Abstract
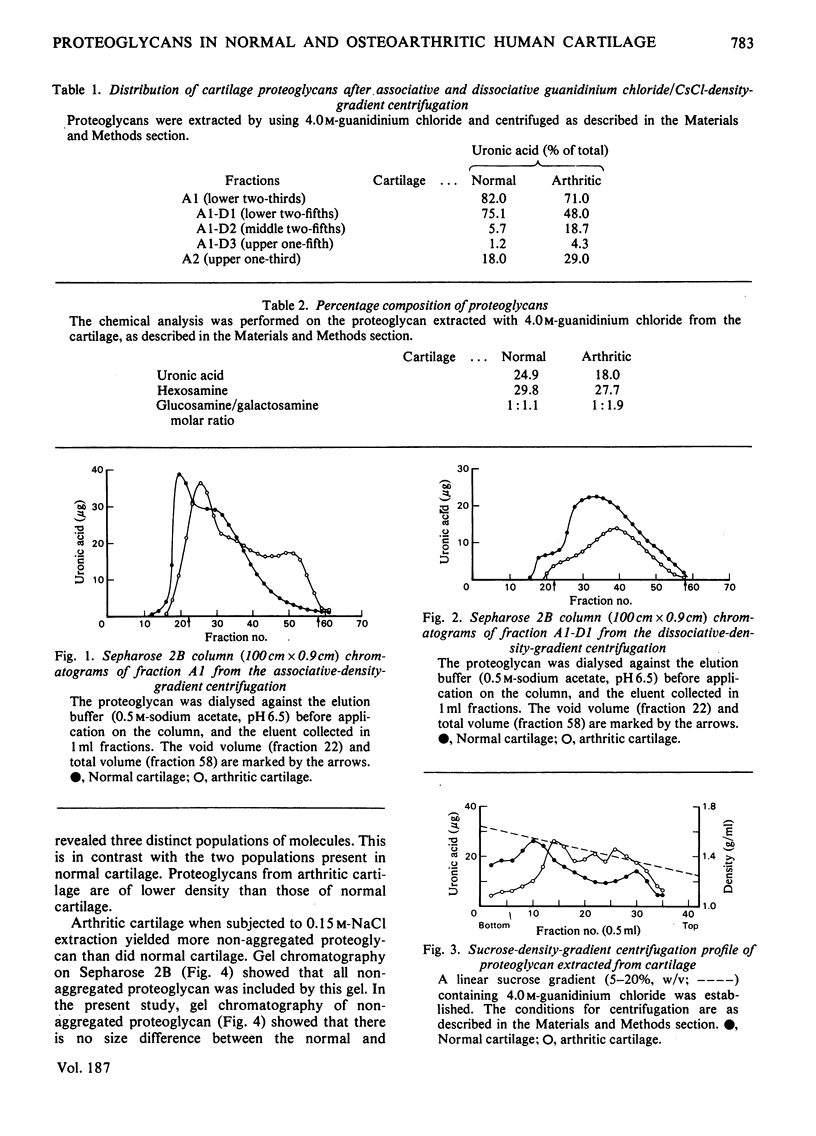
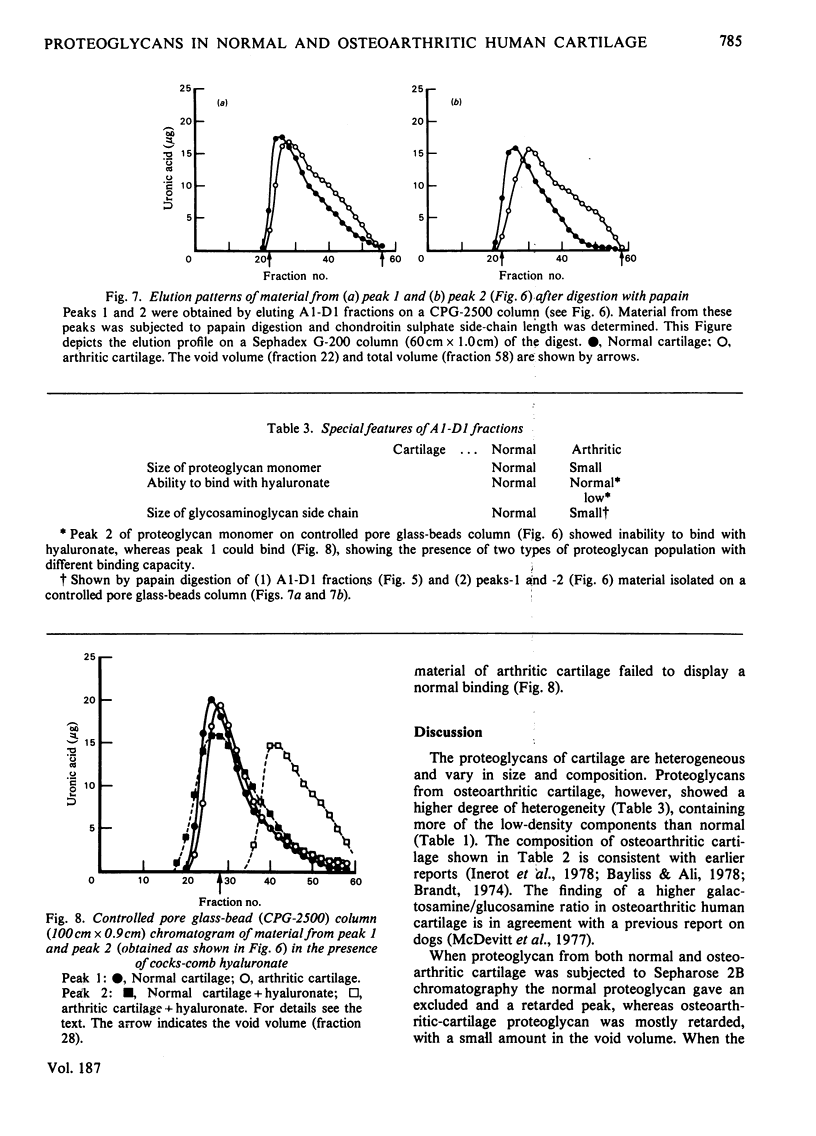
Proteoglycans from osteoarthritic cartilage were compared with those from normal articular cartilage. Normal proteoglycan aggregates are larger in size and more homogeneous than those in osteoarthritis. Proteoglycan monomers from both sources gave two peaks on controlled pore glass-bead chromatography. Although the retarded material from normal cartilage showed an affinity for hyaluronate, the same material from osteoarthritic cartilage did not. The hyaluronate-binding capacity of the material which is partly in the void volume and partly retarded was similar in both types of cartilage. These results suggest that in osteoarthritic cartilage the proteoglycan aggregates are smaller and more heterogeneous and that the chondroitin sulphate side chains are shorter. They also indicate that there are two populations of proteoglycan, one with its hyaluronate-binding-protein region of core protein intact and the other either possessing an inactive binding region or totally lacking it.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D. Enhanced extractability of articular cartilage protoglycans in osteoarthrosis. Biochem J. 1974 Nov;143(2):475–478. doi: 10.1042/bj1430475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Palmoski M. J., Perricone E. Aggregation of cartilage proteoglycans. II Evidence for the presence of a hyaluronate-binding region on proteoglycans from osteoarthritic cartilage. Arthritis Rheum. 1976 Nov-Dec;19(6):1308–1314. doi: 10.1002/art.1780190611. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Palmoski M. Organization of ground substance proteoglycans in normal and osteoarthritic knee cartilage. Arthritis Rheum. 1976 Mar-Apr;19(2):209–215. doi: 10.1002/art.1780190213. [DOI] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Parker H. G., Radin E. L., Krane S. M. Effects of proteolytic enzymes on structural and mechanical properties of cartilage. Arthritis Rheum. 1972 Sep-Oct;15(5):497–503. doi: 10.1002/art.1780150505. [DOI] [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Muir H. Heberden Oration, 1976. Molecular approach to the understanding of osteoarthrosis. Ann Rheum Dis. 1977 Jun;36(3):199–208. doi: 10.1136/ard.36.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Sapolsky A. I., Howell D. S., Woessner J. F., Jr Neutral proteases and cathepsin D in human articular cartilage. J Clin Invest. 1974 Apr;53(4):1044–1053. doi: 10.1172/JCI107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan N. S., Lash J. W. Monomeric and aggregate proteoglycans in the chondrogenic differentiation of embryonic chick limb buds. J Embryol Exp Morphol. 1979 Jan;49:47–59. [PubMed] [Google Scholar]
- Vasan N. S., Lash J. W. Proteogylcan heterogeneity in embryonic chick articular and epiphyseal cartilages. Connect Tissue Res. 1978;6(3):191–199. doi: 10.3109/03008207809152631. [DOI] [PubMed] [Google Scholar]


