Abstract
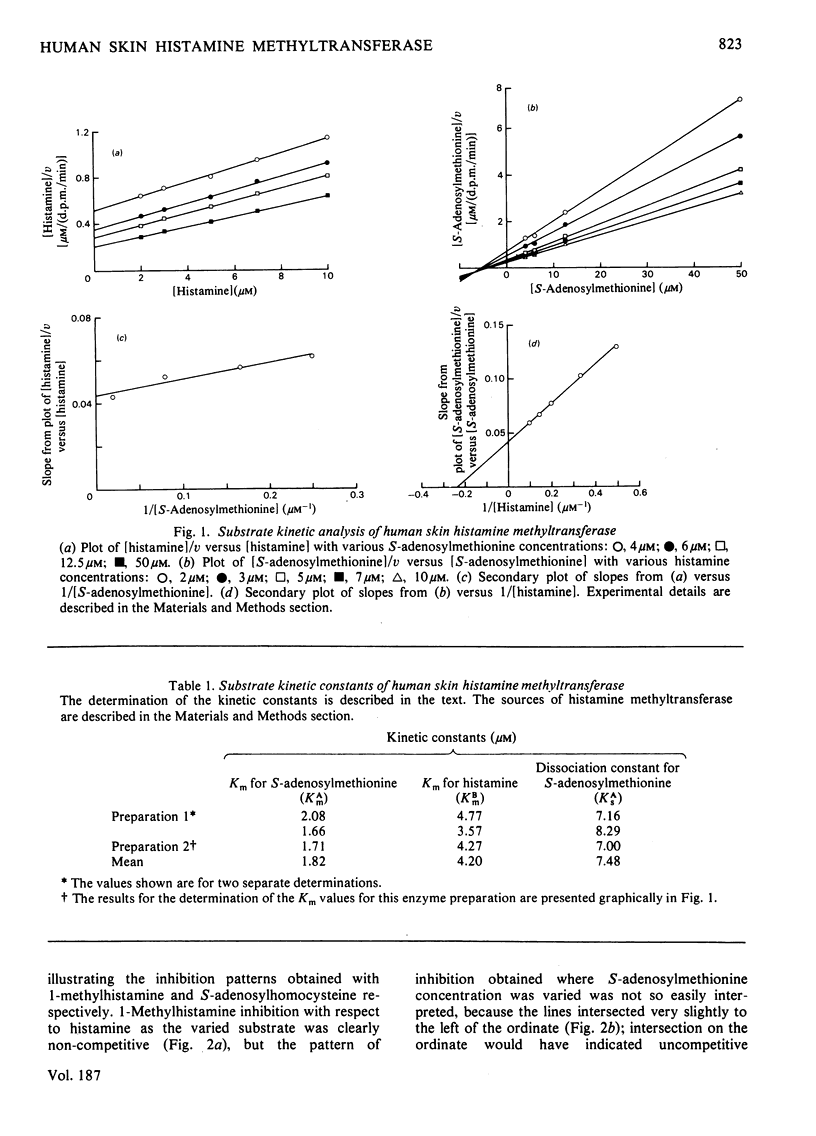
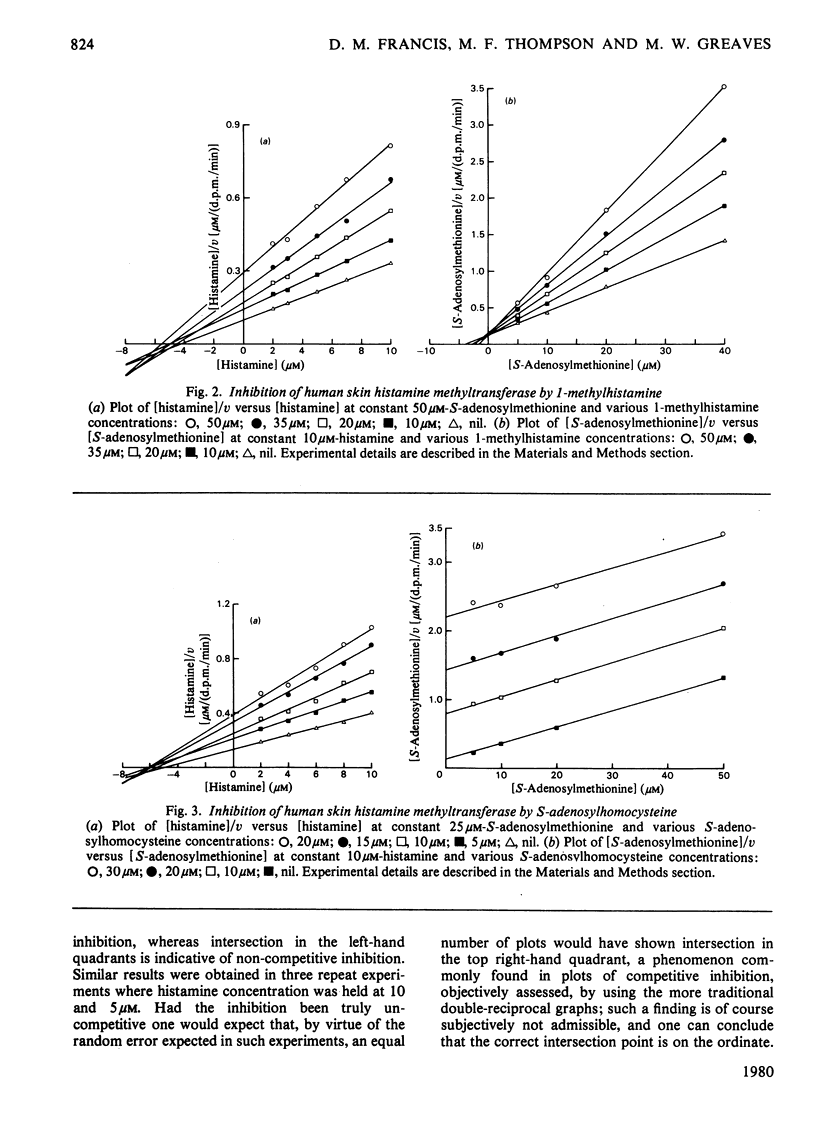
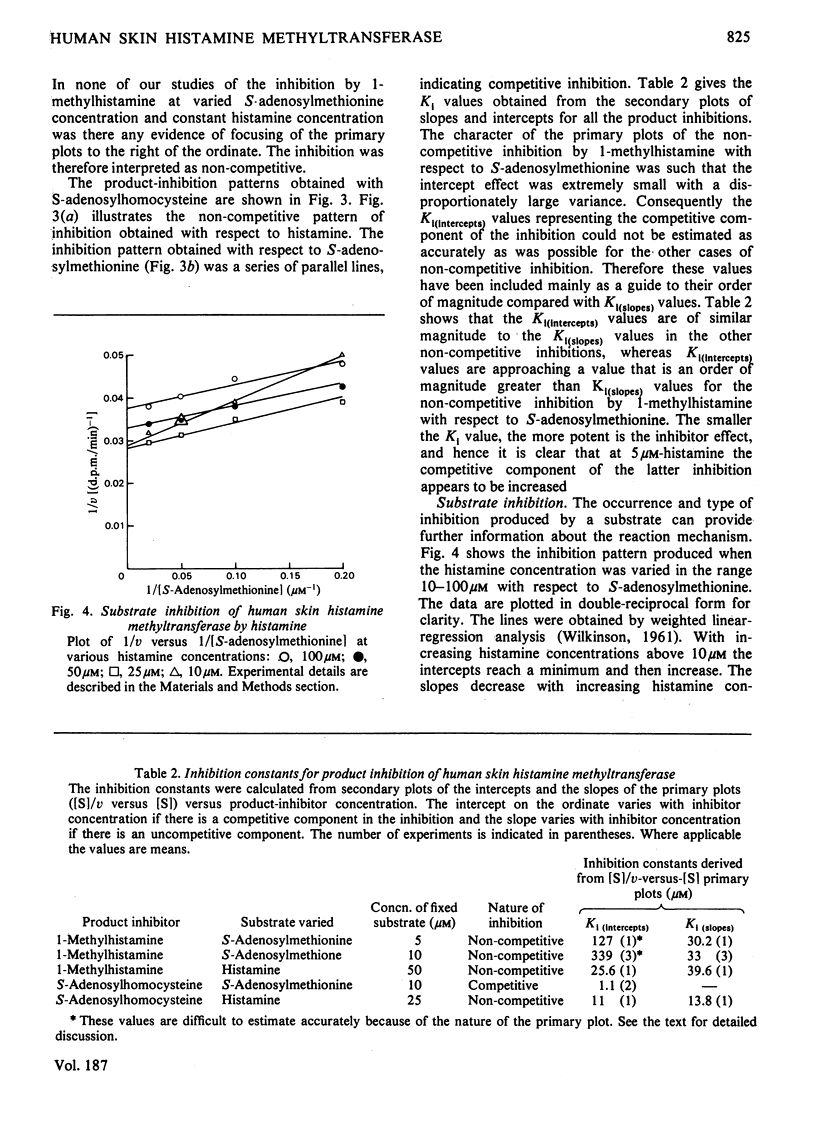
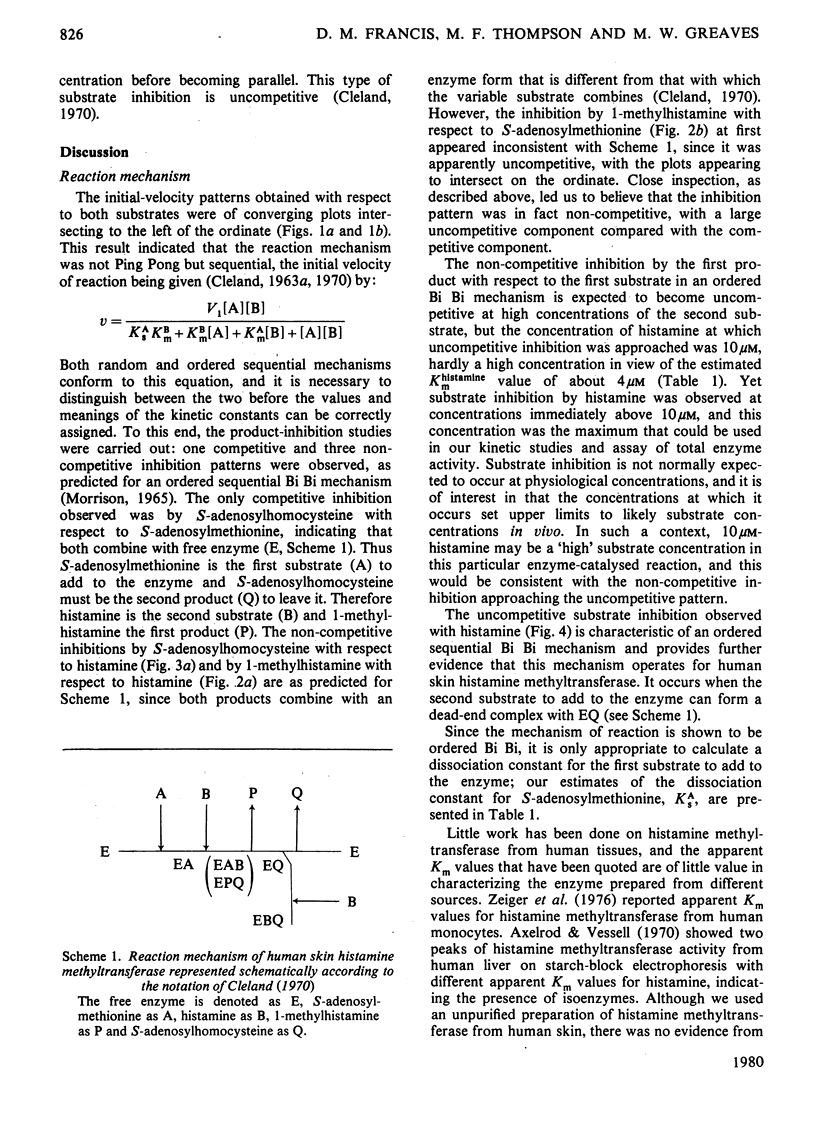
The substrate kinetic properties of histamine methyltransferase from human skin were studied at limiting concentrations of both histamine and S-adenosylmethionine. Substrate inhibition by histamine was observed at concentrations above 10 microM. Primary plots showed evidence of a sequential reaction mechanism. The Michaelis constants were derived from secondary plots of slopes from the primary plots ([S]/v versus [S]) versus reciprocal of the second substrate concentration. The mean Km values for histamine and S-adenosylmethionine were 4.2 and 1.8 microM respectively. Histamine in concentrations of 25-100 microM inhibited enzyme activity uncompetitively with respect to S-adenosylmethionine. No substrate inhibition was observed with S-adenosylmethionine. To elucidate the reaction mechanism further, inhibition by the two products, S-adenosylhomocysteine and 1-methylhistamine, was studied. S-Adenosylhomocysteine inhibited non-competitively with respect to histamine and competitively with respect to S-adenosylmethionine. 1-Methylhistamine inhibited non-competitively with respect to histamine and to S-adenosylmethionine. These results are interpreted as providing evidence for an ordered sequential Bi Bi reaction mechanism, with the methyl-group donor S-adenosylmethionine as the first substrate that adds to the enzyme and histamine as the second substrate. 1-Methylhistamine is the first product to leave the enzyme and S-adenosylhomocysteine is the second. The results are discussed in terms of the possible role that this enzyme could play in the modulation of histamine-mediated reactions in skin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Vesell E. S. Heterogeneity of N-and O-methyltransferases. Mol Pharmacol. 1970 Jan;6(1):78–84. [PubMed] [Google Scholar]
- BROWN D. D., TOMCHICK R., AXELROD J. The distribution and properties of a histamine-methylating enzyme. J Biol Chem. 1959 Nov;234:2948–2950. [PubMed] [Google Scholar]
- Barth H., Niemeyer I., Lorenz W. Speculations about the binding sites of S-adenosyl-L-homocysteine and some of its synthetic analogues to histamine methyltransferase. Agents Actions. 1974 Aug;4(3):186–188. doi: 10.1007/BF01970266. [DOI] [PubMed] [Google Scholar]
- Barth H., Niemeyer I., Lorenz W. Studies on the mode of action of histamine H1- and H2-receptor antagonists on gastric histamine methyltransferase. Agents Actions. 1973 Oct;3(3):138–147. doi: 10.1007/BF01965724. [DOI] [PubMed] [Google Scholar]
- Baudry M., Chast F., Schwartz J. C. Studies on S-adenosylhomocysteine inhibition of histamine transmethylation in brain. J Neurochem. 1973 Jan;20(1):13–21. doi: 10.1111/j.1471-4159.1973.tb12099.x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- Francis D., Greaves M. W., Yamamoto S. Enzymatic histamine degradation by human skin. Br J Pharmacol. 1977 Aug;60(4):583–587. doi: 10.1111/j.1476-5381.1977.tb07538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAYER R. W. Catabolism of physiological quantities of histamine in vivo. Physiol Rev. 1959 Jan;39(1):116–126. doi: 10.1152/physrev.1959.39.1.116. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Snyder S. H., Axelrod J. Sex differences and hormonal control of histamine methyltransferase activity. Biochim Biophys Acta. 1965 Dec 16;111(2):416–421. doi: 10.1016/0304-4165(65)90051-6. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Snyder S. H. Histamine methyltransferase: inhibition and potentiation by antihistamines. Mol Pharmacol. 1972 May;8(3):300–310. [PubMed] [Google Scholar]
- Thithapandha A., Cohn V. H. Brain histamine N-methyltransferase purification, mechanism of action, and inhibition by drugs. Biochem Pharmacol. 1978 Feb 1;27(3):263–271. doi: 10.1016/0006-2952(78)90227-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Francis D., Greaves M. W. Enzymic histamine metabolism in guinea-pig skin and its role in immediate hypersensitivity reactions. Clin Exp Immunol. 1976 Dec;26(3):583–589. [PMC free article] [PubMed] [Google Scholar]
- Zeiger R. S., Yurdin D. L., Colten H. R. Histamine metabolism. II. Cellular and subcellular localization of the catabolic enzymes, histaminase and histamine methyl transferase, in human leukocytes. J Allergy Clin Immunol. 1976 Jul;58(1 Pt 2):172–179. doi: 10.1016/0091-6749(76)90152-4. [DOI] [PubMed] [Google Scholar]



