Abstract
Case summary
A 6-month-old male entire domestic shorthair cat was presented to the ophthalmology department for nasolacrimal duct cannulation and flushing, and castration under general anaesthesia. On pre-anaesthetic assessment, the cat had a heart rate of 90 beats/min (bpm). Clinical examination was unremarkable, although the cat appeared stressed. The echocardiogram was within normal limits. An ambulatory electrocardiogram (ECG) monitor was fitted overnight, and analysis of the ECG revealed a sinus rhythm with a lower than normal heart rate. The mean 1 min rate was 98 bpm. There was a slower than normal sinus rhythm and frequent ventricular escape beats. Differential diagnoses included increased vagal tone and sinoatrial node dysfunction (SND). The latter was suspected as the cat demonstrated signs of stress although an atropine response test was not performed.
Relevant and novel information
SND is relatively common in dogs but extremely rare in cats. To the authors’ knowledge, there is only one affected cat mentioned in the literature. In both dogs and humans, most cases described are in middle-aged and elderly patients. Although the condition is recognised in human infants and fetuses, it has not been reported in dogs under 2 years of age. This case is unusual because SND was suspected in an immature cat. On analysis of the ECG, the heart rate was considerably lower than those previously reported in hospitalised cats; however, findings on physical examination were subtle, highlighting the importance of pre-anaesthetic examination in identifying unexpected abnormalities.
Keywords: Sinoatrial node dysfunction, pre-anaesthetic assessment, cardiology, ambulatory electrocardiogram
Introduction
Sinoatrial node dysfunction (SND) and sick sinus syndrome (SSS) are relatively common in dogs1–15 but rare in cats. This case describes the incidental finding of a bradyarrhythmia, possibly SND, during a pre-anaesthetic assessment of a 6-month-old domestic shorthair cat. To the authors’ knowledge, SND has not been reported in cats or in juvenile animals.
Case presentation
A 6-month-old male entire domestic shorthair cat was presented to the ophthalmology department for nasolacrimal duct cannulation and flushing, and castration under general anaesthesia. A presumptive diagnosis of feline upper respiratory tract infection was made by the referring veterinary surgeon after epiphora and clinical signs in littermates. The aetiology was not identified and the cat was prescribed doxycycline 10 mg/kg PO q24h, topical chloramphenicol 1% and sodium hyaluronate 1.4% q12h, and 0.05 mg/kg meloxicam PO q24h. Upon admission to the hospital, the owner reported intermittent sneezing, respiratory stertor and episodic panting.
On initial physical examination, the cat had a heart rate of 140 beats/min (bpm), a capillary refill time of <2 s, synchronous peripheral pulses and a respiratory rate within normal limits. Thoracic auscultation was unremarkable. On pre-anaesthetic assessment by a veterinary nurse (RVN), a heart rate of 90 bpm was recorded. Signs of stress were noted. The patient was referred to the cardiology department. A multiparameter monitor (uMEC12 Vet; Mindray) was used for continuous monitoring of the electrocardiogram (ECG) before assessment by a cardiologist. A heart rate of 33–80 bpm was observed with no abnormal waveform complexes; however, heart rates <68 bpm could not be confirmed by auscultation and mis-sensing by the monitor was suspected.
Complete blood count (CBC) and serum biochemistry were unremarkable. Feline coronavirus serum antibody ELISA was negative and alpha1-acid glycoprotein was within normal limits (<300 µg/ml). Feline immunodeficiency virus antibody and feline leukaemia virus antigen (SNAP FeLV/FIV Combo; IDEXX) tests were negative, as was the test for Toxoplasma species antibodies (IgG and IgM).
Echocardiography (Vivid E95 Ultra Edition; GE Healthcare), including M-mode, two-dimensional and Doppler studies, was unremarkable (Figure 1). The ECG recorded a sinus rhythm.
Figure 1.
Two-dimensional echocardiogram showing the right parasternal long-axis and short-axis views demonstrating normal atrial and ventricular size, wall thickness and mitral valve morphology. The left atrial:aortic ratio is within normal limits (1:1.3)
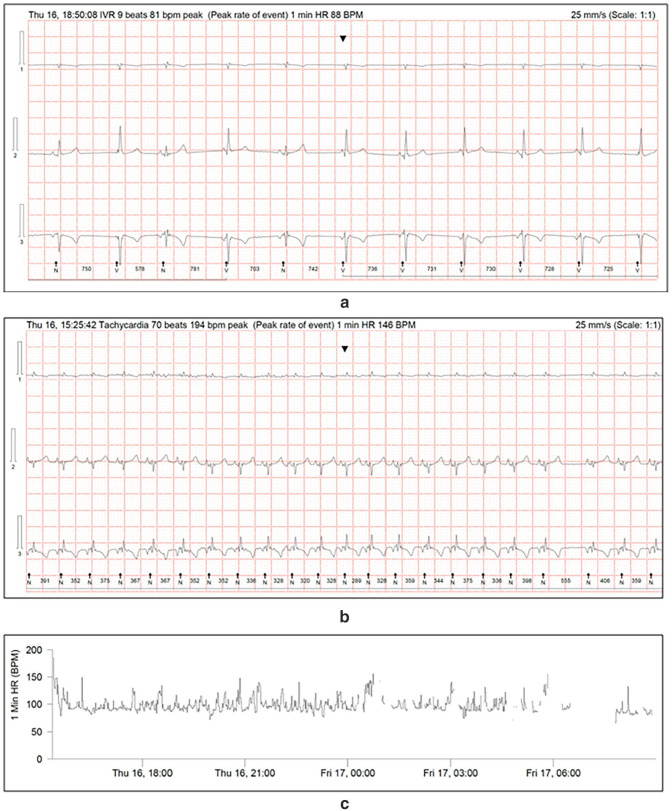
The cat was hospitalised and an ambulatory ECG monitor was fitted for overnight ECG recording (Lifecard CF; Spacelabs Healthcare). An analysis revealed a sinus rhythm with a heart rate lower than normal. This was despite demonstrable signs of stress while kennelled. The mean 1 min rate was 98 bpm (range 74–149). There was a sinus rate lower than normal, with frequent ventricular escape beats (VEBs) totalling 1139 complexes over a period of 17 h and 36 mins, including periods of idioventricular rhythm. A total of 229 episodes consistent with sinus rhythm at a rate considered normal were noted with a maximum 1 min average rate of 149 bpm, an instantaneous rate of 194 bpm and a maximum duration of 366 beats (Figure 2).
Figure 2.
(a) Electrocardiogram (ECG) obtained from the ambulatory ECG monitor showing episodes of idioventricular rhythm, (b) a slower than normal sinus rhythm at the peak rate for this episode and (c) the tachogram with areas of baseline artefact removed
Treatment was not necessary and the owner declined to proceed with the general anaesthesia for elective castration. The owner was advised to disallow outdoor access to prevent unwanted mating. Conscious cannulation and flushing of the nasolacrimal duct were not possible and medical management of epiphora was continued.
On re-examination 4 months later, the cat had a heart rate of 132 bpm with a regular rhythm. Physical examination was otherwise unremarkable. Echocardiography was within the normal limits and the ECG revealed sinus bradycardia. The owner reported no concerns except for persistent mild upper respiratory tract stertor, likely resulting from chronic tissue fibrosis.
Discussion
Heart rates in healthy unrestrained hospitalised cats are reportedly in the range of 128–256 bpm, 16 with means of 150 ± 23 bpm increasing to 187 ± 25 bpm during handling. 17 Lower rates are found in cats in their home environment, in the range of 110–250 bpm, 16 68–294 bpm 18 and 70–303 bpm 19 with means of 132 ± 19 bpm 17 and 157 ± 3.7 bpm. 18 Sinus arrhythmia is abnormal in hospitalised cats but is common at home.17–19 Cats are prone to stress during hospitalisation and increased sympathetic tone is probably responsible for these differences. Our cat had a mean 1 min rate of 98 bpm and a minimum of 74 bpm despite being hospitalised. A rate of 140 bpm at presentation was considered normal, explaining why bradycardia was not initially recognised. Rates this low are uncommon in stressed animals and this perhaps should have been considered abnormal. On re-evaluation, a rate of 90 bpm was auscultated. This was identified by an RVN, highlighting their invaluable role in the monitoring, evaluation and management of patients.
The diagnosis of arrhythmias typically requires ECG analysis. Arrhythmias may not be continuously present and an ambulatory ECG monitor, or cardiac event recorder, is occasionally necessary to achieve diagnosis. 20 Echocardiography is useful to identify structural causes, and serum biochemistry and CBC to eliminate underlying inflammatory disease and electrolyte disturbances. 2 Obtaining a thorough clinical history is important to rule out medication or toxicity as potential causes. 21
In the present cat, echocardiography, serum biochemistry and CBC were within the normal limits. An ECG analysis revealed an underlying sinus rhythm with 1139 VEBs identified, including periods of idioventricular rhythm. Ventricular ectopic beats occur commonly in clinically normal cats but less frequently. Over a 24 h period, Ware 18 identified ranges of 0–59 episodes in all but one cat, which had 729 episodes and suspected enhanced ventricular automaticity, 18 while Hanås et al 19 found ranges of 0–146 complexes with an average of three. 19 In the present patient, significantly more complexes were identified. Possible explanations include elevated vagal tone, myocarditis affecting the sinoatrial node (SAN) or surrounding tissue, and SND.
Sinus arrhythmia and sinus bradycardia are less common in cats than in dogs owing to relatively increased sympathetic drive. Elevated vagal tone can be associated with intracranial lesions and pathology of the abdomen, eye and respiratory tract. 22 The upper respiratory tract signs in the present cat may have been responsible for parasympathetic predominance although the signs of stress raised the suspicion of SND.
SND and SSS are relatively common in humans 23 and dogs1–13,15,24 but rare in cats. To the authors’ knowledge, there is one mention of a cat with SSS in the literature included in a retrospective analysis of outcomes after implantation of an epicardial pacemaker. 25 In dogs, most cases are in middle-aged or elderly animals with females and some breeds overrepresented.1,3,15,24 There are occasional reports in dogs aged as young as 2 years.1,2 In humans, most cases are reported in elderly patients 23 and only occasionally in fetuses, infants and children. 26
This case is unusual because a low heart rate was detected in an apparently stressed cat although a definitive diagnosis of SND was not made because excessive vagal tone was not excluded. In humans, electrophysiological testing can be used to confirm a diagnosis, 3 but is rarely performed in animals owing to requirements for specialist equipment. An atropine response test was warranted to distinguish between intrinsic disease of the SAN and vagally mediated arrhythmias, although the response in dogs is variable. 3 The test was declined because the patient was asymptomatic and the owner elected not to proceed with surgery.
The ECG findings were consistent with reports of SND in dogs and humans where sinus bradycardia or arrest is terminated by VEBs.1,3,21 Electrocardiographic findings in affected patients include sinus bradycardia, episodic sinus arrest, second degree atrioventricular block, VEBs, periods of idioventricular rhythm, paroxysmal supraventricular tachycardia and atrial fibrillation. 27 Junctional or ventricular escape rhythms occur in response to inadequate rates of SAN depolarisation. In the current patient, frequent VEBs and periods of sequential escape complexes were reported.
SND and SSS are characterised by defects in automaticity or conduction of the SAN, resulting in heart rates inadequate to maintain normal physiological function. Patients present without clinical signs if the rate produced by alternative pacemaker cells is sufficient to maintain appropriate cardiac output. Clinically affected individuals develop arrhythmias leading to syncopal episodes, exercise intolerance, weakness and lethargy.1,3 Nomenclature in the literature is inconsistent, but SND generally refers to abnormal conduction in an asymptomatic patient, while SSS describes clinical disease. 1 In this case, episodes of panting were reported, but these were likely to be associated with upper respiratory tract obstruction and were not associated with syncope. It is possible that clinical signs in cats may be observed only with more advanced disease when compared with dogs owing to their increased tendency to modify activity to accommodate pathology.
Most cases of SSS in humans and dogs are idiopathic.21,28 Fibrosis of nodal tissue in humans 21 and fibrous or fibro-fatty infiltration in dogs preventing myocardial conduction is described.24,29 Similar histopathological findings have been identified in dogs with mitral and tricuspid valve regurgitation. 24 Less common causes in humans include myocardial infiltrative disease, 30 including neoplasia, 31 viral destruction of the SAN and central autonomic dysfunction.32–35 Congenital mutations of the alpha-subunit of the cardiac sodium channel (SCN5A) may explain cases identified in fetuses and children. 26 In dogs, breed disposition suggests a genetic basis.1,3,24 Because of the age of the present cat, congenital disease was considered likely.
Despite the history of suspected viral rhinitis, viral myocarditis was thought unlikely. Viral destruction of sinoatrial pacemaker cells is reported in humans with COVID-19 infection33–35 but is not described in domestic animals. The aetiology of the rhinitis was not identified but common causes are feline calicivirus (FCV) and feline herpesvirus-I (FHV-1). 36 Neither are known to cause myocarditis and neither have been identified on PCR of formalin-fixed hearts. 37 Herpes simplex virus encephalitis has been implicated in central autonomic dysfunction in humans depressing SAN depolarisation. 32 FHV-1 and FCV are reported to cause encephalitis in cats; 38 however, in this case, there was no central nervous system involvement. Myocardial infiltrative disease has been reported in cats with lymphoma39–41 and hyper-eosinophilic syndrome. 42 A cardiac troponin assay was warranted to rule out myocardial injury although this was considered unlikely given normal echocardiography findings.
Treatment was not instigated as it does not improve survival in asymptomatic animals. 43 In symptomatic dogs, options include artificial pacemaker implantation and medical management with anticholinergics 1 and cilostazole. 44 Were general anaesthesia not declined, premedication with a parasympatholytic agent, such as atropine, could have been attempted to abolish excessive vagal tone or, in the absence of a response, temporary pacing used to maintain an acceptable heart rate, cardiac output and mean arterial pressure. Drugs that lead to marked bradycardia, such as alpha-2 agonists, should be avoided and opioids that may reduce heart rate even at low doses 45 should be used judiciously.
Cats with increased vagal tone have an excellent prognosis, whereas in animals with SND, the short-term prognosis is often good but there is disease progression. Common sequalae in dogs and humans are associated with congestive heart failure (CHF) and thromboembolic disease is reported in humans.1,21 A retrospective analysis of outcomes in dogs found a mean survival time of 18 months with no difference between symptomatic and asymptomatic patients, or symptomatic cases with or without treatment. 1 The mean age at diagnosis in dogs was 11 ± 3.0 years, and the incidence of cardiac death higher in clinically affected animals despite similar numbers developing CHF. Some dogs survived several years after diagnosis, suggesting that other age-related diseases contributed to their deaths, although these may be associated with poor cardiac output compromising organ function. 1
Conclusions
Although unusual, this case highlights the importance of pre-anaesthetic examination in identifying unexpected abnormalities in apparently healthy young patients. It also raises awareness of SND as a differential diagnosis in cats presenting with bradyarrhythmia. Careful examination of the cardiovascular system in animals undergoing routine neutering procedures, combined with a reduction in sympathetic stimulation by designing a low-stress clinic environment, may lead to more frequent recognition.
Acknowledgments
The authors would like to thank Sophie O’Halloran who initially conducted the pre-anaesthetic assessment and raised concerns about the cat’s heart rate and Kate White for comments on and contributions to the draft manuscript.
Footnotes
Accepted: 26 September 2024
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS Open Reports. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers, tissues and samples) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Florence Hillen  https://orcid.org/0009-0005-1065-5988
https://orcid.org/0009-0005-1065-5988
References
- 1. Ward JL, DeFrancesco TC, Tou SP, et al. Outcome and survival in canine sick sinus syndrome and sinus node dysfunction: 93 cases (2002–2014). J Vet Cardiol 2016; 18: 199–212. [DOI] [PubMed] [Google Scholar]
- 2. Burrage H. Sick sinus syndrome in a dog: treatment with dual-chambered pacemaker implantation. Can Vet J 2012; 53: 565–568. [PMC free article] [PubMed] [Google Scholar]
- 3. Moneva-Jordan A, Corcoran BM, French A, et al. Sick sinus syndrome in nine West Highland white terriers. Vet Rec 2001; 148: 142–147. [DOI] [PubMed] [Google Scholar]
- 4. Kanno N, Suzuki T. Long term effects of cilostazol in a dog with sick sinus syndrome. J Vet Med Sci 2017; 79: 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz Aleixo AS, Alfonso A, Kichise BK, et al. Pacemaker implant in a dog with sick sinus syndrome. Acta Sci Vet 2017; 45: 1–6. [Google Scholar]
- 6. Bogucki S, Noszczyk-Nowak A. Short-term heart rate variability in dogs with sick sinus syndrome or chronic mitral valve disease as compared to healthy controls. Pol J Vet Sci 2017; 20: 167–172. [DOI] [PubMed] [Google Scholar]
- 7. Mosing M. Use of isoproterenol during anaesthesia in a dog with sick sinus syndrome (SSS). Vet Med Austria 2007; 94: 292–295. [Google Scholar]
- 8. Park C, Jung D, Kim H-J, et al. Sick sinus syndrome (SSS) in a Maltese dog concurrent with mitral valve endocardiosis (MVE). J Vet Clin 2005; 22: 396–400. [Google Scholar]
- 9. Crooks AV, Gelzer AR, Oyama MA, et al. Quiet timer blanking in a dog with sick sinus syndrome and a permanent transvenous pacemaker. J Vet Cardiol 2021; 38: 36–43. [DOI] [PubMed] [Google Scholar]
- 10. Kusaba A, Hirakawa A, Kusaba H, et al. Pacemaker implantation in a dog with concurrent sick sinus syndrome and a splenic tumor. J Japan Vet Med Assoc 2016; 69: 607–611. [Google Scholar]
- 11. Ogawa M, Miyakawa H, Hsu HH, et al. A dog presenting with syncope due to two different etiologies. J Adv Vet Anim Res 2022; 9: 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novo Matos J, Silva J, Regada S, et al. Hypertrophic cardiomyopathy in a dog: a systematic diagnostic approach. J Vet Cardiol 2024; 51: 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Kavanagh K. Sick sinus syndrome in a bull terrier. Can Vet J 2002; 43: 46–48. [PMC free article] [PubMed] [Google Scholar]
- 14. Estrada AH, Pariaut R, Hemsley S, et al. Atrial-based pacing for sinus node dysfunction in dogs: initial results. J Vet Intern Med 201; 26: 558–564. [DOI] [PubMed] [Google Scholar]
- 15. Jochman-Edwards CM, Tilley LP, Lichtenberger M, et al. Electrocardiographic findings in Miniature Schnauzers with syncope. J Vet Emerg Crit Care 2002; 12: 253–259. [Google Scholar]
- 16. Quimby JM, Smith ML, Lunn KF. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J Feline Med Surg 2011; 13: 733–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abbott JA. Heart rate and heart rate variability of healthy cats in home and hospital environments. J Feline Med Surg 2005; 7: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware WA. Twenty-four-hour ambulatory electrocardiography in normal cats. J Vet Intern Med 1999; 13: 175–180. [DOI] [PubMed] [Google Scholar]
- 19. Hanås S, Tidholm A, Egenvall A, et al. Twenty-four hour Holter monitoring of unsedated healthy cats in the home environment. J Vet Cardiol 2009; 11: 17–22. [DOI] [PubMed] [Google Scholar]
- 20. Goodwin JK. Holter monitoring and cardiac event recording. Vet Clin North Am Small Anim Pract 1998; 28: 1391–1407. [DOI] [PubMed] [Google Scholar]
- 21. Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 2003; 67: 1725–1732. [PubMed] [Google Scholar]
- 22. Dennis S. Arrhythmias. In: Luis Fuentes V, Johnson LR, Dennis S. (eds). BSAVA manual of canine and feline cardiorespiratory medicine. 2nd ed. Gloucester: BSAVA, 2010, pp 121–114. [Google Scholar]
- 23. Timasheva Y, Badykov M, Akhmadishina L, et al. Genetic predictors of sick sinus syndrome. Mol Biol Rep 2021; 48: 5355–5362. [DOI] [PubMed] [Google Scholar]
- 24. Nakao S, Hirakawa A, Fukushima R, et al. The anatomical basis of bradycardia-tachycardia syndrome in elderly dogs with chronic degenerative valvular disease. J Comp Pathol 2012; 146: 175–182. [DOI] [PubMed] [Google Scholar]
- 25. Frantz EW, Tjostheim SS, Palumbo A, et al. A retrospective evaluation of the indications, complications, and outcomes associated with epicardial pacemakers in 20 cats from a single institution. J Vet Cardiol 2021; 36: 89–98. [DOI] [PubMed] [Google Scholar]
- 26. Benson DW, Wang DW, Dyment M, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 2003; 112: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schamroth L. An introduction to electrocardiography. 7th ed. Oxford: Blackwell Scientific, 1990. [Google Scholar]
- 28. Tilley LP. Essentials of canine and feline electrocardiography: interpretation and treatment. 3rd ed. Philadelphia; London: Lippincott Williams & Wilkins, 1992, pp 184–187. [Google Scholar]
- 29. Machida N, Hirakawa A. The anatomical substrate for sick sinus syndrome in dogs. J Comp Pathol 2021; 189: 125–134. [DOI] [PubMed] [Google Scholar]
- 30. Bejar D, Colombo PC, Latif F, et al. Infiltrative cardiomyopathies. Clin Med Insights Cardiol 2015; 9 Suppl 2: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kondo S, Osanai H, Sakamoto Y, et al. Secondary cardiac lymphoma presenting as sick sinus syndrome and atrial fibrillation which required leadless pacemaker implantation. Intern Med 2021; 60: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braiman D, Konstantino Y, Westreich R. When the brain slows the heart – herpes encephalitis and sinus arrest: a case report. Eur Heart J Case Rep 2021; 5. DOI: 10.1093/ehjcr/ytab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peigh G, Leya MV, Baman JR, et al. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep 2020; 4. DOI: 10.1093/ehjcr/ytaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han Y, Zhu J, Yang L, et al. SARS-CoV-2 infection induces ferroptosis of sinoatrial node pacemaker cells. Circ Res 2022; 130: 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babapoor-Farrokhran S, Batnyam U, Wiener PC, et al. Atrioventricular and sinus node dysfunction in stable COVID-19 patients. SN Compr Clin Med 2020; 2: 1955–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Binns SH, Dawson S, Speakman AJ, et al. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J Feline Med Surg 2000; 2: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meurs KM, Fox PR, Magnon AL, et al. Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc Pathol 2000; 9: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gunn-Moore DA, Reed N. CNS disease in the cat. Current knowledge of infectious causes. J Feline Med Surg 2011; 13: 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carter TD, Pariaut R, Snook E, et al. Multicentric lymphoma mimicking decompensated hypertrophic cardiomyopathy in a cat. J Vet Intern Med 2008; 22: 1345–1347. [DOI] [PubMed] [Google Scholar]
- 40. Shinohara N, MacGregor JM, Calo A, et al. Presumptive primary cardiac lymphoma in a cat causing pericardial effusion. J Vet Cardiol 2005; 7: 65–69. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka S, Suzuki R, Hirata M, et al. Unusual diagnosis of feline cardiac lymphoma using cardiac needle biopsy. BMC Vet Res 2022; 18. DOI: 10.1186/s12917-022-03357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beaumier A, Batista Linhares M, Rush JE, et al. Hypereosinophilic syndrome with cardiac infiltration and congestive heart failure in a cat. J Vet Cardiol 2022; 41: 11–17. [DOI] [PubMed] [Google Scholar]
- 43. Santilli RA, Giacomazzi F, Porteiro Vázquez DM, et al. Indications for permanent pacing in dogs and cats. J Vet Cardiol 2019; 22: 20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwasa N, Nishii N, Takashima S, et al. Long-term management of high-grade atrioventricular block using cilostazole in a cat. JFMS Open Rep 2019; 20. DOI: 10.1177/2055116919878913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robertson SA, Gogolski SM, Pascoe P, et al. AAFP feline anesthesia guidelines. J Feline Med Surg 2018; 20: 602–634. [DOI] [PMC free article] [PubMed] [Google Scholar]