Abstract
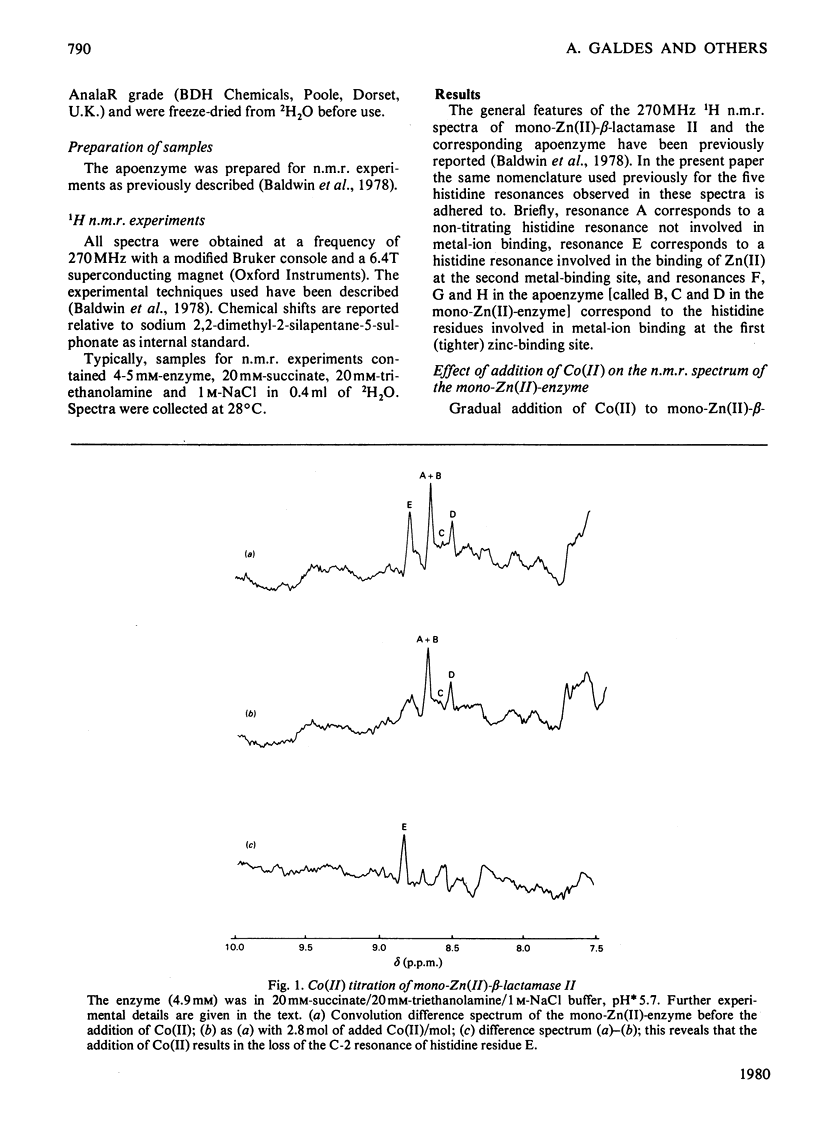
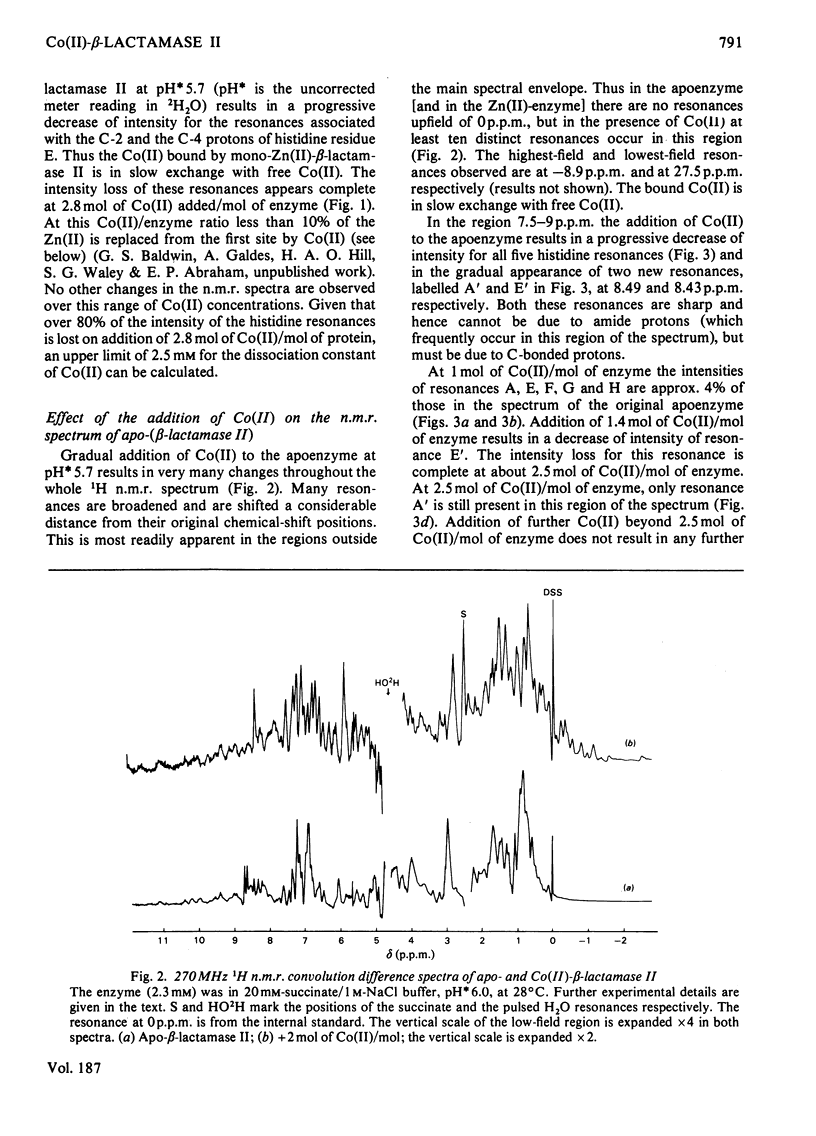
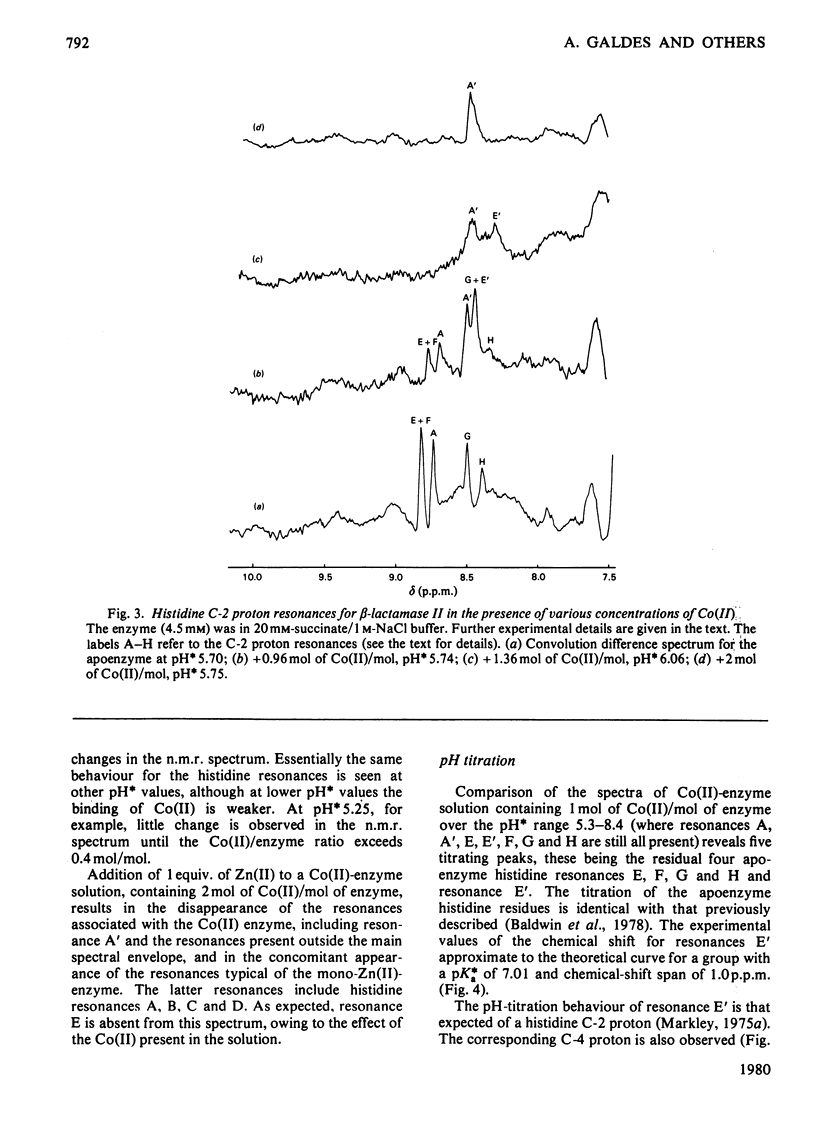
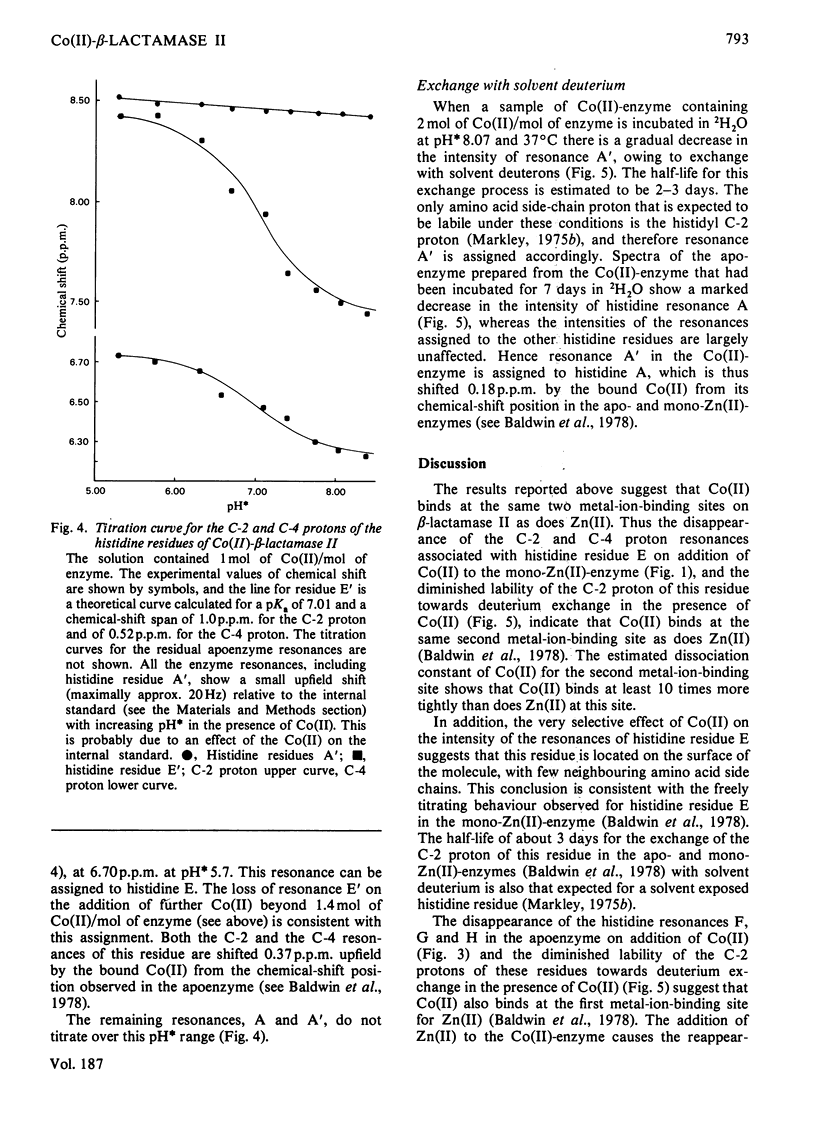
The 1H n.m.r. spectra of beta-lactamase II in the presence of Co(II) were studied. Analysis of the spectra suggests that Co(II) binds at the same two metal-binding sites as does Zn(II). The binding of Co(II) at the first site is much weaker than the binding of Zn(II) at this site, whereas the binding of Co(II) at the second site is tighter than the binding of Zn(II). The binding of Co(II) to the mono-zinc(II)-enzyme caused only one marked change in the spectrum, namely a decrease in the intensity of the resonances assigned to the C-2 and C-4 protons of one histidine residue (residue E). However, when the spectra of the apoenzyme and the Co(II)-enzyme were compared, there were many differences. A significant fraction of the protons in the whole molecule are affected by the binding of Co(II) at the first metal-ion-binding site (where the ligands are the enzyme's sole thiol group and three histidine residues). This may be because the first site is internal, or because of a difference in conformation between the apoenzyme and the mono-Co(II)-enzyme. The second site may be located on the surface of the molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Hill O., Smith B. E., Storm C. B. The proton magnetic resonance spectra of a cobalt (II) azurin. Biochem Biophys Res Commun. 1976 Jun 7;70(3):783–790. doi: 10.1016/0006-291x(76)90660-4. [DOI] [PubMed] [Google Scholar]
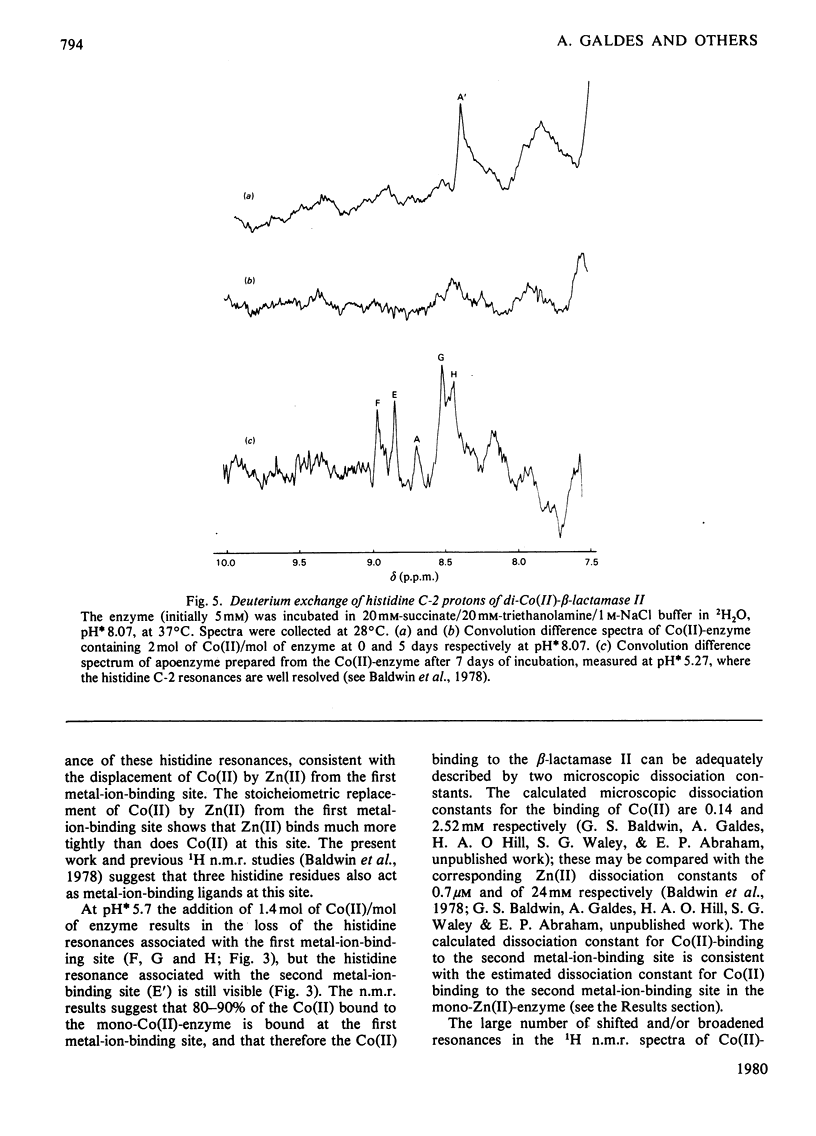
- Baldwin G. S., Galdes A., Hill H. A., Smith B. E., Waley S. G., Abraham E. P. Histidine residues of zinc ligands in beta-lactamase II. Biochem J. 1978 Nov 1;175(2):441–447. doi: 10.1042/bj1750441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Waley S. G., Abraham E. P. Identification of histidine residues that act as zinc ligands in beta-lactamase II by differential tritium exchange. Biochem J. 1979 Jun 1;179(3):459–463. doi: 10.1042/bj1790459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkinski R. E., Agresti D. G., Chen D. M., Glickson J. D. An analysis of the Co2+-induced nuclear magnetic resonance perturbations of hen egg white lysozyme. Biochemistry. 1978 Apr 18;17(8):1463–1468. doi: 10.1021/bi00601a016. [DOI] [PubMed] [Google Scholar]
- Markley J. L. Correlation proton magnetic resonance studies at 250 MHz of bovine pancreatic ribonuclease. I. Reinvestigation of the histidine peak assignments. Biochemistry. 1975 Aug 12;14(16):3546–3554. doi: 10.1021/bi00687a006. [DOI] [PubMed] [Google Scholar]
- Proceedings of the biochemical society. Biochem J. 1966 Jan;98(1):1–16P. [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L. Cobalt substituted zinc metalloenzymes. Adv Exp Med Biol. 1973;40:1–12. doi: 10.1007/978-1-4684-3240-4_1. [DOI] [PubMed] [Google Scholar]


