Abstract
Carbapenems are the last-resort antibiotics used to treat infections caused by bacterial pathogens. Many bacterial pathogens have evolved to produce NDM carbapenemases to hydrolyze carbapenems, posing a great challenge to public health. In this study, we report a multidrug resistant clinical E. coli strain 673. Strain 673 belongs to sequence type (ST) 1431 and carries several plasmids, p673-blaTEM-1B, p673-blaCTX-M-55, p673-blaNDM-5, p673-13272, and p673-6468. p673-blaNDM-5 is an IncHI2/IncHI2A-type plasmid harboring several antibiotic resistance genes, including blaNDM-5, strA, strB, and dfrA. The blaNDM-5 gene was surrounded by two IS26 elements in p673-blaNDM-5, indicating that IS26 could mediate the integration of blaNDM-5 into p673-blaNDM-5. p673-blaCTX-M-55 is an IncFII-type plasmid harboring fosA, aadA1, and blaCTX-M-55. p673-blaTEM-1B is an IncFIB-type plasmid harboring blaTEM-1B and dfrA5. p673-13272 is a ColRNAI-type plasmid that does not carry any drug resistance genes. This is the first report that a blaNDM-5-bearing IncHI2/IncHI2A-type plasmid has emerged in a clinical E. coli strain in China. Our findings suggest that IS26 mediates the integration of blaNDM-5 into p673-blaNDM-5. The spread of blaNDM-5-bearing plasmids is a clinical challenge and endangers public health.
Keywords: NDM-5, IncHI2/IncHI2A plasmid, E. coli
Short Report
Carbapenems are essential antibiotics used in clinical practice. To fight these drugs, many bacterial pathogens can produce NDM carbapenemases. To date, more than 45 NDM variant genes have been identified.1 Among these NDM variant genes, blaNDM-1 and blaNDM-5 are the two predominant NDM carbapenemase genes.2 A recent study revealed that among carbapenem-resistant Escherichia coli strains isolated from China, the majority of these strains carry blaNDM-5 instead of blaNDM-1. These E. coli strains also encode other drug resistance genes, such as blaCTX-M, blaTEM, blaSHV, aadA, and sul2.3 Surveillance studies revealed that in addition to E. coli, other bacterial species, such as Citrobacter freundii, E. fergusonii, Klebsiella pneumoniae, Pluralibacter gergoviae, and Salmonella Typhimurium, also encode blaNDM-5.4–7 Among these diverse bacterial species, E. coli is recognized as the dominant host of blaNDM-5.8 Indeed, according to reports from the China Antimicrobial Resistance Surveillance System (http://www.carss.cn/), E. coli is the dominant gram-negative bacteria isolated in Chinese hospitals from 2020–2022. Among these E. coli strains, approximately 1.5% were carbapenemase producers. Therefore, carbapenem-resistant E. coli is a great challenge in hospitals.
It generally agrees that plasmid is the major vector contributing to the dissemination of blaNDM-5. For example, IncF, IncFII, IncHI2, IncN, and IncX3 plasmids have been reported to harbor blaNDM-5.9–11 Among these different types of plasmids, the IncX3-type plasmid is the most prevalent type of plasmid that carries blaNDM-5.12,13 In addition, blaNDM-5-bearing IncX3-type plasmids can be carried by many bacterial species that are isolates from diverse environments, animals, and humans.11,14 A recent study revealed that the IncX3-type plasmid pX3_NDM-5 has a wide range of bacterial hosts and can be transferred between gram-negative and gram-positive bacteria.15 Moreover, the IncHI2-type plasmid harboring blaNDM-5 has been increasingly reported in China. Bacterial species that carry blaNDM-5-bearing IncHI2-type plasmids were isolated from eggs, chickens, ducks, pig feces, and fish.4,11,16,17 However, neither a clinical isolate a carrying blaNDM-5-bearing IncHI2-type plasmid nor an IncHI2/IncHI2A-type plasmid harboring blaNDM-5 has been reported.
In August 2023, strain 673 was isolated from a urine sample from Zhuhai people’s hospital, Zhuhai city, Guangdong Province, China. This urine sample was obtained from a female patient who had a urinary tract infection. The symptoms include frequent urination and pain while peeing. Ceftriaxone failed to treat the infection. By using 16S rRNA sequencing and MALDI-TOF analysis, strain 673 was identified as E. coli. To determine the antimicrobial susceptibility of the tested strains, a broth dilution method was used as described by the Clinical & Laboratory Standards Institute (CLSI) standard, and the results were interpreted according to the CLSI 2022 guidelines (https://clsi.org).
Antimicrobial susceptibility test data revealed that strain 673 was resistant to carbapenems, including imipenem and ertapenem, indicating that it could be a carbapenemase producer. Strain 673 was resistant to many other drugs, especially cephalosporins, such as ceftriaxone, cefuroxime, ceftriaxone, cefuroxime, ceftazidime, cefepime, and cefoxitin, but was susceptible to amikacin and tigecycline (Table 1). Thus, tigecycline is likely the last choice for treating the infections caused by this multidrug-resistant E. coli strain or other clinical multidrug-resistant pathogenic strains isolated from our hospital.
Table 1.
Minimum Inhibitory Concentrations (MICs, μg/mL) of Different Antibiotics Used for E. Coli Strains and the Interpretation. S, Sensitive; R, Resistant
| Strain | Amikacin | Tigecycline | Ceftriaxone | Cefuroxime | Ceftazidime | Cefepime | Cefoxitin | Ertapenem | Imipenem | Trimethoprim/sulfamethoxazole |
|---|---|---|---|---|---|---|---|---|---|---|
| 673 | 2/S | 0.5/S | ≥64/R | ≥64/R | ≥64/R | ≥32/R | ≥64/R | ≥8/R | ≥16/R | ≥320/R |
| Transconjugant | 1/S | 0.5/S | ≥32/R | ≥32/R | ≥64/R | ≥32/R | 64 | 8/R | 16/R | 320/R |
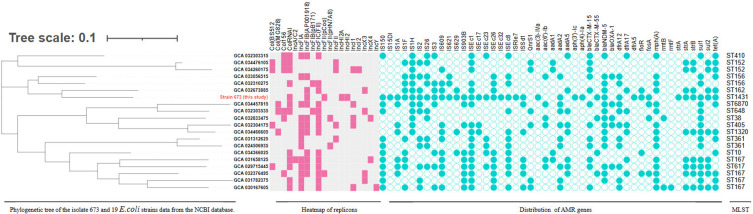
Given that strain 673 is resistant to carbapenems, we used the Illumina and MinION platforms to sequence its genome and to determine which carbapenemase gene was harbored by this strain (GenBank accession numbers CP141196-CP141201). SeroType Finder (https://cge.food.dtu.dk/services/SerotypeFinder/) revealed that the serotype of strain 673 is H19:O8. The complete genome sequences also revealed that strain 673 has a circular chromosome and 5 plasmids. The chromosome is 5,328,997 bp in length with 50.6% GC content, encoding more than 5000 ORFs. MLST analysis of 7 core genes (PubMLST, https://pubmlst.org/), including adk, fumC, gyrB, icd, mdh, purA, and recA, revealed that strain 673 belongs to ST1431 and forms a subclade with an ST162 clinical E. coli strain (GenBank ID: GCA 032673885). ST1431 E. coli strains were isolated from animals and humans,18 but ST1431 is not the pandemic lineage. In addition, strain 673 possesses 20 IS elements, including IS26, IS150, IS3, and IS609, much more than its phylogenetically closely related E. coli strains (Figure 1). Moreover, strain 673 possesses 16 antimicrobial resistance genes and 5 plasmid replicons, making this strain easy to distinguish from its phylogenetically closely related E. coli strains (Figure 1).
Figure 1.
Phylogenetic relationships between strain 673 and 19 E. coli strains. Plasmid replicon types are labeled with pink squares, IS elements and drug resistance genes are labeled with green circles. Adk, fumC, gyrB, icd, mdh, purA, and recA were used as MLST sequences. MEGA version 7.0, which is based on the neighbor-joining method, was used to construct a phylogenetic tree of E. coli strains.
Given that strain 673 was isolated from a urine sample, we wondered which virulence genes were encoded by this strain. Using Virulence Finder (http://www.mgc.ac.cn/VFs/), many virulence genes, such as entF, cfaC, fdeC, iutA, iroN, iucC, and a type VI secretion system (T6SS), were identified. These results reinforced the notion that strain 673 is pathogenic. In addition, some antimicrobial resistance genes, such as floR, sul2, aph(3’)-Ia, were identified on the chromosome of strains 673.
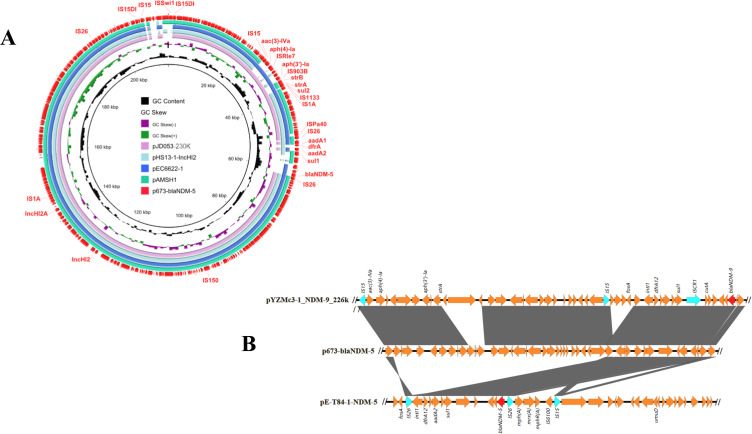
Moreover, strain 673 carries 5 plasmids: p673-blaTEM-1B, p673-blaCTX-M-55, p673-blaNDM-5, p673-13272, and p673-6468. p673-blaNDM-5 is 216,339 bp long and is an IncHI2/IncHI2A-type plasmid. We also found that 11 antibiotic resistance genes, including blaNDM-5, strA, strB, aadA1, sul1, sul2, aadA2, and dfrA, were located in this plasmid. BLAST analysis revealed that p673-blaNDM-5 shares 99.99–100% identity with pAMSH1, pEC6622-1, pJD053-230k, and pHS13-1-IncHI2, with 91–95% coverage. pAMSH1 was carried by an E. coli strain isolated from giant panda feces. Both pEC6622-1 and pHS13-1-IncHI2 were carried by E. coli strains that were isolated from patient feces and a patient, respectively. The host of pJD053-230k is an Escherichia fergusonii strain isolated from cecal contents. These findings indicate that these highly similar plasmids are related to human beings. Among these plasmids, only p673-blaNDM-5 and pEC6622-1 possess blaNDM-5 (Figure 2A). Thus, p673-blaNDM-5 is a novel blaNDM-5-bearing IncHI2/IncHI2A-type plasmid. To the best of our knowledge, this is the first report that a clinical E. coli strain harbors a blaNDM-5-bearing IncHI2/IncHI2A-type plasmid and that an ST1431 E. coli strain harbors blaNDM-5. Moreover, 11 drug resistance genes in p673-blaNDM-5 are flanked by IS5 and IS26, indicating that IS26 and IS5 may mediate the integration of these 11 drug resistance genes into p673-blaNDM-5. BLAST analysis revealed a similar genetic structure in pYZMc3-1_NDM-9_226k, whose host is an E. coli strain isolated from chicken feces. Intriguingly, similar to blaNDM-9 in pYZMc3-1_NDM-9_226k, the blaNDM-5 gene is also surrounded by two IS26 elements in p673-blaNDM-5 and pE-T84-1-NDM5. In p673-blaNDM-5, two IS26 elements form an IS26-xerD-dfrA-orf1-aadA2-qacEΔ1-sul1-orf2-cutA-dsbD-trpF-bleMBL-blaNDM-5-IS26 region (Figure 2B). IS26 may mediate the integration of blaNDM-5 into p673-blaNDM-5 and pE-T84-1-NDM5, and the IS26 element may mediate the spread of blaNDM-5 into other plasmids. To test the transferability of p673-blaNDM-5, a conjugation assay was performed. The results showed that p673-blaNDM-5 could be conjugated to E. coli C600 with an average frequency of 3.46×10−6 and that the transconjugant was resistant to many drugs (Table 1).
Figure 2.
(A) Circular comparative analysis of p673-blaNDM-5 and other plasmids deposited in the GenBank database. Drug resistance genes and insertion sequence elements are labeled at the outermost ring. (B) Linear comparison of blaNDM-5 and blaNDM-9 genetic environments in p673-blaNDM-5 and pYZMc3_NDM-9. Genes are denoted by arrows and are colored on the basis of gene function classification. Red, blaNDM genes; green, IS elements; yellow, other drug resistance genes and nondrug-related genes. Easyfig version 2.2.3 was used to visualize the comparison sequences.
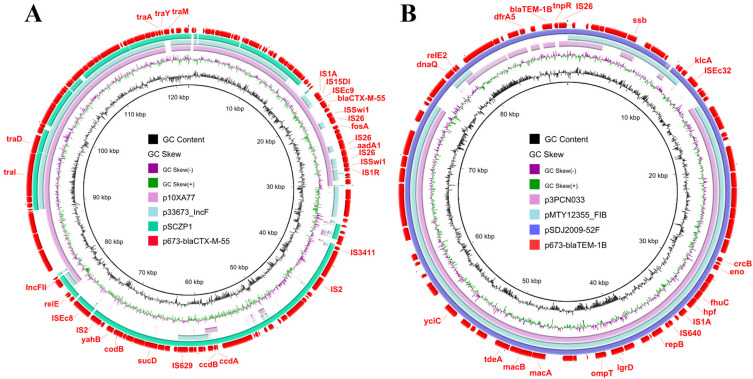
Unlike p673-blaNDM-5, both p673-blaCTX-M-55 and p673-blaTEM-1B possess only 3 and 2 antibiotic resistance genes, respectively. p673-blaCTX-M-55 is 123,043 bp in length and is an IncFII-type plasmid harboring fosA, aadA1, and blaCTX-M-55. This finding is consistent with a previous study showing that blaCTX-M-55-bearing IncFII-type plasmids are prevalent in E. coli strains.19 Moreover, the fosA, aadA1, and blaCTX-M-55 genes are surrounded by IS1A and IS1R elements in p673-blaCTX-M-55 (Figure 3A), indicating that these IS elements may mediate the integration of fosA, aadA1, and blaCTX-M-55 into p673-blaCTX-M-55.
Figure 3.
Circular comparative analysis of p673-blaCTX-M-55 (A), p673-blaTEM-1B (B) and other plasmids deposited in the GenBank database. Drug resistance genes and insertion sequence elements are labeled at the outermost ring.
p673-blaTEM-1B is 89,218 bp long and is an IncFIB-type plasmid harboring blaTEM-1B and dfrA5. BLAST analysis revealed that p673-blaTEM-1B shares high identity with p3PCN033, pMTY12355-FIB, and pSDJ2009-52F, with 83–97% coverage. (Figure 3B). However, p673-blaCTX-M-55 has much lower coverage, approximately 45–74% with other plasmids, p10XA77, p33673-IncF, and pSCZP1 (Figure 3A). Therefore, p673-blaTEM-1B and p673-blaCTX-M-55 are novel plasmids. Notably, similar to p673-blaNDM-5, p673-blaCTX-M-55 possesses more than 10 IS elements, including IS2, IS26, and IS1A, indicating that some exogenous genes could be integrated into p673-blaCTX-M-55 via these mobile elements. Further characterisation of the functions of the exogenous genes would help us to better understand the role of p673-blaCTX-M-55 in strain 673.
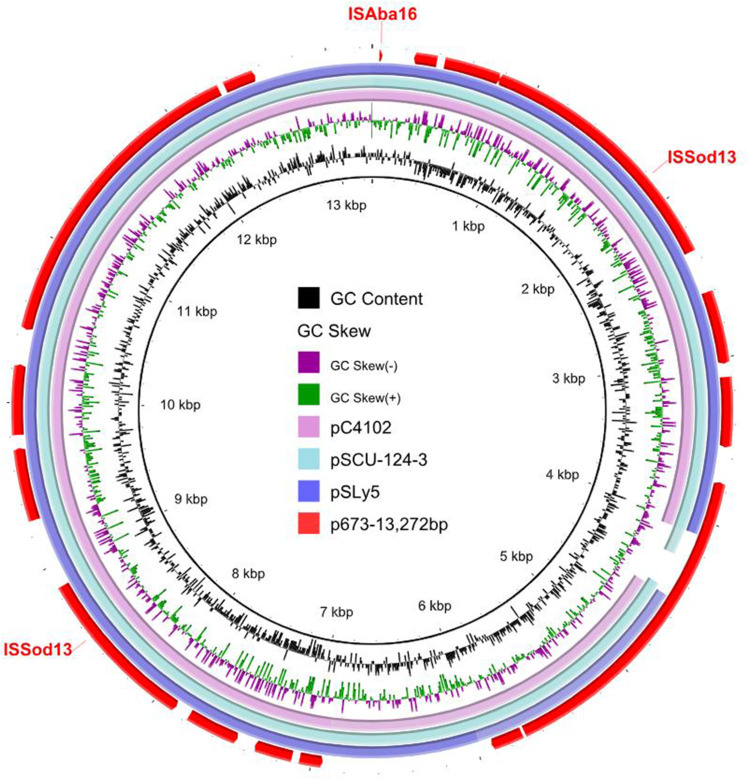
p673-13272 is 13,272 bp in length and is a ColRNAI-type plasmid. BLAST analysis revealed that p673-13272 has high similarity with pC4102, pSCU-124-3, and pSLy5, with 95–99% identity and 95–96% coverage (Figure 4). Unlike p673-blaNDM-5, p673-blaCTX-M-55 and p673-blaTEM-1B, p673-13272 do not possess any drug resistance genes. p673-6468 is 6468 bp in length. Neither the replicon nor the drug resistance gene was identified in this plasmid. The functions of p673-13272 and p673-6468 need further study.
Figure 4.
Circular comparative analysis of p673-13272 and other plasmids deposited in the GenBank database. Insertion sequence elements are labeled at the outermost ring.
In conclusion, E. coli 673 is a clinical strain belonging to ST1431. It carries blaNDM-5 and many other drug resistance genes (Table 2). To the best of our knowledge, this is the first report in which a blaNDM-5-bearing IncHI2/IncHI2A-type plasmid has emerged in a clinical E. coli strain in China. Our findings suggest that IS26 mediates the integration of blaNDM-5 into p673-blaNDM-5. The spread of blaNDM-5-bearing plasmids is a clinical challenge and endangers public health. Given that blaNDM-5-bearing E. coli strains have spread in China, Germany, the Czech Republic, and Zambia,3,20–22 there is an urgent need to conduct surveillances to determine whether blaNDM-5-bearing E. coli strains have spread in other countries and take measures to control their spread.
Table 2.
Drug Resistance Plasmid Replicons and Antimicrobial Resistance Genes of E. Coli 673
| Plasmid | Replicon Type | Drug Resistance Genes |
|---|---|---|
| p673-blaNDM-5 | IncHI2/IncHI2A | blaNDM-5, strA, strB, aadA1, sul1, sul2, aadA2, dfrA, aac(3)-IVa, aph(4)-Ia, aph(3’)-Ia |
| p673-blaCTX-M-55 | IncFII | fosA, aadA1, blaCTX-M-55 |
| p673-blaTEM-1B | IncFIB | blaTEM-1B, dfrA5 |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81650003). Ethical approval was not needed.
Data Sharing Statement
The complete sequences of strain 673 were submitted to the NCBI database under accession numbers CP141196- CP141201.
Ethics Approval Statement and Informed Consent
We confirmed that all experimental protocols were approved by the ethics committee of Zhuhai People’s hospital with Approval number: (2024) Ethical Review (Research) No.15. The informed consent from patient was not a requirement due to the fact that isolation, identification, and characterization of drug-resistant clinical bacteria is a routine work in Zhuhai People’s hospital. We also confirmed that the guidelines outlined in the Declaration of Helsinki were followed and the experiments were carried out in accordance with guidelines and regulations of Zhuhai People’s hospital.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Available from: https://www.ncbi.nlm.nih.gov/pathogens/refgene/#NDM.
- 2.Shen Y, Hu F, Wang Y, et al. Transmission of carbapenem resistance between human and animal NDM-positive Escherichia coli Strains. Engineering. 2022;15:24–33. doi: 10.1016/j.eng.2021.07.030 [DOI] [Google Scholar]
- 3.Y. L, Zhang Y, Sun X, et al. National genomic epidemiology investigation revealed the spread of carbapenem-resistant Escherichia coli in healthy populations and the impact on public health. Genome Med. 2024;16(1):57. doi: 10.1186/s13073-024-01310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YY, Tong L, Yue HY, et al. Occurrence and characterization of NDM-5-producing Escherichia coli from retail eggs. Front Microbiol. 2023;14:1281838. doi: 10.3389/fmicb.2023.1281838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang B, Guan CJ, Lin H, et al. Emergence of co-existence of mcr-1 and blaNDM-5 in Escherichia fergusonii. Int J Antimicrob Agents. 2023;61(3):106742. doi: 10.1016/j.ijantimicag.2023.106742 [DOI] [PubMed] [Google Scholar]
- 6.Zeng SH, Huang YL, Zhang XW, Fu L, Sun ZH, Li XY. Molecular characterization of IncFII plasmid carrying blaNDM-5 in a Salmonella enterica serovar Typhimurium ST34 clinical isolate in China. mSphere. 2023;8(6):e0048023. doi: 10.1128/msphere.00480-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stehling FJ, Stehling EG. Genomic Insights into Pluralibacter gergoviae Sheds Light on Emergence of a Multidrug-Resistant Species Circulating between Clinical and Environmental Settings. Pathogens. 2023;12(11):1335. doi: 10.3390/pathogens12111335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.X. L, Y. F, Shen M, et al. Dissemination of blaNDM-5 gene via an IncX3-type plasmid among non-clonal Escherichia coli in China. Antimicrob Resist Infect Control. 2018;7(1):59. doi: 10.1186/s13756-018-0349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Supplement_7):S521–S528. doi: 10.1093/cid/ciz824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jean SS, Harnod D, Hsueh PR. Global threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. 2022;12:823684. doi: 10.3389/fcimb.2022.823684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv LC, Lu YY, Gao X, et al. Characterization of ndm-5-producing Enterobacteriaceae isolates from retail grass carp (Ctenopharyngodon idella) and evidence of blaNDM-5-bearing IncHI2 plasmid transfer between ducks and fish. Zool Res. 2022;43(2):255–264. doi: 10.24272/j.issn.2095-8137.2021.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian D, Wang B, Zhang H, et al. Dissemination of the bla NDM-5 Gene via IncX3-Type Plasmid among Enterobacteriaceae in Children. mSphere. 2020;5(1):e00699–00619. doi: 10.1128/mSphere.00699-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Q, Berglund B, Zou H, et al. Dissemination of blaNDM-5 via IncX3 plasmids in carbapenem-resistant Enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in Eastern China. Environ Pollut. 2021;273:116370. doi: 10.1016/j.envpol.2020.116370 [DOI] [PubMed] [Google Scholar]
- 14.J. M, Song X, M. L, et al. Global spread of carbapenem-resistant Enterobacteriaceae: epidemiological features, resistance mechanisms, detection and therapy. Microbiol Res. 2023;266:127249. doi: 10.1016/j.micres.2022.127249 [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Ma XD, Zeng LS, et al. Interphylum dissemination of NDM-5-positive plasmids in hospital wastewater from Fuzhou, China: a single-centre, culture-independent, plasmid transmission study. Lancet Microbe. 2023;S2666-5247(23):227–236. [DOI] [PubMed] [Google Scholar]
- 16.Z. M, Zeng Z, Liu J, et al. Emergence of IncHI2 plasmid-harboring blaNDM-5 from porcine Escherichia coli isolates in Guangdong, China. Pathogens. 2021;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao QY, Zhu JH, Cai RM, et al. IS 26 Is Responsible for the Evolution and Transmission of blaNDM -Harboring Plasmids in Escherichia coli of Poultry Origin in China. mSystems. 2021;6(4):e64621. doi: 10.1128/msystems.00646-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiffert SN, Carattoli A, Schwendener S, Collaud A, Endimiani A, Perreten V. Plasmids Carrying blaCMY −2/4 in Escherichia coli from Poultry, Poultry Meat, and Humans Belong to a Novel IncK Subgroup Designated IncK2. Front Microbiol. 2017;15(8):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng S, Luo J, Li X, et al. Molecular Epidemiology and Characteristics of CTX-M-55 Extended-Spectrum β-Lactamase-Producing Escherichia coli From Guangzhou, China. Front Microbiol. 2021;12:730012. doi: 10.3389/fmicb.2021.730012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasange M, Gajdacs M, Muleya W, et al. Genotypic Characterisation and Antimicrobial Resistance of Extended-Spectrum β-Lactamase-Producing Escherichia coli in Humans, Animals, and the Environment from Lusaka, Zambia: public Health Implications and One Health Surveillance. Antibiotics. 2021;13(10):951. doi: 10.3390/antibiotics13100951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chudejova K, Sourenian T, Palkovicova J, Working Group for Monitoring of Antibiotic Resistance, et al. Genomic characterization of ST38 NDM-5-producing Escherichia coli isolates from an outbreak in the Czech Republic. Antimicrob Agents Chemother. 16;2024:e0013324. doi: 10.1128/aac.00133-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hans JB, Pfennigwerth N, Neumann B, et al. Molecular surveillance reveals the emergence and dissemination of NDM-5-producing Escherichia coli high-risk clones in Germany, 2013 to 2019. Euro Surveill. 2023;28(10):2200509. doi: 10.2807/1560-7917.ES.2023.28.10.2200509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete sequences of strain 673 were submitted to the NCBI database under accession numbers CP141196- CP141201.