Recent research has investigated liquid biopsy and circulating proteins, obtainable from serum or plasma, as biomarkers for cost-effective, minimally invasive risk evaluation, early detection, prognosis, treatment modifications, and monitoring disease advancement. 14 Several experimental studies have shown the role of MMPs in the inception, progression, staging, and grading of tumors. 15
This research focused on examining the presence of human MMP-2 and MMP-9 in the blood samples of individuals with breast cancer, as blood is a readily obtainable bodily fluid. The enzyme-linked immunosorbent assay (ELISA) technique was employed to quantify the levels of total MMP-2 and MMP-9. Our findings demonstrate markedly higher levels of circulating MMP-9 and MMP-2 in all breast cancer patients when compared to the healthy control group (P < 0.001). This aligns with prior research indicating raised levels of MMP-2 and MMP-9 in the bloodstream.13,16–20
In analogous research conducted among Egyptian individuals, notably higher levels of mRNA and protein expression of MMP-2 and MMP-9 were observed in cancerous breast tissue in comparison to healthy tissue. Additionally, a notable reduction in average plasma levels of MMP-2 and MMP-9 was observed after the removal of breast carcinoma, indicating their potential as indicators for successful tumor eradication. 21
These findings are in alignment with the expected increase in biomarker levels attributed to their specific involvement in tissue remodeling associated with cancer. MMP-2 (gelatinase-A) and MMP-9 (Gelatinase-B) play a role in collagen restructuring by breaking down the extracellular matrix (ECM), which includes elastin, fibronectin, and vitronectin. They also influence functions beyond the ECM, such as activating pro-TNF-α and transforming growth factor-beta (TGF-β). These gelatinases are predicted to be elevated because of their unique contribution to cancer-related tissue remodeling, affecting processes like tumor cell proliferation, migration, invasion, and metastasis. 22
Metastatic processes often involve the activation of a program that transforms tumor epithelial-mesenchymal cells (EMT), which is triggered by cytokines and factors released by various cells within the tumor microenvironment. Concurrently, there is a breakdown of the extracellular matrix (ECM) due to the production of matrix metalloproteinases (MMPs). This research did not find any significant differences in serum levels of both MMP-2 and MMP-9 between the group of malignant breast cancer (BC) patients and those with metastatic BC (P = 0.91 and P = 0.1, respectively). Similarly, another study indicated no notable variance in the mRNA levels of MMP-2 and MMP-9 genes between BC tissues with or without axillary lymph node metastasis. Nevertheless, the serum levels of MMP-9 were notably higher in M1 patients compared to M0 patients, aligning with findings from other studies.16,19,20,23
In terms of the relationship between serum MMP-2 and MMP-9 levels with clinicopathological variables and standard prognostic factors, no significant correlation was found between MMP-2 serum levels and any clinicopathological characteristics. Previous research has demonstrated conflicting associations with clinicopathological parameters such as patient age, tumor grade, tumor receptor status, and disease stage in breast cancer patients. Some studies have indicated elevated serum MMP-2 levels in patients with advanced tumor stages, while others have shown decreased MMP-2 levels in patients with unfavorable prognostic factors.16,24,25,26 Conversely, there is ongoing debate regarding whether MMP-2 positivity is associated with poorer overall survival in breast cancer patients. 27
Building on the results, there was a notable connection between serum MMP-9 levels and both the histological type of the tumor (P = 0.001) and its size (P = 0.01). Corresponding to these results, previous research has identified higher immunohistochemical scores for MMP-9 in larger tumors, indicating its link to an aggressive breast cancer phenotype, invasion, progression, and poor prognosis. 28 Furthermore, the activity of pro-MMP-9 in clinical stages I, II, III, and IV displayed a significant positive correlation with tumor size. 29 Nevertheless, some studies have not found a significant association between the intensity of MMP-9 immunostaining and tumor size. 30
Furthermore, elevated mRNA levels of MMP-9 have been identified and specifically linked to Invasive Ductal Carcinoma (IDC) in breast cancer. 31 Research involving 113 individuals with non-palpable breast abnormalities revealed notably higher MMP-9 levels in women with invasive ductal carcinoma, indicating its potential as a biomarker. 32 Additional studies have explored the MMP-9 expression in IDC patients, showing differing levels of association with lymph node metastasis or tumor size. 33 This discrepancy could be attributed to variations in experimental techniques and sample sizes.
The debate regarding the link between metalloproteinase expression in blood or tissue and clinical results in different malignant tumors, such as breast cancer, is intricately tied to the regulation of MMPs. This includes factors like mRNA expression, the conversion of the pro-enzyme form into an active state, and the opposing effects of endogenous tissue inhibitors of metalloproteinases (TIMPs). Initially, MMPs are synthesized as zymogens (inactive Pro form) and are activated through various molecular processes. As a result, the levels of active MMP-9 in stromal cells and tumor cells can vary, leading to differences in clinical outcomes. 34
Genetic diversity plays a role in determining MMP expression levels, impacting the susceptibility of individuals to cancer. Research has explored the relationship between MMP-9 and MMP-2 genetic variations and the risk of breast cancer in different populations, yielding contradictory findings.33,35–40 Moreover, discrepancies may stem from variations in methodologies and sampling procedures prior to the quantitative assessment of the specific biomarkers.
Moreover, research has indicated that introducing the MMP-9 gene into existing breast cancer tumor cells can lead to tumor shrinkage by enhancing neutrophil infiltration and activating tumor-associated macrophages (TAM), demonstrating the potential antitumor properties of MMP-9. 41 The antitumor effects of MMPs offer a solid foundation for targeted therapy in clinical settings and hold promise for advancing clinical treatment and prognostic outcomes in the future. 14
Limitation
A key limitation of the study is the use of a case-control design instead of a prospective cohort approach to assess the prognostic impact of the biomarkers. While this design allows for efficient comparisons between metastatic and non-metastatic groups, it does not facilitate longitudinal follow-up, limiting our ability to establish causal relationships and evaluate how biomarker levels may influence progression to metastasis over time. Despite this limitation, our findings provide valuable preliminary insights that should be explored further in prospective studies to confirm their prognostic significance.
Conclusion
Elevated levels of MMP-9 and MMP-2 in the bloodstream of breast cancer (BC) patients indicate their involvement in the remodeling of cancer-associated tissues. The increased presence of MMP-9 in the serum of BC patients is linked to the type of cancer and the size of the tumor, impacting patient survival. This highlights MMP-9 as a potentially valuable prognostic indicator for advanced-stage BC in Egyptian individuals.
Recommendation
Nevertheless, additional extensive studies are required to validate these results. Furthermore, the simultaneous assessment of MMP-9 in both serum and tissue may offer a more effective indication of cancer aggressiveness compared to serum levels alone.
Supplemental Material
Supplemental material, sj-docx-1-iji-10.1177_03946320241304911 for Prognostic impact of matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9) in Egyptian breast cancer patients by Fayrouz A Fouad, Mohamed A Khali, Inas Moaz, Hossam Elmasry, Nada Gheta, Asala Abdeen, Mariam Tantawi, Ganna Elkholy, Shaimaa Rihan, Mahmoud M Kamel, Ayman EL-Meghawry EL-Kenawy, Youssef AS Abdel-Moneim and Abdallah M Gameel in International Journal of Immunopathology and Pharmacology
Acknowledgments
We would like to extend our heartfelt thanks to Doaa Elsayed Abo Kresha for her invaluable assistance in data collection. Her dedication and support played a crucial role in the successful completion of this study. The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-278).
Footnotes
List of abbreviation: Breast cancer (BC), Matrix metalloproteinases (MMPs), epithelial-mesenchymal transition (EMT), extracellular matrix (ECM), metalloproteinase-2 (MMP-2), Triple Negative Breast Cancer (TNBC), Enzyme-linked Immunosorbent Assay (ELISA), immunohistochemistry (IHC), transforming growth factor-beta (TGF-β), Invasive Ductal Carcinoma (IDC), epithelial-mesenchymal cells (EMT), tumor-associated macrophage (TAM), inhibitors of metalloproteinases (TIMPs).
Authors’ contributions: FF, MK, IM, MK, YA, AEE, & AG contributed to the project preparation, study design, submission for protocol and writing draft of the manuscript. HE, MK, & FF were responsible for the management of purchasing tasks and schedules. NG, MK, MT, & GI coordinated specimen collection and transport and implemented a quality policy throughout the laboratory analysis workflow. HE, NG, MK, MT, GI, AEE, and SR contributed to laboratory analysis. Appropriate patient selection and data collection were performed and supervised by FF, HE, YA, and MK. Statistical analysis of data and tabulation of results were accomplished by YA, IM, & SR. All authors have read and approved the final manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have reviewed and approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Taif University, Taif, Saudi Arabia (TU-DSPP-2024- 278).
Ethical approval: The study was conducted in accordance with the ethical guidelines established in the Declaration of Helsinki and received approval from the ethical committee of the Baheya Foundation for Early Detection & Treatment of Breast Cancer (IRB202204260015,16,19,20). Prior to enrollment, all participants provided written informed consent after receiving a thorough explanation of the study’s objectives, procedures, and the potential risks and benefits of their involvement. Participants were assured of the voluntary nature of their participation, and confidentiality of their data was strictly maintained throughout the study.
Informed consent: Informed written consent was obtained from all participants after the study objectives were explained and before blood sampling. Confidentiality of patient data was guaranteed.
Trial registration: *Not applicable.
ORCID iDs: Fayrouz A Fouad  https://orcid.org/0000-0001-6464-8701
https://orcid.org/0000-0001-6464-8701
Mohamed A.khalil  https://orcid.org/0000-0001-8310-3563
https://orcid.org/0000-0001-8310-3563
Hossam Elmasry  https://orcid.org/0000-0001-8273-6246
https://orcid.org/0000-0001-8273-6246
Mahmoud M.Kamel  https://orcid.org/0000-0003-0264-3096
https://orcid.org/0000-0003-0264-3096
Youssef A. S. Abdel-Moneim  https://orcid.org/0000-0002-3320-403X
https://orcid.org/0000-0002-3320-403X
Availability of data and materials: All data and materials are available and can be submitted when needed, Corresponding Author is responsible person who should be contacted if someone wants to request the data from this study.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Arnold M, Morgan E, Rumgay H, et al. (2022) Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast 66: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moaz I, Fouad FA, El-Masry HM, et al. (2023) Associations between serum soluble toll-like receptors 4 and 9 and breast cancer in Egyptian patients. Cancer Control 30: 10732748231204755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ismail GM, Abd El, Hamid AA, Abd El, Naby AG. (2013) Assessment of factors that hinder early detection of breast cancer among females at Cairo University Hospital. World Applied Sciences Journal 23(1): 99–108. [Google Scholar]
- 4. DeSantis CE, Ma J, Gaudet MM, et al. (2019) Breast cancer statistics. CA: A Cancer Journal for Clinicians 69(6): 438–451. [DOI] [PubMed] [Google Scholar]
- 5. Seyfried TN, Huysentruyt LC. (2013) On the origin of cancer metastasis. Critical Reviews in Oncogenesis 18(1–2): 43–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Jia M, Wang JH, et al. (2019) Association of MMP9-1562C/T and MMP13-77A/G polymorphisms with non-small cell lung cancer in southern Chinese population. Biomolecules 9(3): 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jabłońska-Trypuć A, Matejczyk M, Rosochacki SJ. (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of Enzyme Inhibition and Medicinal Chemistry 31(sup1): 177–183. [DOI] [PubMed] [Google Scholar]
- 8. Huang H. (2018) Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 18(10): 3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Qiu Z, Li F, et al. (2017) The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncology Letters 14(5): 5865–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webb AH, Gao B, Goldsmith ZK, et al. (2017) Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 17(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang H, Li H. (2021) Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 21(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean AG, Arner TG, Sunki GG, et al. (2011) Epi Info™, a database and statistics program for public health professionals. CDC, Atlanta, GA, USA. [Google Scholar]
- 13. Patel S, Sumitra G, Koner BC, et al. (2011) Role of serum matrix metalloproteinase-2 and -9 to predict breast cancer progression. Clinical Biochemistry 44(10–11): 869–872. [DOI] [PubMed] [Google Scholar]
- 14. Veyssière H, Bidet Y, Penault-Llorca F, et al. (2022) Circulating proteins as predictive and prognostic biomarkers in breast cancer. Clinical Proteomics 19(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, Yan T, Cai Y, et al. (2023) Expression of matrix metalloproteinases and their association with clinical characteristics of solid tumors. Gene 850: 146927. [DOI] [PubMed] [Google Scholar]
- 16. Sheen-Chen SM, Chen HS, Eng HL, et al. (2001) Serum levels of matrix metalloproteinase 2 in patients with breast cancer. Cancer Letters 173(1): 79–82. [DOI] [PubMed] [Google Scholar]
- 17. La Rocca G, Pucci-Minafra I, Marrazzo A, et al. (2004) Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. British Journal of Cancer 90(7): 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Somiari S, Somiari RI, Heckman C, et al. (2006) Circulating MMP2 and MMP9 in breast cancer—Potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. International Journal of Cancer 119(6): 1403–1411. [DOI] [PubMed] [Google Scholar]
- 19. Wu Z, Wu Q, Yang J, et al. (2008) Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. International Journal of Cancer 122(9): 2050–2056. [DOI] [PubMed] [Google Scholar]
- 20. Sung H, Choi JY, Lee SA, et al. (2012) The association between the preoperative serum levels of lipocalin-2 and matrix metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC Cancer 12(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alrehaili AA, Gharib AF, Karam RA, et al. (2019) Clinical significance of plasma MMP-2 and MMP-9 levels as biomarkers for tumor expression in breast cancer patients in Egypt. Molecular Biology Reports 47(2): 1153–1160. [DOI] [PubMed] [Google Scholar]
- 22. Vandenbroucke RE, Libert C. (2014) Is there new hope for therapeutic matrix metalloproteinase inhibition? Nature Reviews Drug Discovery 13(12): 904–927. [DOI] [PubMed] [Google Scholar]
- 23. Tang D, Piao Y, Zhao S, et al. (2014) Expression and correlation of matrix metalloproteinase-9 and heparanase in patients with breast cancer. Medical Oncology 31(7): 1–18. [DOI] [PubMed] [Google Scholar]
- 24. Talvensaari-Mattila A, Pääkkö P, Turpeenniemi-Hujanen T. (2003) Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. British Journal of Cancer 89(7): 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu SC, Yang SF, Yeh KT, et al. (2006) Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clinica Chimica Acta 371(1–2): 92–96. [DOI] [PubMed] [Google Scholar]
- 26. Lv M, Xiaoping X, Cai H, et al. (2011) Cytokines as prognstic tool in breast carcinoma. Frontiers in Bioscience 16(1): 2515. [DOI] [PubMed] [Google Scholar]
- 27. Ren F, Tang R, Zhang DX, et al. (2015) Overexpression of MMP family members Functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLOS ONE 10(8): e0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vizoso F, González L, Corte MD, et al. (2007) Study of matrix metalloproteinases and their inhibitors in breast cancer. British Journal of Cancer 96(6): 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanković S, Konjević G, Gopčević K, et al. (2010) Activity of MMP-2 and MMP-9 in sera of breast cancer patients. Pathology - Research and Practice 206(4): 241–247. [DOI] [PubMed] [Google Scholar]
- 30. Mohammadizadeh F, Bagherian-Dehkordia M. (2021) Relationship between matrix metalloproteinase-9 and some clinicopathological prognostic factors of breast carcinoma. American Journal of Clinical and Experimental Immunology 10(1): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 31. Merdad A, Karim S, Schulten HJ, et al. (2014) Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Research 34(3): 1355–1366. [PubMed] [Google Scholar]
- 32. Provatopoulou X, Gounaris A, Kalogera E, et al. (2009) Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer 9(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Süllü Y, Demirağ G, Yıldırım AA, et al. (2011) Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carcinoma of the breast. Pathology - Research and Practice 207(12): 747–753. [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Min K, Kim DH, et al. (2018) High TNFRSF12A level associated with MMP-9 overexpression is linked to poor prognosis in breast cancer: Gene set enrichment analysis and validation in large-scale cohorts. PLOS ONE 13(8): e0202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Oliveira VA, Chagas DC, Amorim JR, et al. (2020) Association between matrix metalloproteinase-9 gene polymorphism and breast cancer in Brazilian women. Clinics 75: e1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang X, Jin G, Li J, et al. (2015) Association between four MMP-9 polymorphisms and breast cancer risk: A meta-analysis. Medical Science Monitor 21: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu T, Zhang S, Qiu D, et al. (2020) Association between matrix metalloproteinase 9 polymorphisms and breast cancer risk: An updated meta-analysis and trial sequential analysis. Gene 759: 144972. [DOI] [PubMed] [Google Scholar]
- 38. Abd Elmaogoud Ragab Ibrahim F, Essam Elfeky S, Haroun M, et al. (2020) Association of matrix metalloproteinases 3 and 9 single nucleotide polymorphisms with breast cancer risk: A case-control study. Molecular and Clinical Oncology 13(1): 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dofara SG, Chang SL, Diorio C. (2020) Gene polymorphisms and circulating levels of MMP-2 and MMP-9: A review of their role in breast cancer risk. Anticancer Research 40(7): 3619–3631. [DOI] [PubMed] [Google Scholar]
- 40. Yan C, Sun C, Lu D, et al. (2022) Estimation of associations between MMP9 gene polymorphisms and breast cancer: Evidence from a meta-analysis. The International Journal of Biological Markers 37(1): 13–20. [DOI] [PubMed] [Google Scholar]
- 41. Leifler KS, Svensson S, Abrahamsson A, et al. (2013) Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. Journal of Immunology 190(8): 4420–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-iji-10.1177_03946320241304911 for Prognostic impact of matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9) in Egyptian breast cancer patients by Fayrouz A Fouad, Mohamed A Khali, Inas Moaz, Hossam Elmasry, Nada Gheta, Asala Abdeen, Mariam Tantawi, Ganna Elkholy, Shaimaa Rihan, Mahmoud M Kamel, Ayman EL-Meghawry EL-Kenawy, Youssef AS Abdel-Moneim and Abdallah M Gameel in International Journal of Immunopathology and Pharmacology