Abstract
Objective:
Adverse Childhood Experiences (ACEs), such as physical, emotional, and sexual abuse trigger inflammatory changes and have been associated with many causes of morbidity and mortality, including autoimmune diseases. Although Multiple Sclerosis (MS) is a debilitating neurological autoimmune disease, literature linking ACEs and MS is understudied. The aim of this review was to examine the 1) state of the literature, and 2) relationships between childhood adversity and the prevalence and physical clinical features of MS (e.g., age at onset, relapses, pain, fatigue, disability).
Methods:
A comprehensive search was preformed through five databases and by hand using the ancestry and descendancy approach for connections to papers published through January 20th, 2022. Studies were screened by independent reviewers using Rayyan.ai, and critically appraised for both quality and reporting transparency.
Results:
Twelve studies examined relationships between any ACE(s) and the prevalence or physical clinical features of MS. There was considerable variance in the measurement of stressors, confounders, and categorization of MS; however most studies (n = 10) demonstrated an association between ACEs and MS (alone or grouped with other similar diagnoses), or physical clinical features.
Conclusion:
Although there are few studies in this area, it is of quickly growing interest.
These results should be cautiously interpreted, yet highlight the need for continued work to disentangle and discern true associations.
Keywords: Child maltreatment, Neglect, Childhood trauma questionnaire (CTQ), Stress and adversity inventory (STRAIN), Multiple sclerosis
Adverse childhood experiences (ACEs), such as abuse or household dysfunction (e.g., parental substance abuse) are a public health crisis that affects about two thirds of the United States (U.S.) population [1]. This crisis costs the U.S. $2 trillion dollars from short- and long-term consequences, such as acute and chronic health care costs [2]. Traumatic stress can drive individuals to develop maladaptive fight, flight, or freeze responses [3,4]. Within the limbic system, which is responsible for emotion such as fear conditioning, the amygdala can become enlarged and hypersensitive to perceiving threats [5,6]. When a real or perceived threat is detected, the amygdala triggers the Hypothalamic Pituitary Adrenal (HPA) axis in the brain which signals the release of stress hormones and pro-inflammatory cytokines [3,4]. This is the beginning of the inflammation cascade which creates wear and tear within the body from repeated activation, or an inability of the body to shut off these functions once the threat dissipates [7,8]. Due to this wear and tear, individuals living in a pro-inflammatory state have increased risk of many leading causes of mortality, such as obesity, diabetes, stroke, Alzheimer’s disease, and as the body’s cells turn against themselves, autoimmune diseases can develop [9–11].
Multiple Sclerosis is an immune-modulated demyelinating inflammatory disease of the central nervous system that affects between 2.5 and 2.8 million people globally [12,13]. A disproportionate number of people with MS (PwMS), 1 million, reside in the U.S. [14]. The U.S. has the second highest prevalence rate at 288 cases per 100,000 people, compared to an average global rate of 36 cases per 100,000 people [14]. Globally, MS is most frequently diagnosed in White females, yet when other races or men are diagnosed, they report higher disease burden [15,16]. The average age at onset is 36, and in numerous countries MS is the leading cause of non-traumatic neurologic disability in young adults [14,17].
Multiple Sclerosis symptoms and disability arise from demyelination, axonal injury, and loss. In a healthy individual, the myelin sheath normally surrounds and protect neurons, facilitating electrical impulse conduction [18]. With MS, the body’s inflammatory process attacks the myelin sheath, damaging axons and inhibiting the communication between the brain and body [18,19]. Common symptoms include discoordination, pain, fatigue, and spasticity progressing to complete loss of function in the corresponding part of the body (e.g., limb, bladder). Between 85 and 90% of individuals present with a relapsing remitting form and can maintain a relatively stable functional level between relapses (i.e., exacerbations characterized by new or worsening symptoms) [12]. During a relapse, function can suddenly decline to the point of paralysis in different parts of the body [20]. Progressive forms of MS are characterized by steadily worsening symptoms, higher disease burden and few to no relapses. While there isn’t a cure, there are numerous Disease Modifying Therapies (DMTs) that may reduce relapses and delay MS progression.
The exact etiology of MS is unknown but considered to be an interaction of genetics and environmental factors, such as vitamin-D, viral/bacterial exposure, smoking, and obesity [12] [21]. Latitude of birth or long-term residence has been associated with risk of MS in a gradient pattern that is lowest at the equator and increases with distance away from the equator [22,23]. This pattern is thought to derive from the varying amounts of sunlight available at different latitudes, therefore impacting the amount of circulating serum vitamin D in an individual [22]. Similarly, birth month has been associated with risk of MS through the amount of sunlight available to the mother during pregnancy [24,25].
Adult stress has been associated with MS relapse rates [26–28]. One way stress is thought to impact MS directly is through the HPA axis inflammatory response involving many cytokines, which play an integral role in MS pathogenesis and disease course [27]. Traumatic stressors could also impact MS indirectly through maladaptive coping behaviors, such as substance use or overeating. Smoking tobacco and obesity are known risk factors for MS, and both involve inflammatory processes [29–31]. Albeit limited, researchers have also begun to explore the longer-term effects of stressors during childhood, MS onset and clinical features. In one small study, ACEs were significantly correlated with a younger age at MS onset and decreased reading recognition [32]. Another study associated childhood physical and emotional abuse and neglect with adult MS relapse rates [33]. There are similar gender patterns in both the rates of some traumatic childhood stressors and MS. Girls are disproportionately affected by sexual abuse at a rate of 1 in 7 compared to 1 in 25 boys [34,35]. Similarly, MS disproportionately affects women two to three times more than men [12].
Research exploring relationships between traumatic childhood stressors and MS onset or physical clinical features is of growing interest. The objective of this review was to assess the state of published research regarding relationships between childhood adversity and the prevalence and/or physical clinical features (e.g., age at onset, relapse rates, pain, fatigue, disability) of MS.
1. Methods
1.1. Search strategy
Five North American and European databases (PubMed, Embase, CINAHL, Scopus, PsycINFO) were searched in accordance with best practice systematic processes [36] and tracked using preferred reporting methods [37]. A comprehensive approach was used, with over 50 terms in Boolean search logic. Examples include ACEs, child sexual abuse, maltreatment, incarceration, foster, divorce, psychiatric trauma, teen, youth, adolescent, Multiple Sclerosis, demyelinating autoimmune diseases of the CNS, disseminated sclerosis, autoimmunity, neuroimmunomodulation (Appendix A)
1.2. Eligibility criteria
Two investigators used Rayyan.ai [38] to screen articles independently and blindly, first by title and abstract followed by full text. Once complete, blinding was removed and the two investigator’s results were compared. A third investigator consulted on any discrepancies. Original search criteria approved original human research published in English in peer reviewed journals any time before March 5th, 2020. The article must have included at least one childhood traumatic stressor aligned with the widely recognized ACE tool (e.g., sexual/physical/emotional abuse, neglect, household mental illness/substance abuse, parental separation/divorce/incarceration) [39], and an outcome of MS or a physical MS clinical feature. Exclusion criteria was met if the article 1) reported only trauma >18 years old or lifetime trauma without also specifically evaluating childhood trauma, 2) was not a research study, 3) was done on animals, 4) did not include MS (alone or grouped) or MS clinical features as an outcome (e.g., biomarker, telomere length). Included studies were then entered into ResearchRabbit.ai [40] to hand search using the ancestry and descendancy approach for connections to any new papers published until January 20th, 2022.
1.3. Data extraction and critical appraisal
A detailed data extraction tool was used to evaluate and compare content. Examples include the trauma survey instrument, number and types of stressors, results reported by gender, confounding factors (e.g., education), and critical covariates (e.g., latitude) that could impact relationships with MS. Google maps was used to acquire latitude ranges of sample locations (maps.google.com). Two reviewers independently assessed quality with National Institutes of Health (NIH) open access tools that guide assessment for the risk of bias [41]. Criteria varied by the study design, for example, use of concurrent controls in a case control study, or if loss to follow up is <20% for cohort studies. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used to evaluate reporting transparency [42]. Most of the combined STROBE tool is universal, while some items apply per study design, such as stating the follow up time for cohort studies. Ratings from both tools were categorized into good, fair, poor.
2. Results
2.1. Overview and characteristics
Of the 4068 records identified in the initial search, 1485 duplicates were removed. From the remaining 2583 results, 34 articles progressed to full text review. Nine met all inclusion criteria and were included in this review (Fig. 1). An additional three papers were identified via handsearching. Critical appraisal revealed 11 articles of good quality and nine of high transparency (Table 1).
Fig. 1.
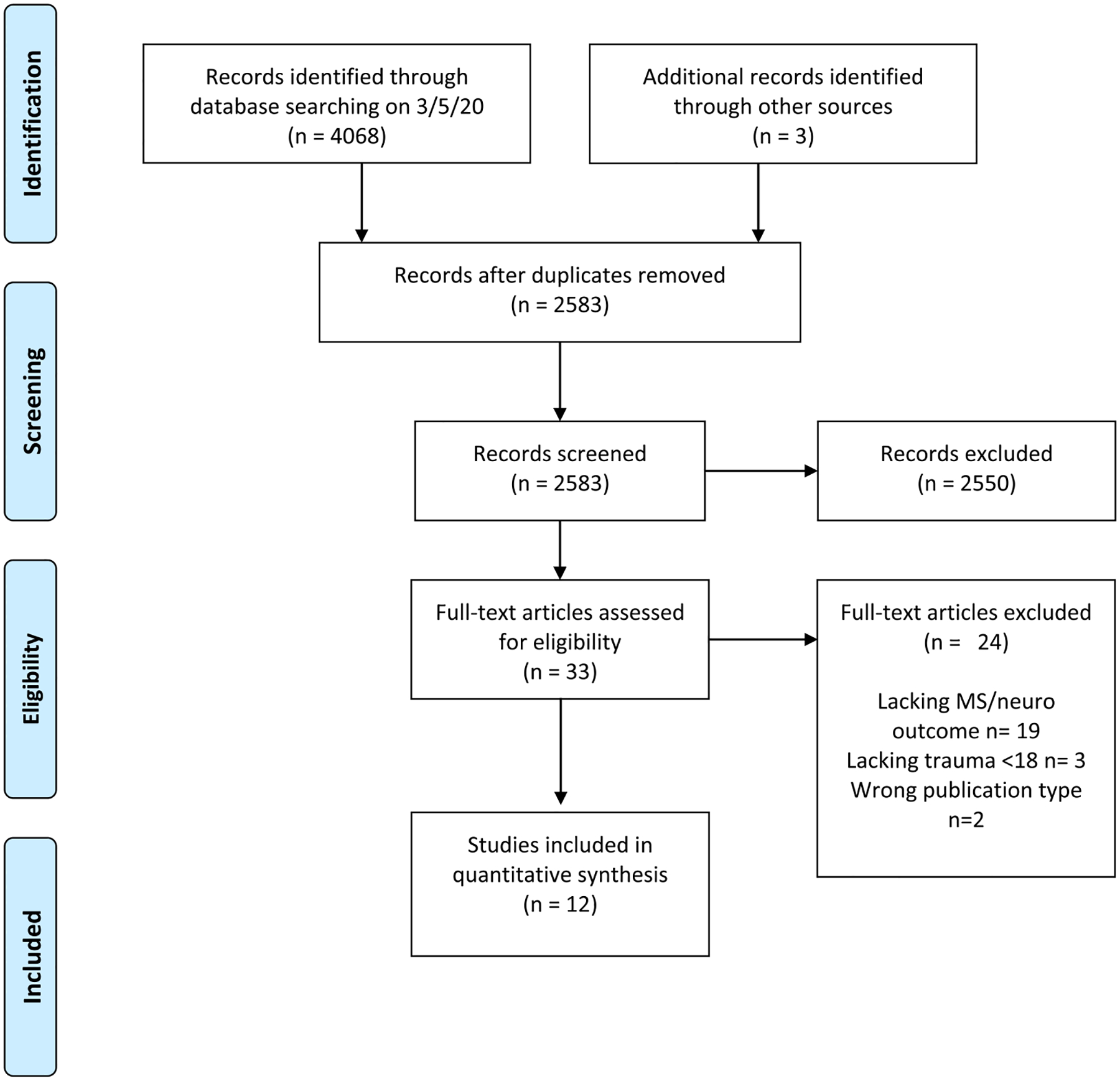
PRISMA flow diagram.
Table 1.
| Author (Year) | Sample & Study Characteristics: Total n = MS n= gender, age, year (s) data collected Location (latitude) Data type and representation Critical appraisal | Traumatic stressors: Tool & Life stage measured | Relevant health outcomes | Report final results by gender | Critical MS covariates reported | Key findings (all significant unless otherwise marked NS) |
|---|---|---|---|---|---|---|
| Cazassa et al., (2020) | Total n = 330 MS n = NR Female n = 238 Male n = 91 Transgender n = 1 Mean age 32.2 (SD 13.6) in 2019 Brazil (−33–5.) National Sample Cross sectional data Quality: good Transparency: high |
STRAINa (child & adulthood) CTQb (Childhood) |
Prevalence of AI diagnosis, MS on list of 19 diseases | Yes | Gender Age Race SES Negative affect |
2.8% more likely to have any AI disease for each additional lifetime stressor detected (IRR = 1.028, CI = 1.008–1.048, p = .006) Risk of any AI is greater for those who experienced chronic stress over acute life events (p < .001) Risk of any AI is greater for those who experienced adulthood stress over early life stress (p < .001) |
| Dube et al., (2009) | Total n = 15,358 MS n = 17 Female n = 8293 MS Female = 12 Male n = 7064 MS Male = 5 Mean age 56 (SD 15) 2005 (Admission data) 1995–97 (ACE data) San Diego, CA. 32.7–33.1) Local sample Retrospective longitudinal data Quality: good Transparency: high |
ACEc (childhood) | First admission for AI disease, MS on list of 21 diseases | Yes | Age Sex Race |
With every increase in ACE Score the likelihood of admission for any AI disease increased 20% for those 19–64 years old (p < .05), and 10% for those >65 years old (p = .08) NS relationship between ACEs and admission for diseases of mixed Th1/Th2 type (included MS and 6 other diseases) |
| Goodwin & Stein (2004) | Total n = 8098 MS n = NR Females = NR Males = NR Age range 15–54 in 1990–92 U.S. (20–70) Nationally representative Cross sectional data Quality: good Transparency: moderate |
(childhood) Neglect (no specification of type) Physical abuse Sexual abuse |
Neurological disorders, MS on list of 3 | Yes | Age Race Gender Income Education Anxiety Depression Substance dependence |
2.2 greater odds of neuro disorders after physical abuse (combined male and female) (OR = 2.2, CI = 1.1–4.5 p < .05) |
| Horton et al., (2022) | Control n = 1185 MS n = 1422 MS female = 1124 MS male = 298 Mean birth year 1958 (SD 8.8) in 2006–14 Northern California (37–42) Cross sectional data Quality: good Transparency: high |
(childhood into adulthood, 0–20) Modified ACEsd from Coddington’s Life Event Record *not including: emotional neglect, sexual abuse, parental substance use or incarceration |
MS risk & clinical features | No | Sex Race Birth year Years since MS onset Additional factors in sensitivity analysis: Education Parental homeowner status at participant age 10 Family history of MS |
Experiencing 4+ ACEs did not increase the risk of MS *(OR = 1.01, CI = 0.87–1.18) Experiencing 4+ ACEs was associated with: *2 year earlier age at MS onset (β = −1.99, CI = −3.62– −0.37, p = .02) *greater odds of needing walking aids (OR = 1.52, CI = 1.03–2.24, p = .03) *both of these findings lost significance when adjusting for multiple testing comparisons. |
| MacDonald et al., (2021) | Total n = 577 MS n = 232 Female n = 434 MS Female = 191 Male n = 142 MS Male = 41 Mean age 54.6 (14) in 2017–19 Winnipeg region, Manitoba Canada (49–51) Cross sectional data Quality: good Transparency: high |
CTQb | MS clinical feature using immune mediated inflammatory disease population (including MS, IBD, and RA) | Yes | Age Sex Race Education Marital status, Disease activity, Anxiety & Depression symptoms |
While adjusting for sociodemographics: 3.32 greater odds of pain catastrophizing after exposure to 1 or more types of maltreatment (OR = 3.32, CI = 1.89–5.85, p < .001) 2.53 greater odds of pain catastrophizing in females (OR = 2.53, CI = 1.19–5.40, p = .016) while further adjusting for anxiety and depression symptoms: 2.55 greater odds of pain catastrophizing after exposure to 1 or more types of maltreatment (OR = 2.55, CI = 1.39–4.67, p < .002) 2.05 greater odds of pain catastrophizing in females but NS (OR = 2.05, CI = 0.91–4.59, p = .08) |
| Neilsen et al., (2014) | Total n = 2,973,993 MS n = 3260 Age range 0–43 in 2011 Denmark (54.5–58) Nationally registry Historical prospective longitudinal data Quality: good Transparency: high |
(childhood) Parental divorce Death of parent Death of sibling |
MS prevalence | Tested for homogeneity | Sex Age Calendar period, Education, Income |
13% increased risk of developing MS after parental divorce (RR = 1.13, CI = 1.04–1.23) 11% elevated risk of MS after any of the 3 life events (RR = 1.11, CI = 1.03–1.20) 17% elevated but NS risk of MS after >1 life event (RR = 1.17, CI = 0.09–1.49) |
| Pust et al., (2020) | Total n = 571 MS n = 571 MS Female = 438 MS Male = 133 Mean age 43 (SD 10.9) in 2018–19 Germany (47–55) National sample Cross sectional data Quality: good Transparency: high |
CTQb (childhood) | MS clinical feature | No | None | Emotional neglect and emotional abuse had indirect effects on fatigue via emotional disturbances in childhood (e. g., alexithymia, impaired autonomy & performance) and current severity of depression, anxiety, and disability. |
| Riise et al., (2011) | Total n = 68,325 MS n = 292 All female nurses Aged 25–55 in 1982–2003 U.S. (20–70) National sample Prospective longitudinal Quality: good Transparency: high |
(childhood <11 & adolescence 11–17) Physical abuse, Sexual abuse | MS prevalence | No, female only sample | Age Ethnicity Latitude of birth, BMI Smoking |
Elevated but NS risk of MS after repeated sexual abuse in childhood (OR = 1.51, CI = 0.90–2.55) Elevated but NS risk of MS after repeated sexual abuse in adolescence (OR = 1.25, CI = 0.70–2.23) |
| Shaw et al., (2017) | Total n = 67 MS n = 67 MS Female = 52 MS Male = 15 Age range 22–69 in 2014–15 New York, U.S. (40.4–41) Local sample Cross sectional data Quality: fair Transparency: moderate |
ACEc (childhood) | MS clinical features | No | None reported | As ACE score increases the age at MS onset decreases (r = −0.30, p = .04) As ACE score increases, reading ability as proxy for premorbid IQ decreases (r = −0.25, p = .04) |
| Slavich & Shields (2018) | Total n = 205 MS n = NR Female = 107 Male = 96 Transgender = 2 Age range 19–68 in 2016 U.S. (20–70) National sample Cross sectional data Quality: good Transparency: high |
STRAINa (child & adulthood) CTQb (Childhood) |
Prevalence of AI diagnosis, MS on list of 19 Diseases | Yes | Gender Age Race SES Negative affect |
3.8% more likely to have any AI disease for each additional lifetime stressor detected (RR = 1.038, CI = 1.013–1.055, p < .001) |
| Spitzer et al., (2012) | Control n = 885 MS n = 234 MS Female = 73% Age range 18–50 in 2009 Germany (47–55) Local sample Cross sectional data Quality: good Transparency: high |
CTQb (childhood) | MS Prevalence & clinical features | Yes | Sex Age Education Current depression MS type |
3.4 greater odds of developing MS after emotional abuse (OR = 3.4, 95% CI = 2.0–5.7, p < .001) 2.2 the odds of MS after sexual abuse (OR = 2.2, CI = 1.1–4.2, p < .05) Twice the odds of MS after emotional neglect (OR = 2.0, CI = 1.3–3.2, p < .01) Relapse rates significantly higher in survivors of severe abuse compared to non-abused MS patients (F = 5.4, p = .022, d = 0.44) |
| Van Houdinhove et al., (2001) | Total n = 242 MS n = 26 Mean age 40 (SD 9) in 1998 Belgium (49.5–51.5) Local sample Cross sectional data Quality: Good Transparency: moderate |
QBEe (child <14 & adulthood) | MS/RA control group (ACE prevalence in MS group) | No | Control groups were matched & did not have overt visible symptoms | Prevalence rates for MS/RA group: Emotional neglect 30.8% Emotional abuse 13.5% Physical abuse 11.5% Sexual harassment 11.5% Sexual abuse 7.7% |
AI (Autoimmune), RA (Rheumatoid Arthritis), IBD (Inflammatory Bowel Disease), SES (Socioeconomic Status), BMI (Body Mass Index), CI (95% confidence Interval), NS (Non-Significant), NR (Not Reported), SD Standard Deviation.
STRAIN (Stress and Adversity Inventory) reported emotional abuse, emotional neglect, household substance use, parental mental illness, parental separation, physical abuse, physical neglect, sexual abuse, witnessed violence (count, severity, duration, frequency).
CTQ (Childhood Trauma Questionnaire) reported emotional abuse, emotional neglect, physical abuse, physical neglect, sexual abuse (count, severity).
ACE (Adverse Childhood Experiences) reported emotional abuse, household substance use, household mental illness, household incarceration, parental separation, physical abuse, sexual abuse, witnessed violence against mother (count).
Modified ACEs reported death of parent/sibling, life threatening illness of parent/sibling, parental divorce, parental remarriage, went to live with other family members, significant physical or verbal abuse, family lost their home / had to move, victim of violent crime, placed in foster care (count).
QBE (Questionnaire on Burdening Experiences) reported emotional abuse, emotional neglect, physical abuse, sexual harassment, sexual abuse (count, severity, frequency).
Eight studies collected and presented primary data [32,33,43–48] and four studies utilized existing datasets [49–52]. Data was collected through a wide range of years from 1982 until 2019, with three studies using prospective longitudinal data (Table 1). Half focused on MS by specifically using MS patients as their sample [32,33,44,46,51,52], while five had MS on a list of 3–21 possible autoimmune or neurological diseases grouped together [43,45,47,49,50]. One study focused on patients with fibromyalgia but used rheumatoid arthritis and PwMS as a control group [48].
2.2. Traumatic stressor measurement tools
The most prevalent tool used was the Childhood Trauma Questionnaire - Short Form (CTQ) (n = 5) [33,43,45–47], followed by the ACE tool [32,49]. However, two of the three studies that used the CTQ used it to validate the Stress and Adversity Inventory (STRAIN) [43,47]. Remaining four studies modified their own questions or used criteria available in databases, such as divorce from a national registry [44,50–52]. Four studies reported adult or adolescent specific stressors in addition to childhood stressors [43,47,48,52]. All studies have a binary (yes/no) or count indicating trauma exposure, while five included details of frequency and severity [33,43,47,48,52]. Two studies that used the STRAIN also reported duration [43,47].
2.3. Traumatic stressor typology
The types of traumas reported ranged from two to nine, with physical and sexual abuse being the most prevalent across studies (Table 1). The two studies that used the STRAIN [43,47] were the most comprehensive regarding number of stressors and additional descriptive variables (e.g., frequency, duration), followed by the studies that used the ACE or modified ACEs from another tool [32,44,49]. For example, in addition to other stressors outside the scope of this review, the STRAIN tool included emotional abuse/neglect, household substance use, parental mental illness, parental separation, physical abuse/neglect, sexual abuse, witnessed violence. The ACE tool captured eight closely aligned types of stressors (e.g., emotional abuse, household substance use). The CTQ included five traumatic stressors (e.g., emotional abuse/neglect, physical abuse/neglect), as did the Questionnaire on Burdening Experiences (e.g., emotional abuse/neglect, sexual abuse/harassment) [48]. All remaining studies developed their own items and assessed a range of two to four traumatic stressors [50–52]
2.4. Life stages
Most studies (n = 9) used the traditional age threshold of 18 years old to differentiate between childhood and adulthood. Some studies assessed only the childhood period, some made a specific distinction for adolescence, and others additionally assessed stressors into adulthood (Table 1). There were further inconsistencies between the three studies that deviated from the traditional threshold. Riise et al., (2011) defined childhood trauma under 11 years old, and adolescence was deemed between 11 and 17 years old [52]. Van Houdinhove et al., (2001) defined childhood as under 14 years old, and traumatic stressors that occurred after that were classified into the adult category [48]. Horton et al., (2022) captured stressors from ages 0–20, and further compared ages 0–10 and 11–20 in some analyses [44].
2.5. Critical MS covariates reported
There was wide variation in number and type of covariates specific to MS (e.g., gender, age, Body Mass Index [BMI], smoking) included in the 9 studies. Number of covariates ranged from zero [32,46] to nine [51]. Most studies reported controlling for demographics such as age (n = 10), and race/ethnicity (n = 8). Only one study accounted for latitude of birth or long-term residence [52]. Likewise, only one study reported the calendar period or otherwise accounting for sunlight differences based on seasonality [51]. Latitude and seasonality were more commonly included in studies examining MS risk compared to studies examining disease features. Riise et al., (2011) was the only study that reported controlling for BMI and was one of only two studies that reported accounting for substance use or smoking [50,52]. None of the studies accounted for DMTs which may have reduced relapses or disability and thus may have impacted findings.
2.6. Gender
Half the articles reported results by gender [33,43,45,47,49,50]. One sample was comprised solely of women and therefore could not report results by gender [52]. One study tested for homogeneity and after finding no significant differences, reported results together [51]. Only two studies reported any non-binary gender [43,47].
2.7. Relevant MS health outcomes
There were inconsistencies of categorizing MS in some studies that did not use an MS specific sample, but had MS mixed with other diagnoses. For example, MS was classified under a neurological category when there was also an autoimmune category that may have also been applicable [50]. Most studies reported MS prevalence or risk, while five assessed physical clinical features such as age at onset, disability/walking ability, pain catastrophizing, and fatigue [32,33,44–46].
2.8. Key findings on the relationship of traumatic stressors and MS
The majority of studies included in this review cautiously support a relationship between childhood trauma and MS or its clinical features. Regarding MS prevalence, the significant risks ranged from 3.8% increase per each stressor experienced (from a list of 55 stressors) for having any autoimmune disease [47], to a 17% increased risk of developing MS after at least one stressful life event [51] (Table 1). The significant odds-ratios ranged from 2.0, or twice the odds of MS after emotional neglect, to 3.4, or nearly three and a half times the odds of MS after emotional abuse [33].
Regarding clinical features, PwMS who reported higher rates of traumatic stressors had earlier onset (r = −0.25, p = .04) [32]. Survivors of severe abuse had higher relapse rates (F = 5.4, p = .022, d = 0.44) [33]. There were 2.55 greater odds of pain catastrophizing for PwMS with exposure to at least one type of trauma (p = .002) [45]. In a path model, emotional abuse and neglect both indirectly impacted MS fatigue via emotional disturbances (i.e., maladaptive schemas) in childhood and current severity of anxiety, depression, and disability [46]. Horton et al., 2022 found that PwMS that experienced four or more modified ACEs had an earlier onset and greater odds of needing walking aids, however both associations lost significance when adjusting for multiple testing comparisons [44].
Two other studies found non-significant results when assessing physical and sexual abuse and MS prevalence in a sample of female nurses [52], and while assessing ACE score and hospitalizations for the category of autoimmune disease inclusive of MS [49]. However, Dube et al., (2009) did find that every additional ACE significantly increased the likelihood for being admitted for any autoimmune disease by 10–20% depending on age [49].
3. Discussion
Studies examining the relationship between traumatic childhood stressors and adult health outcomes continue to garner much interest. This review contributes to the field by examining the state of the peer reviewed literature linking these stressors and Multiple Sclerosis and its disease features. Although there were few studies, most were high quality, utilizing national and/or large samples. However, there was considerable heterogeneity in methodology, including inconsistencies in traumatic stressor measurement and MS classification. This issue, compounded by the scarcity of the literature, makes comparison across studies difficult. Yet, most studies cautiously support a significant relationship between traumatic childhood stressors and either MS prevalence or clinical outcomes, which warrants further investigation. The emerging state of the science leaves much room to build upon the reviewed work. As researchers move to advance this field, it is crucial to be aware of and address gaps left in the literature.
Even though the landmark ACE study was published in 1998 [9], a comprehensive assessment of adversity or traumatic stressors is a relatively new trend in the field. This is demonstrated by variation of assessment over time. For example, the two oldest studies assessed fewer traumatic stressors [48,50], while newer studies assessed more [43,44]. Notably, even comparisons between studies using the same trauma measurement tool can be challenging because of the evolving nature of what is considered a traumatic stressor. For example, the ACE tool used by Dube et al., (2009) [49] and Shaw et al., (2017) [32], asks if someone at least five years older than the participant sexually abused them, which could have missed traumatized individuals. Of all familial sexual abuse, sibling sexual abuse is the most common form [53]. Additionally, Kreinert and Walsh (2011) found that of the police reports of sibling sexual abuse in their study, over 25% involved siblings that were only one to four years apart [53].
Overall, many of the studies in this review examined a limited number of traumatic stressors and did not capture additional information such as the severity, duration, or frequency. Thus, they are likely under-estimating relationships between childhood trauma and MS. More recently there has been a shift to broaden the criteria of what is considered a significant enough stressor to elicit the stress response and possible malfunctions of the HPA axis. Some researchers have included discrimination, poverty, and witnessing community violence [54]. Researchers should be careful to include a comprehensive assessment of traumatic stressors in future studies and be mindful of exclusion criteria.
The proportion of MS cases within study samples was an additional barrier to clear results. Of the studies that had MS in a mixed diagnoses group or as a control group, only two reported the number of MS cases which comprised only 0.01% and 10% of the total samples [48,49]. Perhaps the small number of PwMS simply means the analyses were under-powered to detect truly meaningful relationships to MS. Reports of non-significant results should therefore not be disregarded or deemed unworthy of further investigation in an MS focused sample.
Only one study assessing MS risk reported considerations for latitude of birth or long-term residence in study design or statistical analysis. This is a major gap in the literature as one of the few consistent facts about the etiology of MS is that the prevalence rate increases with the distance away from the equator [23,55]. It is therefore difficult to determine if the statistical risks of MS differ between studies due to a latitude discrepancy, for example, between Denmark at approximately 54–57 degrees [51] and Brazil at approximately −5 to −33 degrees [43], or if differences are due to other confounding factors. Latitude naturally causes variability of results in the literature and should therefore be considered in sampling design and be controlled for in statistical analyses to provide the most valuable and comparable information between studies. Similarly, other covariates that impact MS like treatment type/potency, BMI, and substance use (e.g., smoking tobacco, using cannabis for symptom management), were not well accounted for.
While a narrow majority of studies did report results by gender, there was still a lack of careful analysis by gender. Nearly half of the studies either did not assess both genders or report an analysis of each gender. Analyzing the sample as a whole could lead to significant results in one gender being disguised by nonsignificant results of other gender(s). Further, a large majority of the studies did not give a non-binary gender option. This could introduce more variability if the most accurate gender option is not available for participants select. Considering that rates of both MS and traumatic stressors vary by gender, it should be expected that gender data be collected with a non-binary option, and when sample size allows for it, all genders should be evaluated separately in sub-analyses to give more granular information.
The relationships found in this review may be underestimated by issues surrounding disclosure. Items on any traumatic stressor questionnaire are extremely sensitive and not all participants may feel comfortable revealing this information. A wealth of literature supports that research participants are likely to underreport child abuse and neglect due to stigma [56,57]. Non-disclosure could also stem from a social desirability bias, memory issues, coping through denial, or from the events not being seen as trauma due to how the question is asked. Since the data collection ranges as far back as 1982, there could also be variability in rates of disclosure. More contemporary media coverage and evolving culture has potentially made study participants in the newer studies more likely to reveal their sensitive information. This could help explain why the newer studies are finding significant results. Despite different criteria, classifications, sample composition, and potential bias, over 83% of studies found significant associations to MS or its physical clinical features. Although this seems encouraging, it must also be interpreted within the context of a relatively small number of studies which contain diverse methodological limitations.
This review revealed that five studies have assessed relationships between traumatic childhood stressors and physical clinical features of MS. Four of these studies found significant results despite using different measurement tools, covariates, and being conducted at different latitudes. Although they did not meet inclusion criteria for this review, additional recent studies demonstrate increasing interest in the relationships between childhood trauma and mental health features of MS. Eliam-Stock et al., (2021) found that ACEs predicted the initial reaction to, and increased anxiety over the first year following MS diagnosis [58]. Wan et al., (2022) found that childhood emotional abuse more than doubled the odds for women to have an immune mediated inflammatory disease (IMID), which included MS, Rheumatoid Arthritis, and Inflammatory Bowel Disease [59].
These findings suggest that there may be significant relationships between childhood trauma and MS risk and physical clinical features. Yet, additional rigorous studies are needed to support these preliminary findings, address the gaps, and expand to other features such as sleep, BMI, conversion time from first MS attack to MS onset, coping mechanisms, and comorbidities. There may be factors associated with both childhood trauma and MS that may have confounding, indirect, or cumulative effects. For example, a child may attempt to cope with trauma by smoking or eating, and smoking tobacco and obesity relate to MS and clinical features. Similarly, an adult with a history of trauma may have a lower stress tolerance or poorer coping skills at the time of MS diagnosis. The additional stress burden may then impact their disease management and clinical features such as medication adherence, pain catastrophizing or fatigue. Trauma informed clinical care, stressor screening at diagnosis, and referral to support services may enhance the clinician-patient therapeutic alliance and allow for better MS management (58,60).
4. Limitations
Despite a strong search strategy, relatively few papers were identified. Studies had much heterogeneity in methods and measurement, including mixed diagnoses categories where MS was a small portion, so interpretations must be cautious. The result was a global sample of studies from Germany, U.S., Brazil, Denmark, Canada, and Belgium. However, cultural differences of stressor experience and coping may be confounding factors. Some studies were cross sectional which limits inference about causality. However, MS typically onsets between the ages of 30–50 and childhood trauma occurs before age 18, increasing the likelihood of assumed temporal ordering. Most studies relied on retrospective self-reporting of sensitive stressors, thus were limited by recall and social desirability bias. A related concern is the impact of “effort after meaning” where some PwMS may report trauma to give meaning or find explanation for their disease [46]. However, significant results were found when using a prospective longitudinal registry-based design with objective adversity measures which would not be as susceptible to recall or reporting bias [51]. This review was limited by excluding gray literature or publications in languages other than English.
5. Implications and future directions
Although this review cannot fully tease out all factors impacting the relationships between childhood trauma and MS, it does highlight gaps to inform future studies to promote efficient growth in this area. More studies are needed that focus on samples of PwMS, and that comprehensively measure stressors (e.g., cumulative, severity). Researchers should be careful to analyze data by gender, especially if assessing correlations to specific traumatic stressors. Potential confounders that may interact with both childhood adversity and MS such as gender or education should be carefully considered when designing analytic strategies and including covariates. Further, latitude, seasonality, BMI, DMTs, and substance use should be included when appropriate to enhance understanding of true risk and impacts on clinical features.
Regarding outcomes, previous research has shown those with childhood trauma have increased sensitivity to pain, a symptom that burdens MS patients [61,62]. Anxiety, depression, and fatigue are closely intertwined and have been widely recognized as results of trauma [46,62]; PwMS experience high rates of these symptoms [63]. Underlying factors like mental health may mediate the relationship between childhood trauma and MS clinical features [46]. Similarly, whether stemming from emotional or physical pain, or an imbalance of neurotransmitters in the brain, those with trauma are more likely to use and abuse various substances [4,62,64]. PwMS are often prescribed pain medication and may use marijuana for multi-symptom relief [61]. Pain and substance use should be explored in future research to tease out the relationship that trauma has on the symptomatology and coping styles of those with MS.
Regarding design, additional longitudinal data is needed. It can be difficult and expensive to prospectively research participants before and/or after MS onset and still capture a sample size substantial enough to investigate intricate longitudinal relationships. Technological advancements can help address this. Patient Centered Outcomes Research Institute (PCORI) is a nonprofit that started creating a large network of clinical data in 2013, PCORnet, by connecting health systems and providers [65]. Electronic health record data can be aggregated from the 68 million patients throughout the 348 health systems to create a nationally representative longitudinal data [65]. Other organizations have started building MS specific databases as resources for MS research, such as the iConquerMS database, which also participates in PCORnet [66]. Data sharing networks have potential to improve researcher access, producing impactful, national and timely results, decreasing in person recruitment burden typically localized to one health system.
The American Academy of Neurology’s (AAN) position statement in 2012 supports screening patients for past and present abuse [67,68]). Although screening for past and present abuse may not offer a comprehensive assessment of an individual’s complete ACE history, abuse screening is recommended to help survivors understand how their stressor history may impact their health and behaviors, and because resources such as safety planning, counseling, or advocacy may be beneficial for their health and wellbeing [68]. However, evidence suggests that formal screening practices for abuse are inconsistent [60,67]. The findings from this review align with the AAN’s position and support screening practices for not only abuse but other adverse experiences as well. This review and future work that expands upon it may help facilitate future adoption of screening/referral practices in neurology settings. More sensitive, rigorous, quality research may also help facilitate widespread policy changes that support screening and referral practices. For example, Medi-Cal insurance in California recently created ACE specific billing codes and incentivized screening by reimbursing $29 for child and adult ACE screening [69,70]. Increases in population health and economic savings as a result, may motivate other insurance providers to similarly incentivize stressor screening.
6. Conclusions
This review evaluated the state of the literature surrounding traumatic childhood stressors and MS including its clinical features. Despite limitations, there is some evidence to support that childhood trauma may impact neuro-immune disease such as MS. More research, especially with prospective longitudinal designs, is needed to better understand these relationships. Future work should consider confounders, covariates, and other biopsychosocial clinical features such as sleep, substance use, and comprehensive mental health comorbidity. By expanding upon this foundation of knowledge, preventative and mitigating efforts, such as implementing trauma informed clinical care and screening/referrals to support services, could help promote health across the lifespan.
Acknowledgements
The authors are grateful to Kate Saylor of the University of Michigan Library for her guidance throughout the search process, and Janis Miller of the University of Michigan School of Nursing for her thoughtful feedback during project initiation.
Funding
C. Polick was supported by NIH/NINR grant #T32NR016914 Complexity: Innovations for Promoting Health and Safety (CIPHS).
Appendix A
| PubMed search strategy |
|---|
| “Child of Impaired Parents”[Mesh] OR “Adverse Childhood Experiences”[Mesh] OR “Adult Survivors of Child Adverse Events”[Mesh] OR “Child Abuse”[Mesh] OR “Child Abuse, Sexual”[Mesh] OR “Child, Foster”[Mesh] OR “adverse childhood”[tiab] OR “Childhood Trauma”[tiab] OR ((child[tiab] OR children[tiab] OR childhood[tiab] OR youth[tiab] OR teen[tiab] OR teenager[tiab] OR adolescent[tiab] OR adolescence[tiab]) AND (stressor[tiab] OR stressors[tiab] OR violence[tiab] OR foster[tiab] OR neglect[tiab] OR neglected[tiab] OR maltreatment[tiab] OR maltreated[tiab] OR abuse[tiab] OR abused[tiab] OR molestation[tiab] OR “adverse experience”[tiab] OR “adverse experiences”[tiab] OR divorce[tiab] OR incarceration[tiab] OR incarcerated[tiab] OR “psychiatric trauma”[tiab] OR “parent child conflict”[tiab] OR “serious life events”[tiab] OR “stressful life events”[tiab])) “Autoimmune Diseases”[Mesh] OR “Neuroimmunomodulation”[Mesh] OR “Immune System”[Mesh] OR “Demyelinating Autoimmune Diseases, CNS”[Mesh] OR “Multiple Sclerosis”[Mesh] OR “Disseminated Sclerosis”[tiab] OR Autoimmune[tiab] OR “Multiple Sclerosis”[tiab] OR Autoimmunity [tiab] OR Diabetes[tiab] OR lupus[tiab] OR “Rheumatoid Arthritis”[tiab] NOT (animals[mesh] NOT humans[mesh]) |
| CINAHL search strategy |
| (MH “Children of Impaired Parents+”) OR (MH “Adverse Childhood Experiences”) OR (MH “Child Abuse+”) OR (MH “Child Abuse Survivors”) OR (MH “Child, Foster”) OR TI(“adverse childhood” OR “Childhood Trauma”) OR AB (“adverse childhood” OR “Childhood Trauma”) OR TI((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”)) OR AB((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”)) (MH “Autoimmune Diseases+”) OR (MH “Immune System+”) OR (MH “Multiple Sclerosis”) OR TI(“Disseminated Sclerosis” OR Autoimmune OR “Multiple Sclerosis” OR Autoimmunity OR Diabetes OR lupus OR “Rheumatoid Arthritis”) OR AB(“Disseminated Sclerosis” OR Autoimmune OR “Multiple Sclerosis” OR Autoimmunity OR Diabetes OR lupus OR “Rheumatoid Arthritis”) |
| PsychINFO search strategy |
| DE “Childhood Adversity” OR DE “Foster Care” OR DE “Foster Children” OR DE “Child Abuse” OR DE “Battered Child Syndrome” OR DE “Child Neglect” OR ((DE “Early Experience” OR DE “Early Childhood Development”) AND (DE “Exposure to Violence” OR DE “Violence” OR DE “Dating Violence” OR DE “Domestic Violence” OR DE “Gun Violence” OR DE “Intimate Partner Violence” OR DE “Patient Violence” OR DE “Political Violence” OR DE “School Violence” OR DE “Violent Crime” OR DE “Workplace Violence” OR DE “Verbal Abuse” OR DE “Sexual Abuse” OR DE “Physical Abuse” OR DE “Divorce” OR DE “Incarceration” OR DE “Family Conflict” OR DE “Marital Conflict”)) OR TI(“adverse childhood” OR “Childhood Trauma”) OR AB(“adverse childhood” OR “Childhood Trauma”) OR TI((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”)) OR AB((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”)) DE “Immunologic Disorders” OR DE “Celiac Disease” OR DE “Guillain-Barre Syndrome” OR DE “Immune System” OR DE “Bone Marrow” OR DE “Spleen” OR DE “Multiple Sclerosis” OR TI (“Disseminated Sclerosis” OR Autoimmune OR “Multiple Sclerosis” OR Autoimmunity OR Diabetes OR lupus OR “Rheumatoid Arthritis”) OR AB(“Disseminated Sclerosis” OR Autoimmune OR “Multiple Sclerosis” OR Autoimmunity OR Diabetes OR lupus OR “Rheumatoid Arthritis”) |
| Embase search strategy |
| ‘child of impaired parents’/exp. OR ‘childhood adversity’/exp. OR ‘childhood trauma survivor’/de OR ‘child abuse survivor’/exp. OR ‘child abuse’/exp. OR ‘foster child’/exp. OR (“adverse childhood” OR “Childhood Trauma” OR ((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”))):ti,ab ‘autoimmune disease’/exp. OR ‘immunomodulation’/exp. OR ‘immune system’/exp. OR ‘multiple sclerosis’/exp. OR ‘demyelinating disease’/de OR ‘demyelination’/exp. OR ‘chronic inflammatory demyelinating polyneuropathy’/exp. OR ‘acute disseminated encephalomyelitis’/exp. OR (“Disseminated Sclerosis” OR Autoimmune OR “Multiple Sclerosis” OR Autoimmunity OR Diabetes OR lupus OR “Rheumatoid Arthritis”):ti,ab |
| Scopus search strategy |
| (((“adverse childhood” OR “Childhood Trauma” OR ((child OR children OR childhood OR youth OR teen OR teenager OR adolescent OR adolescence) AND (stressor OR stressors OR violence OR foster OR neglect OR neglected OR maltreatment OR maltreated OR abuse OR abused OR molestation OR “adverse experience” OR “adverse experiences” OR divorce OR incarceration OR incarcerated OR “psychiatric trauma” OR “parent child conflict” OR “serious life events” OR “stressful life events”))) AND (“Disseminated Sclerosis” OR autoimmune OR “Multiple Sclerosis” OR autoimmunity OR diabetes OR lupus OR “Rheumatoid Arthritis”))) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “sh”) OR LIMIT-TO (DOCTYPE, “Undefined”)) AND (LIMIT-TO (LANGUAGE, “English”)) |
Footnotes
Declaration of Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1].Centers for Disease Control and Prevention, Preventing Adverse Childhood Experiences [cited 2022; Available from: https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html, 2022.
- [2].Peterson C, Florence C, Klevens J, The economic burden of child maltreatment in the United States, 2015, Child Abuse Negl. 86 (2018) 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Juster RP, McEwen BS, Lupien SJ, Allostatic load biomarkers of chronic stress and impact on health and cognition, Neurosci. Biobehav. Rev 35 (1) (2010) 2–16. [DOI] [PubMed] [Google Scholar]
- [4].McEwen BS, et al. , Mechanisms of stress in the brain, Nat. Neurosci 18 (10) (2015) 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Edwards D, Childhood sexual abuse and brain development: a discussion of associated structural changes and negative psychological outcomes, Child Abuse Rev. 27 (3) (2018) 198–208. [Google Scholar]
- [6].McEwen BS, Nasca C, Gray JD, Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex, Neuropsychopharmacology 41 (1) (2016) 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McEwen BS, Stellar E, Stress and the individual: mechanisms leading to disease 153, 1993, pp. 2093–2101. [PubMed] [Google Scholar]
- [8].Rosemberg M-AS, Li Y, Seng J, Allostatic load: a useful concept for advancing nursing research, J. Clin. Nurs 26 (2017) 5191–5205, 10.1111/jocn.13753. [DOI] [PubMed] [Google Scholar]
- [9].Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS, Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study, Am. J. Prev. Med 14 (4) (1998) 245–258, 10.1016/j.amepre.2019.04.001. [DOI] [PubMed] [Google Scholar]
- [10].Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Slavich G, Chronic inflammation in the etiology of disease across the life span, Nat. Med 25 (2019) 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kochanek KDM, Sherry L, Xu Jiaquan, Arias Elizabeth, Deaths: Final Data for 2017, Retrieved from, https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf, 2019. [PubMed]
- [12].Drori T, Chapman J, Neurological disorders, in: Mosaic of Autoimmunity: The Novel Factors of Autoimmune Diseases, Academic Press, San Diego, CA, 2019, pp. 533–536. [Google Scholar]
- [13].Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, LaRocca NG, The prevalence of MS in the United States. A population-based estimate using health claims data, Neurology 92 (10) (2019) e1029–e1040, 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Multiple Sclerosis International Federation, Atlas of MS, Retrieved from, www.atlasofms.org, 2021.
- [15].National Multiple Sclerosis Society, What Causes MS?, Retrieved from, https://www.nationalmssociety.org/What-is-MS/What-Causes-MS, 2021.
- [16].Tyshkov CD, Charvet LE, Krupp LB, Multple sclerosis in children, in: Clinical Neuroimmunology: Multiple Sclerosis and Related Disorders, Springer Nature Switzerland AG, Cham, Switzerland, 2020. [Google Scholar]
- [17].Versini M, Jeandel P, Rosenthal E, Shoenfeld Y, Obesity in autoimmune disease: not a passive bystander, in: The Mosiac of Autoimmunity: The Novel Factors of Autoimmune Diseases, Academic Press, San Diego, CV, 2019, pp. 339–368. [Google Scholar]
- [18].Campbell G, Mahad DJ, Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis, Federat. Eur. Biomed. Soc 592 (7) (2018) 1113–1121, 10.1002/1873-3468.13013. [DOI] [PubMed] [Google Scholar]
- [19].Franklin R, Ffrench-Constant C, Remyelination in the CNS: from biology to therapy, Nat. Rev. Neurosci 9 (2008) 839–855, 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- [20].Barin L, Salmen A, Disanto G, Babačíc H, Calabrese P, Chan A, von Wyl V, The disease burden of multiple sclerosis from the individual and population perspective: which symptoms matter most? Multiple sclerosis and related disorders 25 (2018) 112–121. [DOI] [PubMed] [Google Scholar]
- [21].National Multiple Sclerosis Society, Who gets MS? (Epidemiology), Retrieved from, https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS, 2021.
- [22].Roostaei T, De Jager PL, Epidemiology and Genetics. In Clinical Neuroimmunology: Multiple Sclerosis and Related Disorders, Second ed., Springer Nature Switzerland AG, Cham, Switzerland, 2020. [Google Scholar]
- [23].Tao C, Simpson S, Mei VD, Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis, J. Neurol. Neurosurg. Psychiatry 87 (12) (2016) 1343–1349. [DOI] [PubMed] [Google Scholar]
- [24].Ismailova K, Poudel P, Parlesak A, Frederiksen P, Heitmann B, Vitamin D in early life and later risk of multiple sclerosis—a systematic review, meta-analysis, PLoS One 14 (8) (2019), e0221645, 10.1371/journal.pone.0221645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Handel AE, Giovannoni G, Ebers GC, Ramagopalan S, Environmental factors and their timing in adult-onset multiple sclerosis, Nat. Rev. Neurol 6 (2010) 156–166. [DOI] [PubMed] [Google Scholar]
- [26].Artemiadis AK, Anagnostouli MC, Alexopoulos EC, Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review, Neuroepidemiology 36 (2011) 109–120. [DOI] [PubMed] [Google Scholar]
- [27].Sharif K, Watad A, Krosser A, Coplan L, Amital H, Afek A, Shoenfeld Y, Psychological stress and the kaleidoscope of autoimmune diseases, in: Mosaic of Autoimmunity: The Novel Factors of Autoimmune Diseases, Academic Press, San Diego, CA, 2019, pp. 321–329. [Google Scholar]
- [28].Mitsonis CI, Zervas IM, Mitropoulos PA, Dimopoulos NP, Soldatos CR, Potagas CM, Sfagos CA, The impact of stressful life events on risk of relapse in women with multiple sclerosis: a prospective study, Eur. Psychiatry 23 (7) (2008) 497–504. [DOI] [PubMed] [Google Scholar]
- [29].Gianfrancesco MA, Barcellos LF, Obesity and multiple sclerosis susceptability: a review, J. Neurol. Neuromed 1 (7) (2016) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Perricone C, Versini M, Ben-Ami D, Gertel S, Watad A, Segel M, Shoenfeld Y, Smoke and autoimmunity: the fire behind the disease, in: Mosiac of Autoimmunity: The Novel Factors of Autoimmune Disease, Academic Press, San Diego, CA, 2019. [Google Scholar]
- [31].Rosso M, Chitnis T, Association between cigarette smoking and multiple sclerosis, JAMA Neurol. 77 (2) (2020) 245–253, 10.1001/jamaneurol.2019.4271. [DOI] [PubMed] [Google Scholar]
- [32].Shaw MT, Pawlak NO, Frontario A, Sherman K, Krupp LB, Charvet LE, Adverse childhood experiences are linked to age of onset and reading recognition in multiple sclerosis, Front. Neurol 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spitzer C, Bouchain M, Winkler LY, Wingenfeld K, Gold SM, Grabe HJ, Heesen C, Childhood trauma in multiple sclerosis: a case-control study, Psychosom. Med. J. Biobehav. Med 74 (2012) 312–318. [DOI] [PubMed] [Google Scholar]
- [34].Gewirtz-Meydan A, Finkelhor D, Sexual abuse and assault in a large National Sample of children and adolescents, Child Maltreatment 25 (2) (2020) 203–214, 10.1177/1077559519873975. [DOI] [PubMed] [Google Scholar]
- [35].Scoglio AA, Kraus SW, Saczynski J, Jooma S, Molnar BE, Systematic review of risk and protective factors for revictimization after child sexual abuse, Trauma Violence Abuse 1–13 (2019), 10.1177/1524838018823274. [DOI] [PubMed] [Google Scholar]
- [36].Nelson HD, Systematic Reviews to Answer Health Care Questions, Lippincott, Williams & Wilkiins, Philedelphia, PA, 2014. [Google Scholar]
- [37].Moher D, Liberati A, Tetzlaff J, Altman DG, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med. 6 (7) (2009), 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ouzzani Mourad, Hammady Hossam, Fedorowicz Zbys, Elmagarmid Ahmed, Rayyan — a web and mobile app for systematic reviews, Syst. Rev 5 (2016) 210, 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH, The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology, Eur. Arch. Psychiatry Clin. Neurosci 256 (3) (2006) 174–186, 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Research Rabbit, Retrieved from, https://researchrabbitapp.com, 2022.
- [41].National Institutes of Health: National Heart, L., and Blood Institute, Study Quality Assessment Tools, Retrieved from, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, 2021.
- [42].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Int. J. Surg 12 (12) (2014) 1495–1499, 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- [43].Cazassa M, Oliveira MS, Spahr C, Shields G, Slavich G, The stress and adversity inventory for adults (adult STRAIN) in Brazilian Portuguese: initial validation and Links with executive function, sleep, and mental and physical health, Front. Psychol (2020), 10.3389/fpsyg.2019.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Horton MK, Shao X, Bellesis K, Chinn T, Schaefer C, Lisa F Barcellos, Case-control study of adverse childhood experiences and multiple sclerosis risk and clinical outcomes, PLoS One 17 (1) (2022), e0262093, 10.1371/journal.pone.0262093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].MacDonald TM, Fisk JD, Bernstein CN, El-Gabalawyd R, Hitchon CA, Kornelsen J, Marrie RA, The association between childhood maltreatment and pain catastrophizing in individuals with immune-mediated inflammatory diseases, J. Psychopom. Res 145 (2021), 110479, 10.1016/j.jpsychores.2021.110479. [DOI] [PubMed] [Google Scholar]
- [46].Pust GA, Bettmers C, Randerath J, Rahn AC, Heesen C, Schmidt R, Gold SM, Fatigue in multiple sclerosis is associated with childhood adversities, Front. Psychiatry 11 (2020), 10.3389/fpsyt.2020.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Slavich GM, Shields GS, Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult STRAIN): an overview and initial validation, Psycosom. Med 80 (1) (2018) 17–27, 10.1097/PSY.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Van Houdenhove B, Neerinckx E, Lysens R, Vertommen H, Van Houdenhove L, Onghena P, D’Hooghe MB, Victimization in chronic fatigue syndrome and fibromyalgia in tertiary care: a controlled study on prevalence and characteristics, Psychosomatics 42 (1) (2001) 21–28, 10.1176/appi.psy.42.1.21. [DOI] [PubMed] [Google Scholar]
- [49].Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB, Cumulative childhood stress and autoimmune diseases in adults, Psychosom. Med 71 (2) (2009) 243–250, 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goodwin R, Stein M, Association between childhood trauma and physical disorders among adults in the United States, Psychol. Med 34 (3) (2004) 509–520, 10.1017/S003329170300134X. [DOI] [PubMed] [Google Scholar]
- [51].Nielsen NM, Pedersen BV, Stenager E, Koch-Henriksen N, Frisch M, Stressful life-events in childhood and risk of multiple sclerosis: a Danish nationwide cohort study, Mult. Scler. J 20 (12) (2014) 1609–1615, 10.1177/1352458514528761. [DOI] [PubMed] [Google Scholar]
- [52].Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, Ascherio A, Stress and the risk of multiple sclerosis, Am. Acad. Neurol 76 (22) (2011), 10.1212/WNL.0b013e31821d74c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Krienert JL, Walsh JA, Sibling sexual abuse: an empirical analysis of offender, victim, and event characteristics in National Incident-Based Reporting System (NIBRS) data, 2000–2007, J. Child Sexual Abuse 20 (4) (2011) 353–372, 10.1080/10538712.2011.588190. [DOI] [PubMed] [Google Scholar]
- [54].Cronholm PF, Forke CM, Wade R, Bair-Merritt MH, Davis M, Harkins-Schwarz M, Fein JA, Adverse childhood experiences: expanding the concept of adversity, J. Prev. Med 49 (3) (2015) 354–361. [DOI] [PubMed] [Google Scholar]
- [55].Dobson R, Giovannoni G, Ramagopalan S, The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude, J. Neurol. Neurosurg. Psychiatry 84 (4) (2013) 427–430. [DOI] [PubMed] [Google Scholar]
- [56].Church C, Andreassen OA, Lorentzen S, Melle I, Childhood trauma and minimization/denial in people with and without a severe mental disorder, Front. Psychol 8 (2017) 1276, 10.3389/fpsyg.2017.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Finkelhor D, Current Controversies on Family Violence, Second Edition ed., Sage Publications, Thousand Oaks, California, 2005. [Google Scholar]
- [58].Eilam-Stock T, Links J, Khan NZ, Bacon TE, Zuniga G, Laing L, Sammarco C, Sherman K, Charvet L, Adverse childhood experiences predict reaction to multiple sclerosis diagnosis, Health Psychol. Open 8 (2) (2021) 1–11, 10.1177/20551029211052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wan A, Bernstein CN, Graff LA, Patten SB, Sareen J, Fisk JD, Marrie RA, Childhood maltreatment and psychiatric comorbidity in immune-mediated inflammatory disorders, Psychosom. Med 84 (1) (2022) 10–19, 10.1097/PSY.0000000000001025. [DOI] [PubMed] [Google Scholar]
- [60].Polick CS, Polick SR, Stoddard SA, Braley TJ, Slavich GM, The importance of assessing life stress exposure in multiple sclerosis: a case report, Mult. Sclerosis Related Disorder 54 (2021), 103145, 10.1016/j.msard.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Braley TJ, Whibley D, Alschuler KN, Ehde DM, Chervin RD, Clauw DJ, Kratz AL, Cannabinoid use among Americans with MS: current trends and gaps in knowledge, Multiple Sclerosis J. Exper. Translat. Clin 6 (3) (2020), 2055217320959816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sachs-Ericsson N, Cromer K, Hernandez A, Kendall-Tackett K, A review of childhood abuse, health, and pain-related problems: the role of psychiatric disorders and current life stress, J. Trauma & Dissociat 10 (2) (2009) 170–188, 10.1080/15299730802624585. [DOI] [PubMed] [Google Scholar]
- [63].Henry A, Tourbah A, Camus G, Deschamps R, Mailhan L, Castex C, Montreuil M, Anxiety and depression in patients with multiple sclerosis: the mediating effects of perceived social support, Mult. Sclerosis Related Disorder 27 (2019) 46–51, 10.1016/j.msard.2018.09.039. [DOI] [PubMed] [Google Scholar]
- [64].Edalati H, Krank MD, Childhood maltreatment and development of substance use disorders: a review and a model of cognitive pathways, Trauma Violence Abuse 17 (5) (2016) 454–467, 10.1177/1524838015584370. [DOI] [PubMed] [Google Scholar]
- [65].PCORnet., The National Patient-Centered Clinical Research Network. Research & Results, Retrieved from, https://pcornet.org, 2021.
- [66].iConquerMS, How we can assist your research: iConquerMS Cohort descriptions, Retrieved from, https://www.iconquerms.org/how-we-can-assist-your-research, 2020.
- [67].Roque A, Weinberg J, Hohler Depold A, Evaluating exposure to abuse and violence in neurological patients, Neurologist 19 (1) (2013) 7–10, 10.1097/NRL.0b013e31827c6c26. [DOI] [PubMed] [Google Scholar]
- [68].Schulman EA, Hohler AD, The American Academy of Neurology position statement on abuse and violence, Am. Acad. Neurol 78 (6) (2012), 10.1212/WNL.0b013e318245d21c. [DOI] [PubMed] [Google Scholar]
- [69].Underwood E, Screen for childhood trauma triggers debate, Science 367 (6477) (2020) 498, 10.1126/science.367.6477.498. [DOI] [PubMed] [Google Scholar]
- [70].ACEs Aware, Billing & Payment 2022, Available from, https://www.acesaware.org/learn-about-screening/billing-payment/#.


