Abstract
Purpose:
This study aimed to explore the influence of high altitude on myopia, macular choroidal thickness (mCT), and macular retinal thickness (mRT) in adolescents.
Methods:
Two schools, one in Shanghai (at sea level) and one in Shigatse, Tibet (more than 4000 m above sea level), were selected. Refractive error was measured by an autorefractor instrument and subjective refraction, and mCT and mRT were measured at three concentric circles by optical coherence tomography. Student’s t-test, Chi-square test, and multiple linear regression analyses were used to analyze the data.
Results:
A total of 1114 participants (657 and 457 in Shanghai and Tibet, respectively) were enrolled in this cross-sectional study. The average age of the participants was 18.81 ± 1.10 years, and 44.34% were males. The spherical equivalent (SE) of adolescents in Shanghai was significantly lower than that of adolescents in Tibet (-4.14 ± 2.37 D and -2.12 ± 1.87 D, P < 0.01). The prevalence of myopia and high myopia among adolescents in Shanghai (94.52%, 19.48%) was significantly higher than those among adolescents in Tibet (44.74%, 2.41%) (P < 0.01). The mCT of Tibetan adolescents was significantly thicker than that of Shanghai adolescents (295.80 ± 62.46 μm and 218.71 ± 61.42 μm, P < 0.01), especially the central mCT. The mRT of Tibetan adolescents was also thicker than that of Shanghai adolescents (301.42 ± 23.26 μm and 281.04 ± 12.24 μm, P < 0.01).
Conclusions:
Compared with Shanghai adolescents, the choroid of Tibet adolescents is thicker, and the myopia prevalence is lower. It is speculated that increased altitude is associated with the thickening of mCT and a low myopia prevalence.
Key words: Adolescents, choroidal thickness, high altitude, myopia, retinal thickness
Myopia, the blurring of distant vision, is a common eye disease present worldwide.[1] Complications associated with progressive myopia, including myopic maculopathy and high-myopic-associated optic neuropathy, have become common reasons for irreversible vision loss and blindness.[2,3] By 2050, the prevalence of myopia and high myopia is estimated to be 4.76 billion (49.8% of the global population) and 1 billion (9.8% of the global population), respectively.[4] Therefore, myopia prevention and control have attracted attention worldwide. However, presently, myopia research mainly focuses on low-altitude areas with convenient transportation and dense populations, and there are a few research studies on myopia in high-altitude regions with sparse populations.
An altitude of more than 1500 m is generally considered a high altitude.[5] The prevalence of myopia among adolescents in high-altitude regions is reportedly significantly lower than that in adolescents in low-altitude regions.[6,7] Myopia prevalence was reported to be 53.80% in children and adolescents living in Chongqing, China (average altitude: 500 m), and 43.86% in Qamdo, China (average altitude: 3500 m), and high myopia prevalence was 7.04% and 1.30%, respectively.[6] Myopia prevalence in adolescents was 56.92% in Tianjin, China (average altitude: 6 m), and 28.51% in Naidong, China (average altitude: 3500 m).[7] These studies suggest that the lower prevalence of myopia may be related to a lower educational level, less eye use at close range, and more outdoor activities at high altitudes. However, it ignores the influence of the environment of the plateau (solar radiation, ultraviolet radiation, and anoxia) on the retina and choroid in the fundus, leading to myopia. Furthermore, the high-altitude areas in these studies were approximately 3500 m above sea level or below it, and research on myopia in adolescents at ultra-high altitudes of more than 4000 m is scarce.
The thickness of the retina and choroid impacts myopia development.[8] Stretching of the ocular wall associated with myopia can cause gradual thinning of the choroid and retina, resulting in the progression of retinopathy.[9] Moreover, increased choroidal thickness is considered a protective factor of myopia.[10] Low oxygen levels at high altitudes may cause a significant decrease in oxygen saturation in the fundus, which is accompanied by obvious changes in the choroid or retina.[11,12] However, these studies were conducted in humans exposed acutely to extreme altitudes, and the long-term effects of altitude on choroidal thickness have revealed controversial results. One study compared adolescents living at different altitudes (1535, 1917, and 2936 m above sea level) and showed that choroidal thickness increases with altitude.[13] In contrast, another study showed that choroidal thickness is thinner at higher altitudes (Ağrı City, Turkey; average altitude: 1,630 m) than at lower altitudes (Ordu City, Turkey; average altitude: 5 m).[14] Additionally, one study reported no significant effect of altitude on choroidal thickness.[12] However, in all these studies, the highest altitude was less than 3000 m, and the altitude difference between regions was not significant. Studies on the effects of ultra-high altitude (more than 4000 m) on the retina or choroid and those that investigate the effects of altitude on myopia, retina, and choroid simultaneously are still lacking.
Therefore, we identified and selected two regions in China with extremely contrasting altitudes: Shigatse, Tibet, and Shanghai. The Qinghai–Tibet plateau, known as the third pole and roof of the world, is not only the highest plateau worldwide but also the highest place for human habitation in China, with a population of more than 10 million.[15] Shigatse in Tibet is located on this plateau, and the average altitude is more than 4000 m, and the average oxygen level is only 65% of the oxygen level at sea level. With an average altitude of only 4 m above sea level, Shanghai’s natural environment contrasts sharply with that of Tibet. Therefore, by comparing adolescents living in Tibet and Shanghai, this study simultaneously explored the influence of high altitude on myopia, choroid thicknesses, and retinal thicknesses.
Methods
Setting and participants
Random cluster sampling was applied in this study. One school in Shanghai (at sea level) and one in Shigatse, Tibet (4000 m above sea level), were selected for this research. A total of 1266 adolescents studying in 2016 were enrolled, including 703 in Shanghai and 563 in Tibet. The protocol for this cross-sectional study was approved by the Ethics Committee of Shanghai General Hospital and followed the tenets of the Declaration of Helsinki. All participants understood the study protocol and provided signed informed consent.
The inclusion criteria were as follows: (1) best-corrected visual acuity ≥0.8; (2) cooperation with ophthalmic examinations; and (3) living in the same place for at least 10 years. The exclusion criteria were as follows: (1) presence of ophthalmic diseases, such as dominant strabismus, cataract, glaucoma, choroidopathy, and retinopathy; (2) the presence of combined systemic diseases; and (3) a previous history of ocular trauma or ocular surgery. Briefly, except for myopia-associated optic disk and peripapillary changes, the participants had no other ocular abnormalities. Only the right eye of each participant was assessed.
Apparatus and methods
All participants underwent the same ophthalmic examinations, including axial length (AL), refractive error, choroidal thickness, and retinal thickness assessments. AL was measured using optical low-coherence reflectometry (Lenstar; Haag-Streit AG, Koeniz, Switzerland). Refractive error was measured by an autorefractor instrument (model KR-8900; Topcon, Tokyo, Japan) and subjective refraction without mydriatics. The spherical equivalent (SE) was calculated as the sum of the spherical plus and half of the cylinder plus. According to the SE of the participants, all participants were divided into four groups: M1 (non-myopia): SE ≥-0.5D; M2 (mild myopia): -0.5D > SE≥-3D; M3 (moderate myopia): -3D > SE≥-6D; and M4 (high myopia): SE<-6D.
Macular retinal thickness (mRT) and macular choroidal thickness (mCT) were measured by swept-source optical coherence tomography (SS-OCT; model DRI OCT-1 Atlantis; Topcon). The mRT was defined as the vertical distance between the inner boundary membrane and the retinal pigment epithelium interface. The mCT was defined as the vertical distance from the outermost hyper-reflective line of the retinal pigment epithelium to the inner margin of the sclera [Fig. 1a]. Each scan was divided into three concentric circles with nine regions as follows: a central circle (diameter: 1 mm), an inner circle (diameter: 3 mm), and an outer circle (diameter: 6 mm). The mRT and mCT were measured by OCT at nine regions: the fovea and the nasal (N), temporal (T), superior (S), and inferior (I) orientations at 1500 and 3000 μm from the fovea [Fig. 1b]. Before OCT image collection, AL and spherical and cylindrical power diopters were input into the system to adjust the magnification factors. All measurements were conducted by technicians who were experienced in taking OCT images. The mean values calculated after three repeated measurements of each participant’s mCT and mRT were finally recorded. The OCT was performed twice for the first 50 participants in each region to assess measurement reproducibility. Images with a signal strength index of 60 or less were excluded from the analysis. Besides, due to the diurnal and seasonal variation in choroidal thickness, all OCT measurements were performed simultaneously between 9:00 and 11:00 am for a consecutive week.
Figure 1(Original).
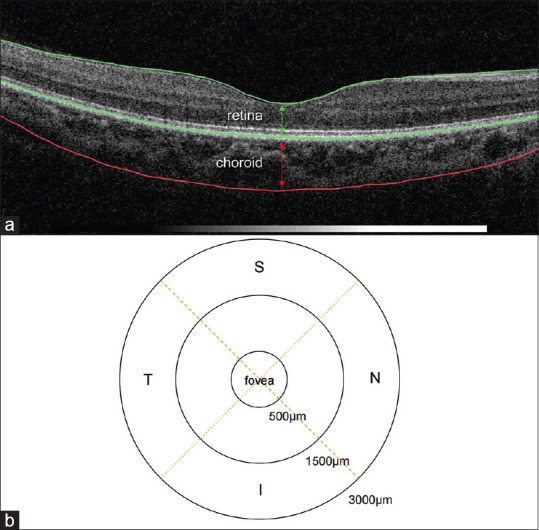
Definition of certain thickness and locations. (a) The two layers in the figure, from top to bottom, represent the retina and the choroid. (b) The nine locations for thickness detection. N, nasal; T, temporal; S, superior; I, inferior
Statistical analyses
All analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) Statistics version 26.0 software. All data were entered twice, independently by two research associates, and all discrepancies were adjudicated. Baseline characteristics were shown as counts or proportions for categorical data and as means ± standard deviation for continuous data. The distribution of all variables was examined for normality using the Kolmogorov − Smirnov test. Student’s t-test and Chi-square test were used to compare continuous and categorical variables, respectively. The effects of altitude, sex, age, and SE (independent variables) on mCT or mRT (dependent variables) and the effects of altitude, sex, age, mCT, and mRT (independent variables) on SE (dependent variables) were assessed using multiple linear regression analyses. The subjects were divided into a non-myopic group (M1) and a myopic group (M2, M3, and M4), and multiple linear regression analyses and interaction analyses were conducted to analyze the SE and related factors of these two groups. Statistical significance was set as P < 0.05 (two-sided).
Results
General characteristics
Of the 1266 students enrolled in the study, 67 adolescents were excluded due to a best-corrected visual acuity of < 0.8 and 85 due to abnormal reading of thickness data. Finally, 1114 students were included in the analysis (657 in Shanghai and 457 in Tibet). The average age of the participants was 18.81 ± 1.10 years, and 44.34% were males. The overall myopia rate was 85.55%, the prevalence of high myopia was 12.48%, and the average SE was -3.32 ± 2.39D [Table 1].
Table 1.
Demographic and ocular characteristics of the study population
| Shanghai (n=657) | Tibet (n=457) | P | Total (n=1114) | |
|---|---|---|---|---|
| Age, year | 18.77±1.20 | 18.86±0.92 | 0.15 | 18.81±1.10 |
| Male, n (%) | 302 (45.97) | 192 (42.01) | 0.19 | 494 (44.34) |
| Myopia, n (%) | 621 (94.52) | 332 (72.65) | <0.01 | 953 (85.55) |
| AL, mm | 25.27±1.10 | 23.90±1.07 | <0.01 | 24.71±1.28 |
| SE, D | -4.14±2.37 | -2.12±1.87 | <0.01 | -3.32±2.39 |
| M1, n (%) | 36 (5.48) | 125 (27.35) | <0.01 | 161 (14.45) |
| M2, n (%) | 199 (30.28) | 210 (45.95) | <0.01 | 409 (36.71) |
| M3, n (%) | 294 (44.74) | 111 (24.29) | <0.01 | 405 (36.36) |
| M4, n (%) | 128 (19.48) | 11 (2.41) | <0.01 | 139 (12.48) |
| mCT, µm | ||||
| Central circle | 221.66±69.24 | 360.46±71.70 | <0.01 | 278.60±97.97 |
| Inner circle | 222.15±64.83 | 300.94±61.71 | <0.01 | 254.47±74.43 |
| Outer circle | 214.55±57.29 | 274.50±66.30 | <0.01 | 239.14±67.87 |
| Average | 218.71±61.42 | 295.80±62.46 | <0.01 | 250.34±72.53 |
| mRT, µm | ||||
| Central circle | 228.05±18.01 | 280.30±35.03 | <0.01 | 249.49±36.81 |
| Inner circle | 305.05±14.08 | 319.39±24.61 | <0.01 | 310.93±20.36 |
| Outer circle | 270.27±12.54 | 288.72±32.56 | <0.01 | 277.84±24.69 |
| Average | 281.04±12.24 | 301.42±23.26 | <0.01 | 289.40±20.26 |
Continuous variables are shown as mean±SD, and categorical variables are shown as n (%). Continuous variables are analyzed by Student’s t-test, and categorical variables are compared by the Chi-square test. AL, axial length; SE, spherical equivalent; M1, non-myopia (SE ≥-0.5 D); M2, mild myopia (-0.5 D>SE ≥-3 D); M3, moderate myopia (-3 D>SE≥-6 D); M4, high myopia (SE <-6 D); mCT, macular choroidal thickness; mRT, macular retinal thickness
The myopia prevalence of adolescents in Tibet was significantly lower than that in Shanghai (P < 0.01). Moderate myopia was the main myopia type among Shanghai adolescents (44.74%). The prevalence and SE of myopia were 94.52% and -4.14 ± 2.37D. Mild myopia was the main myopia type among Tibetan adolescents (45.95%). The prevalence and SE of myopia were 72.65% and -2.12 ± 1.87D. Moreover, the prevalence of high myopia among Tibetan adolescents was significantly lower than that among Shanghai adolescents (2.41% and 19.48%, respectively, P < 0.01). Results are shown in Table 1.
The influencing factors of choroid thickness
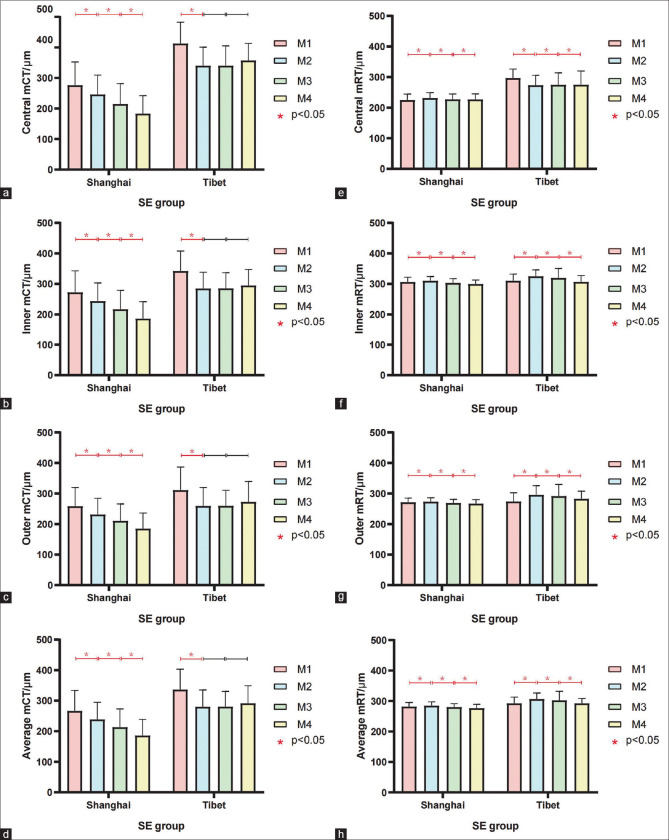
The central, inner, outer, and average mCTs of the Tibetan adolescents were significantly more than those of the Shanghai adolescents [Table 1]. Central mCT showed the largest difference among all mCTs between the adolescents in the two regions (360.46 ± 71.70 μm and 221.66 ± 69.24 μm, respectively), and this difference gradually decreased toward the periphery [Table 1]. To exclude the effect of myopia on mCT, this study further divided the participants into four groups according to their SE. The results showed that the mCT of Tibetan adolescents was significantly more than that of Shanghai adolescents in all groups. Moreover, the mCT of the Shanghai adolescents gradually reduced from the M1 group to the M4 group, whereas there was no significant difference in the mCT among the mild, moderate, and high myopia groups in the Tibetan adolescents [Fig. 2a-d]. Finally, we analyzed the influencing factors of mCT through multiple linear regression analysis [Table 2] and found that mCT was still more in the Tibetan adolescents than in the Shanghai adolescents. The influence of region on mCT was greater in the central choroid (coefficient = 117.13) than in the inner (coefficient = 59.79) and outer (coefficient = 44.29) circles.
Figure 2.
Comparison of central mCT (a), inner mCT(b), outer mCT(c) and average mCT(d) in different SE groups in Shanghai and Tibet adolescents. Comparison of central mRT (e), inner mRT(f), outer mRT(g) and average mRT(h) in different SE groups in Shanghai and Tibet adolescents
Table 2.
Multivariate regression analyses for mCT
| Total | Shanghai | Tibet | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Coefficient | P | Coefficient | P | Coefficient | P | |
| Central | ||||||
| Constant | 165.31 | <0.01 | 222.93 | <0.01 | 557.99 | <0.01 |
| Region | 117.13 | <0.01 | - | - | - | - |
| Sex | -2.89 | 0.47 | -8.89 | 0.08 | 6.49 | 0.34 |
| Age | -0.61 | 0.74 | 3.07 | 0.14 | -9.71 | <0.01 |
| SE | 10.85 | <0.01 | 10.93 | <0.01 | 11.59 | <0.01 |
| Inner | ||||||
| Constant | 225.12 | <0.01 | 230.81 | <0.01 | 491.04 | <0.01 |
| Region | 59.79 | <0.01 | - | - | - | - |
| Sex | -2.97 | 0.42 | -8.83 | 0.06 | 5.14 | 0.38 |
| Age | -1.00 | 0.55 | 2.48 | 0.21 | -9.47 | 0.02 |
| SE | 9.54 | <0.01 | 10.02 | <0.01 | 9.24 | <0.01 |
| Outer | ||||||
| Constant | 236.60 | <0.01 | 223.93 | <0.01 | 477.08 | <0.01 |
| Region | 44.29 | <0.01 | - | - | - | - |
| Sex | -4.21 | 0.24 | -9.54 | 0.02 | 3.53 | 0.71 |
| Age | -1.44 | 0.37 | 2.10 | 0.23 | -10.13 | <0.01 |
| SE | 7.93 | <0.01 | 8.23 | <0.01 | 8.02 | <0.01 |
| Average | ||||||
| Constant | 223.56 | <0.01 | 226.87 | <0.01 | 492.27 | <0.01 |
| Region | 59.27 | <0.01 | - | - | - | - |
| Sex | -3.51 | 0.32 | -9.15 | 0.04 | 4.57 | 0.44 |
| Age | -1.15 | 0.47 | 2.39 | 0.20 | -9.79 | 0.02 |
| SE | 8.97 | <0.01 | 9.33 | <0.01 | 8.96 | <0.01 |
mCT, macular choroidal thickness; SE, spherical equivalent
The influencing factors of retinal thickness
The central, inner, outer, and average mRTs of the Tibetan adolescents were significantly more than those of the Shanghai adolescents. The difference in the mRT between the adolescents in the two regions was largest in the central mRT (280.30 ± 35.03 μm and 228.05 ± 18.01 μm, respectively) and gradually decreased toward the periphery [Table 1]. The retinal thickness was the highest in the M2 group and showed a trend of first increasing and then decreasing with changes in the SE [Fig. 2e-h]. Finally, we analyzed the influencing factors of mRT through multiple linear regression analysis [Table 3]. Similar to the mCT, the mRT was more in the Tibetan adolescents than in the Shanghai adolescents, and the influence of region on mRT in the central retina (coefficient = 50.10) was also more than that in the inner (coefficient = 13.10) and outer (coefficient = 18.73) circles.
Table 3.
Multivariate regression analyses of mRT
| Total | Shanghai | Tibet | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Coefficient | P | Coefficient | P | Coefficient | P | |
| Central | ||||||
| Constant | 194.62 | <0.01 | 229.64 | <0.01 | 327.74 | <0.01 |
| Region | 50.10 | <0.01 | - | - | - | - |
| Sex | -7.18 | <0.01 | -5.99 | <0.01 | -7.27 | 0.03 |
| Age | -0.03 | 0.97 | 0.52 | 0.37 | -1.59 | 0.37 |
| SE | 1.21 | <0.01 | 0.52 | 0.07 | 2.82 | <0.01 |
| Inner | ||||||
| Constant | 291.83 | <0.01 | 308.24 | <0.01 | 312.20 | <0.01 |
| Region | 13.10 | <0.01 | - | - | - | - |
| Sex | -6.47 | <0.01 | -5.93 | <0.01 | -9.51 | <0.01 |
| Age | 0.70 | 0.18 | 0.66 | 0.13 | 1.02 | 0.41 |
| SE | 0.71 | 0.01 | 1.54 | <0.01 | -1.44 | 0.02 |
| Outer | ||||||
| Constant | 248.74 | <0.01 | 273.69 | <0.01 | 273.58 | <0.01 |
| Region | 18.73 | <0.01 | - | - | - | - |
| Sex | -4.53 | <0.01 | -3.68 | <0.01 | -8.67 | 0.01 |
| Age | 0.51 | 0.42 | 0.34 | 0.39 | 1.21 | 0.46 |
| SE | -0.07 | 0.82 | 1.00 | <0.01 | -2.85 | <0.01 |
| Average | ||||||
| Constant | 261.88 | <0.01 | 284.15 | <0.01 | 296.76 | <0.01 |
| Region | 19.71 | <0.01 | - | - | - | - |
| Sex | -5.68 | <0.01 | -4.94 | <0.01 | -8.89 | <0.01 |
| Age | 0.53 | 0.27 | 0.50 | 0.19 | 0.81 | 0.49 |
| SE | 0.42 | 0.08 | 1.19 | <0.01 | -1.60 | 0.01 |
mRT, macular retinal thickness; SE, spherical equivalent
The influencing factors of SE
Table 4 shows further analysis of the SE and its related factors. The results of the multiple linear regression analysis showed that the average mCT (coefficient = 0.008, P < 0.01) and region (coefficient = -2.486, P < 0.01) are relevant factors of SE. Interaction analysis showed that the effect of mCT on SE was greater in Shanghai than in Tibet (coefficient = 0.005, P = 0.01). By dividing the participants into the non-myopic and myopic groups, the SE and related factors of the two groups were analyzed by multiple linear regression analysis, and an interaction analysis was further conducted. The results showed that in the non-myopic group, there was no significant difference in the influence of mCT on SE between Shanghai and Tibetan adolescents (P = 0.76). In the myopic group, the change in mCT had no significant effect on the SE of Tibetan adolescents (P = 0.85); however, in the Shanghai adolescents, the mCT was related to SE, and the SE increased by 0.01D for every 1 μm increase in the choroid thickness (P < 0.01).
Table 4.
Multivariate regression analyses for SE
| Total | Non-myopia | Myopia | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Coefficient | P | Coefficient | P | Coefficient | P | |
| Constant | -6.399 | <0.01 | 0.619 | 0.07 | -8.153 | <0.01 |
| Age | 0.057 | 0.31 | -0.003 | 0.85 | 0.038 | 0.51 |
| Sex | -0.304 | 0.02 | -0.03 | 0.29 | -0.078 | 0.55 |
| Average mRT | 0.004 | 0.24 | -0.003 | 0 | 0.015 | <0.01 |
| Average mCT | 0.008 | <0.01 | 0.001 | <0.01 | 0.000 | 0.85 |
| Region | -2.486 | <0.01 | -0.044 | 0.76 | -3.321 | <0.01 |
| Region*mCT | 0.005 | 0.01 | <0.01 | 0.71 | 0.010 | <0.01 |
SE, spherical equivalent
Discussion
This cross-sectional study assessed the effect of altitude on choroidal and retinal thickness by comparing adolescents in Shanghai and Tibet. Our results showed that the mCT of the Tibetan adolescents was more than that of the Shanghai adolescents, especially at the central circle (360.46 ± 71.70 and 221.66 ± 69.24 μm, respectively). A previous study compared adolescents living at different altitudes (1535, 1917, and 2936 m) and found that choroidal thickness increased with altitude, which was consistent with the results of our study.[13] However, in that study, the difference in central mCT among adolescents in the three regions was not as significant as that in our study (277 ± 56 μm, 280 ± 52 μm, and 302 ± 62 μm, respectively). This may be related to the fact that the highest altitude in that study was <3000 m and the altitude difference among the three regions was not significant.
The phenomenon of mCT increasing with altitude has been previously discussed in many studies, and it is possibly related to the compensation of hypoxia at high altitudes. The choroid is rich in blood vessels and important for providing oxygen to the outer retina and the anterior optic nerve.[16] Retinal arterial oxygen saturation was significantly associated with peripheral arterial oxygen saturation and central mCT.[12] At high altitudes, the intraocular environment, including the retina, is significantly hypoxic.[12] Therefore, choroidal blood flow velocity and blood flow increase with an increase in altitude; thus, the higher the altitude, the more obvious the increase in choroidal blood flow.[11] Changes in choroidal blood flow may alter choroidal thickness through associated changes in blood vessel diameters, especially in mammals and primates whose choroids lack lacunae.[17,18] Therefore, we speculated that such long-term changes in choroidal blood flow can lead to an increase in the choroidal thickness in people living in high-altitude regions. Additionally, the fovea, where vision is most acute, requires more oxygen than the rest of the retina, which is consistent with the observation of the most significant changes in central mCT. This phenomenon further supports the hypothesis that choroidal thickening is compensation for hypoxia present in plateau regions. Moreover, we found that the mRT increased slightly at high altitudes, which was most obvious in the central circle (280.30 ± 35.03 μm and 228.05 ± 18.01 μm in the Tibetan and Shanghai adolescents, respectively), which may also be due to hypoxia compensation. Similar to the mCT, reduced retinal oxygen saturation leads to a compensatory increase in retinal blood flow, ultimately leading to changing the mRT. The greater oxygen demand in the fovea may be related to the more significant thickening in the central mRT.[11,19]
The prevalence of myopia and high myopia among Tibetan adolescents was significantly lower than that among Shanghai adolescents. We speculated that choroidal thickness could be an important internal factor affecting the differences in myopia prevalence. Myopia occurrence and development may be related to retina and sclera anoxia.[20] Choroidal blood flow is the main source of oxygen and nourishment supply for the outer five layers of the retina and the adjacent sclera. Reduced choroidal blood flow creates a relative hypoxic environment and induces a series of changes in the sclera and retina, finally accelerating axial elongation and promoting the onset and progression of myopia.[20] Increased choroidal blood flow may ameliorate scleral hypoxia, thereby inhibiting axial elongation and myopia development.[21] Additionally, synthesis activity in the choroid is related to choroidal thickness. Changes in choroid thickness may regulate long-term scleral changes and are closely related to scleral remodeling.[22] Therefore, choroidal thickening is considered to be a protective factor for the prevention and control of the acceleration of myopia.[23,24] Myopia prevention and control measures, such as orthokeratology, atropine administration, and low-intensity laser therapy, can increase choroidal thickness and slow myopia progression in children, suggesting that choroidal blood flow is involved in the occurrence and development of myopia.[10,23,25] Therefore, the compensatory increase in blood flow and choroidal thickening at high altitudes may prevent or delay myopia development by maintaining a stable intraocular environment. Moreover, the results of the multiple regression and interaction analyses of SE deserve attention. For the myopic participants, mCT change had a significant impact on SE in the Shanghai adolescents (P < 0.01), whereas the change in choroidal thickness had no significant effect on SE in the Tibetan adolescents (P = 0.85). This may be related to the fact that although the mCT in the Tibetan participants was significantly more than that in the Shanghai participants, there was no statistically significant difference in the mCT in the mild, moderate, and high myopia groups in the Tibetan participants (P > 0.05). Among the Shanghai participants, the mCT of the mild, moderate, and high myopia groups showed statistically significant differences (P < 0.01), and adolescents with mild myopia had a thicker choroid. Therefore, we propose the following speculation: On the one hand, people with a relatively thick choroid themselves may be less likely to be myopic than those with a thin choroid. On the other hand, with some myopia prevention and control measures (such as atropine and low-intensity laser treatment), people with more significant choroidal thickening may be less prone to myopia than people without significant choroidal thickening.
In addition to the lack of oxygen, other natural conditions at the plateau, such as high solar radiation and ultraviolet radiation, may also affect the retina and choroid of the fundus, thereby affecting the occurrence and development of myopia. In high-altitude regions, sunshine hours are long, and ultraviolet and solar radiation are strong.[26] Studies showed that prolonged or high-intensity outdoor light may have a protective effect on myopia in adolescents.[27] Besides, ultraviolet exposure can affect axial elongation of the eye and prevent myopia progression.[28,29] Therefore, the effect of high altitude on the choroid and retina and the protective effect on myopia may also be related to high sun exposure and ultraviolet rays.
By comparing adolescents in Shanghai and Tibet, this study explored the influence of extreme altitudes on the fundus and myopia and supplemented the data on myopia, retinal thickness, and choroidal thickness of adolescents at ultra-high altitudes. However, there are some limitations in this study. Due to the special natural and social environment of high altitudes, this study failed to include other genetic and environmental factors of myopia, such as school time, outdoor exposure, near work time, the use of electronic devices, economic situation, and genetic background, in the analysis.[30] We only explored the possible correlation among high-altitude environment, choroidal thickness, retinal thickness, and myopia.
Conclusion
In conclusion, this is the first study to simultaneously explore the effects of ultra-high altitude on myopia, mCT, and mRT. This study found that the mCT and mRT were more in individuals residing in ultra-high-altitude regions, and the prevalence of myopia in Tibetan adolescents was lower than that in Shanghai adolescents. Changes in choroidal thickness in the natural environment at high altitude may be associated with myopia development.
Abbreviation
AL: axial length
SE: spherical equivalent
SS-OCT: swept-source optical coherence tomography
mRT: macular retinal thickness
mCT: macular choroidal thickness.
Ethics approval and consent to participate
All ophthalmic examinations in this study were noninvasive. The study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University, and followed the tenets of the Declaration of Helsinki. All participants understood the study protocol and provided signed informed consent.
Consent for publication
The manuscript is approved by all authors for publication.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China under grants 81970811 and 82371072 and the Domestic Science and Technology Cooperation Project of Shanghai Municipal Science and Technology Commission under grant 21015800700.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X, et al. Myopia. Nat Rev Dis Primers. 2020;6:99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62:6. doi: 10.1167/iovs.62.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Song P, Han H, Feng H, Hui Y, Zhou T, Meng W, et al. High altitude relieves transmission risks of COVID-19 through meteorological and environmental factors:Evidence from China. Environ Res. 2022;212:113214. doi: 10.1016/j.envres.2022.113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Xiang Y, Zhu L, Zheng S, Ji Y, Lv B, et al. Myopia progression and associated factors of refractive status in children and adolescents in Tibet and Chongqing during the COVID-19 pandemic. Front Public Health. 2022;10:993728. doi: 10.3389/fpubh.2022.993728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian X, Liu B, Wang J, Wei N, Qi X, Li X, et al. Prevalence of refractive errors in Tibetan adolescents. BMC Ophthalmol. 2018;18:118. doi: 10.1186/s12886-018-0780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang X, Lv L, Xiang Y. Research progress of retinal and choroidal thickness and blood flow in myopia. Acta Med Univ Sci Technol Huazhong. 2021;50:793–9. [Google Scholar]
- 9.Matsumura S, Kuo AN, Saw SM. An update of eye shape and myopia. Eye Contact Lens. 2019;45:279–85. doi: 10.1097/ICL.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 10.Ye L, Shi Y, Yin Y, Li S, He J, Zhu J, et al. Effects of atropine treatment on choroidal thickness in myopic children. Invest Ophthalmol Vis Sci. 2020;61:15. doi: 10.1167/iovs.61.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch MM, Merz TM, Barthelmes D, Petrig BL, Truffer F, Bloch KE, et al. New insights into ocular blood flow at very high altitudes. J Appl Physiol (1985) 2009;106:454–60. doi: 10.1152/japplphysiol.90904.2008. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Yang D, Sun Y, Xie Y, Zhang Z, Li S, et al. Retinal vessel oxygen saturation and vessel diameter in healthy individuals during high-altitude exposure. Acta Ophthalmol. 2019;97:279–86. doi: 10.1111/aos.13897. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Yang Y, Li YT, Huo CD, Wu WM, Wang JQ, et al. Research on choroidal thickness of adolescents lived in different high altitude areas. Chin J Optom Ophthalmol? Vis Sci. 2019;21:285–90. [Google Scholar]
- 14.Gok M, Karaman S, Erdem B. Evaluation of macular and choroidal thickness in healthy residents living at high altitude. Indian J Ophthalmol. 2022;70:1650–5. doi: 10.4103/ijo.IJO_2079_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Xin Z, Huang Y, Yu J. Climate suitability assessment on the Qinghai-Tibet Plateau. Sci Total Environ. 2022;816:151653. doi: 10.1016/j.scitotenv.2021.151653. [DOI] [PubMed] [Google Scholar]
- 16.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Zhang H, Zhang Y, Wang Q, Wang Y, Li ZW. Relationship between aquaporin-1 protein expression and choroidal thickness during the recovery of form-deprivation myopia in guinea pigs. Curr Eye Res. 2020;45:705–12. doi: 10.1080/02713683.2019.1689275. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wildsoet CF. RPE and choroid mechanisms underlying ocular growth and myopia. Prog Mol Biol Transl Sci. 2015;134:221–40. doi: 10.1016/bs.pmbts.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin C, Wang J, Zhang W, Wang L, Peng X. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS) Eye (Lond) 2014;28:415–21. doi: 10.1038/eye.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Zhang G, Zhou X, Xu R, Wang S, Guan Z, et al. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. 2019;60:3074–83. doi: 10.1167/iovs.18-26397. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Zhang S, Zhang G, Chen Y, Lei Y, Xiang J, et al. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2020;61:25. doi: 10.1167/iovs.61.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wang L, Xu Y, Pang Z, Mu G. The influence of the choroid on the onset and development of myopia:From perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 2021;99:730–8. doi: 10.1111/aos.14773. [DOI] [PubMed] [Google Scholar]
- 23.Xiong F, Mao T, Liao H, Hu X, Shang L, Yu L, et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int. 2021;2021:8915867. doi: 10.1155/2021/8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du DX, Song JK, Bi HS. Research progress of choroidal thickness and myopia prevention and control. Int Eye Sci. 2022;22:592–6. [Google Scholar]
- 25.Walline JJ, Greiner KL, McVey ME, Jones-Jordan LA. Multifocal contact lens myopia control. Optom Vis Sci. 2013;90:1207–14. doi: 10.1097/OPX.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Tian Q, Zhang X, Xu J, Tang G, Li R, et al. Prevalence of refractive error and visual acuity among school children in the plateau region of Qinghai, China. Int J Gen Med. 2021;14:5795–805. doi: 10.2147/IJGM.S326046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Sankaridurg P, Wang J, Chen J, Naduvilath T, He M, et al. Time outdoors in reducing myopia:A School-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022;129:1245–54. doi: 10.1016/j.ophtha.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Prepas SB. Light, literacy and the absence of ultraviolet radiation in the development of myopia. Med Hypotheses. 2008;70:635–7. doi: 10.1016/j.mehy.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–9. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philipp D, Vogel M, Brandt M, Rauscher FG, Hiemisch A, Wahl S, et al. The relationship between myopia and near work, time outdoors and socioeconomic status in children and adolescents. BMC Public Health. 2022;22:2058. doi: 10.1186/s12889-022-14377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]