Abstract
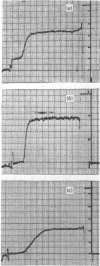
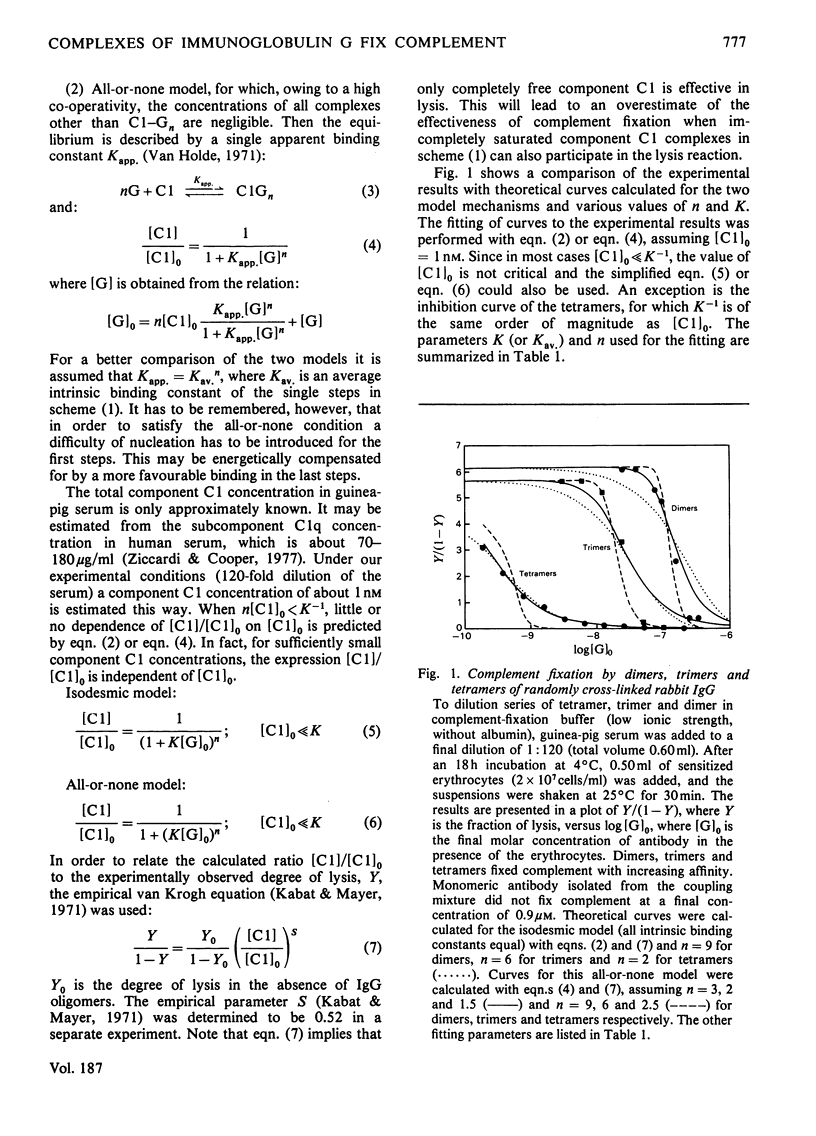
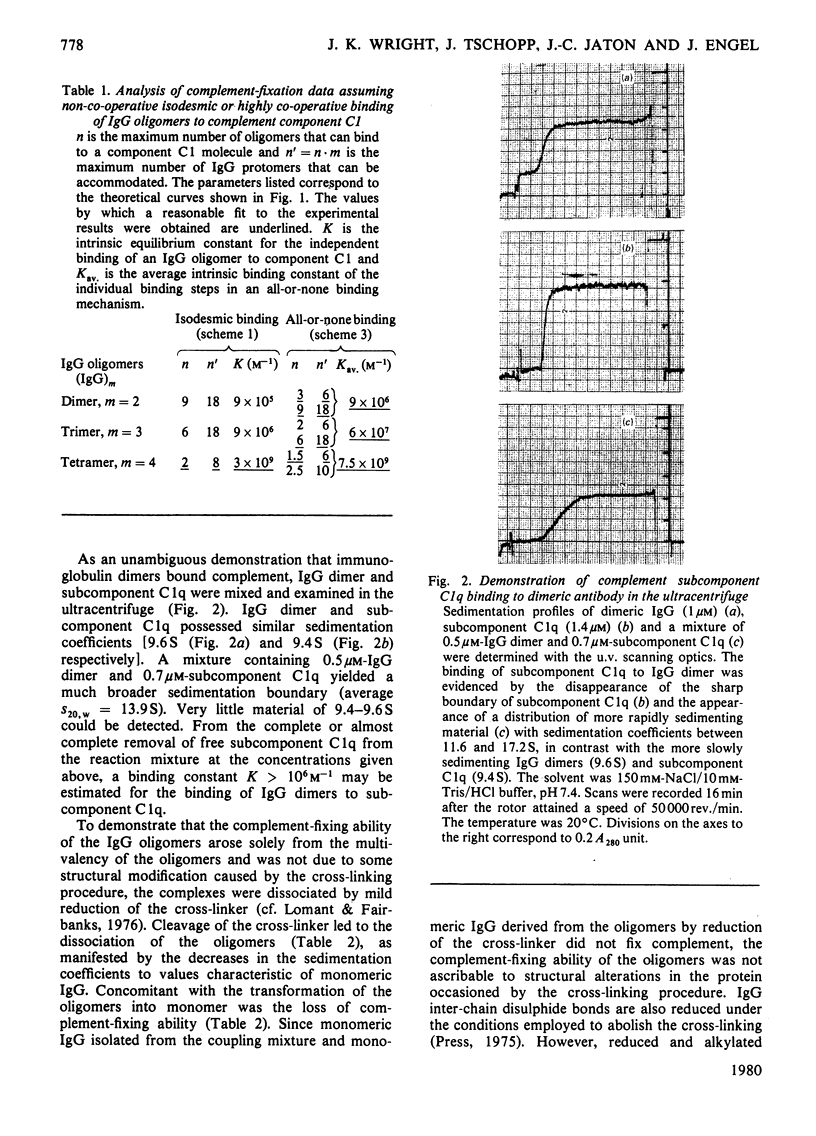
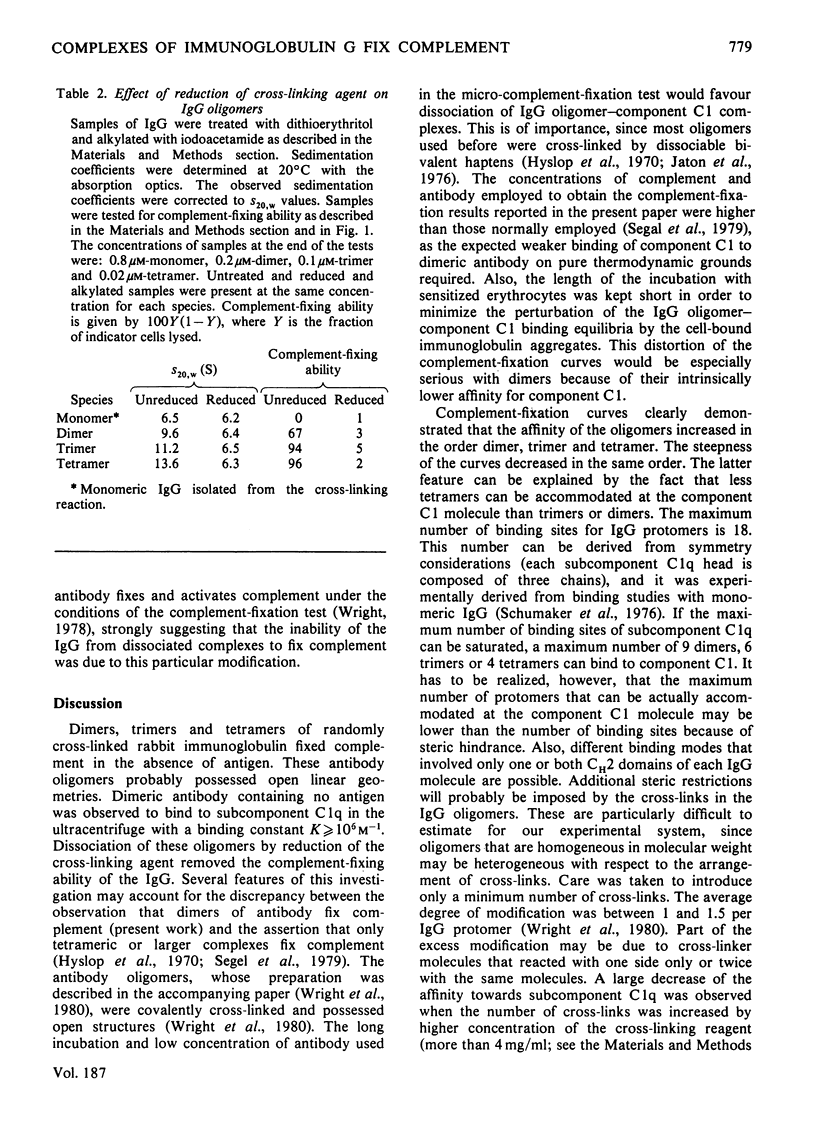
The binding of pure dimers, trimers and tetramers of randomly cross-linked non-immune rabbit immunoglobulin G to the first component and subcomponent of the complement system, C1 and C1q respectively, was studied. These oligomers possessed open linear structures. All three oligomers fixed complement with decreasing affinity in the order: tetramer, trimer, dimer. Complement fixation by dimeric immunoglobulin exhibited the strongest concentration-dependence. No clear distinction between a non-co-operative and a co-operative binding mechanism could be achieved, although the steepness of the complement-fixation curves for dimers and trimers was better reflected by the co-operative mechanism. Intrinsic binding constants were about 10(6)M-1 for dimers, 10(7)M-1 for trimers and 3 X 10(9)M-1 for tetramers, assuming non-co-operative binding. The data are consistent with a maximum valency of complement component C1 for immunoglobulin G protomers in the range 6-18. The binding of dimers to purified complement subcomponent C1q was demonstrated by sedimentation-velocity ultracentrifugation. Mild reduction of the complexes by dithioerythritol caused the immunoglobulin to revert to the monomeric state (S20,w = 6.2-6.5S) with concomitant loss of complement-fixing ability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimeh S. N., Bing D. H., Painter R. H. A simple method for the isolation of the subcomponents of the first component of complement by affinity chromatography. J Immunol. 1974 Jul;113(1):225–234. [PubMed] [Google Scholar]
- BARANDUN S., KISTLER P., JEUNET F., ISLIKER H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Koshland M. E. Activation of antibody Fc function by antigen-induced conformational changes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5111–5115. doi: 10.1073/pnas.72.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Koshland M. E. Evidence for a long-range conformational change induced by antigen binding to IgM antibody. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5682–5686. doi: 10.1073/pnas.74.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Hyslop N. E., Jr, Dourmashkin R. R., Green N. M., Porter R. R. The fixation of complement and the activated first component (C1) of complement by complexes formed between antibody and divalent hapten. J Exp Med. 1970 Apr 1;131(4):783–802. doi: 10.1084/jem.131.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaton J. C., Huser H., Braun D. G., Givol D., Pecht I., Schlessinger J. Conformational changes induced in a homogeneous anti-type III pneumococcal antibody by oligosaccharides of increasing size. Biochemistry. 1975 Dec 2;14(24):5312–5315. doi: 10.1021/bi00695a014. [DOI] [PubMed] [Google Scholar]
- Jaton J. C., Huser H., Riesen W. F., Schlessinger J., Givol D. The binding of complement by complexes formed between a rabbit antibody and oligosaccharides of increasing size. J Immunol. 1976 May;116(5):1363–1366. [PubMed] [Google Scholar]
- Lancet D., Pecht I. Kinetic evidence for hapten-induced conformational transition in immunoglobin MOPC 460. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3549–3553. doi: 10.1073/pnas.73.10.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Matsushima M., Marquart M., Jones T. A., Colman P. M., Bartels K., Huber R. Crystal structure of the human Fab fragment Kol and its comparison with the intact Kol molecule. J Mol Biol. 1978 Jun 5;121(4):441–459. doi: 10.1016/0022-2836(78)90393-5. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]
- Metzger H. The effect of antigen on antibodies: recent studies. Contemp Top Mol Immunol. 1978;7:119–152. doi: 10.1007/978-1-4757-0779-3_4. [DOI] [PubMed] [Google Scholar]
- Press E. M. Fixation of the first component of complement by immune complexes: effect of reduction and fragmentation of antibody. Biochem J. 1975 Jul;149(1):285–288. doi: 10.1042/bj1490285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Givol D., Hochman J., Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2775–2779. doi: 10.1073/pnas.72.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Calcott M. A., Spiegelberg H. L., Müller-Eberhard H. J. Ultracentifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry. 1976 Nov 16;15(23):5175–5181. doi: 10.1021/bi00668a035. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Guyer R. L., Plotz P. H. Complement fixation by model immune complexes free in solution and bound onto cell surfaces. Biochemistry. 1979 May 1;18(9):1830–1835. doi: 10.1021/bi00576a031. [DOI] [PubMed] [Google Scholar]
- Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. K., Engel J., Brandt D. C., Jaton J. C. Independence of the binding of domain-specific ligands to Fab and Fc suggests that antigen-induced effects in IGG antibodies are not allosteric. FEBS Lett. 1978 Jun 1;90(1):79–83. doi: 10.1016/0014-5793(78)80302-0. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Engel J., Jaton J. C. Selective reduction and proteolysis in the hinge region of liganded and unliganded antibodies: identical kinetics suggest lack of major conformational change in the hinge region. Eur J Immunol. 1978 May;8(5):309–314. doi: 10.1002/eji.1830080505. [DOI] [PubMed] [Google Scholar]
- Wright J. K. Reduced immunoglobulin G activates complement system with decreased cooperativity. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1284–1290. doi: 10.1016/0006-291x(78)91360-8. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Tschopp J., Jaton J. C. Preparation and characterization of chemically defined oligomers of rabbit immunoglobulin G molecules for the complement binding studies. Biochem J. 1980 Jun 1;187(3):767–774. doi: 10.1042/bj1870767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. The subunit composition and sedimentation properties of human C1. J Immunol. 1977 Jun;118(6):2047–2052. [PubMed] [Google Scholar]