Abstract
Background
In recent years, dexamethasone (Dex) has been used to treat acute respiratory distress syndrome (ARDS) in patients with COVID-19 and achieved promising outcomes. Venovenous extracorporeal membrane oxygenation (VV ECMO) support for patients with ARDS has increased significantly worldwide. However, it remains unknown whether Dex could improve the efficiency of VV ECMO to reduce lung injury. Here, we investigate the combined efficiency of VV ECMO and Dex in rats with acute lung injury (ALI).
Methods
We established VV ECMO in oleic acid (OA)-treated ALI rats and administered Dex. We conducted HE staining and evaluated lung and bronchoalveolar lavage (BAL) fluid cytokines to assess lung injury and inflammation. Furthermore, we investigated the activation of Hippo/YAP signalling in alveolar epithelial type II cell (AT2)-mediated alveolar epithelial repair using quantitative PCR, Western blotting and immunofluorescence. In vitro, the human alveolar epithelial cell line A549 was used to investigate the key role of YAP in alveolar epithelial cell differentiation.
Results
VV ECMO combined with Dex alleviated OA-induced lung injury and pulmonary inflammation. Pulmonary oedema and exudation were significantly alleviated, and the lung and BAL levels of IL-6, IL-8 and TNF-α were significantly reduced compared with those observed with ECMO alone. In addition, VV ECMO combined with Dex treatment protected alveolar epithelial cells by activating Hippo/YAP signalling. In vitro, Dex promoted YAP expression and alveolar epithelial cell differentiation, whereas YAP knockdown inhibited YAP-mediated differentiation.
Conclusions
Our findings suggest that adjuvant Dex treatment during VV ECMO could alleviate ALI and pulmonary inflammation by activating the Hippo/YAP signalling pathway, which promoted alveolar regeneration and AT2 differentiation.
Keywords: ARDS, Critical Care, Inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have shown that both venovenous extracorporeal membrane oxygenation support and dexamethasone administration could alleviate lung injury. However, it remains unclear whether adjuvant dexamethasone during venovenous extracorporeal membrane oxygenation could reduce lung injury.
WHAT THIS STUDY ADDS
Venovenous extracorporeal membrane oxygenation (VV ECMO) combined with dexamethasone activates Hippo/YAP signalling which promotes alveolar regeneration and differentiation. Adjuvant dexamethasone during VV ECMO alleviates acute lung injury and pulmonary inflammation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
For patients with severe acute respiratory distress syndrome who require venovenous extracorporeal membrane oxygenation support, adjuvant dexamethasone administration may help reduce lung injury and improve clinical outcomes.
Introduction
Acute lung injury (ALI) and its severe condition, acute respiratory distress syndrome (ARDS), are catastrophic respiratory diseases with high morbidity and mortality rates.1,3 Based on oxygenation criteria, the incidence of ARDS in COVID-19 patients ranges from 20% to 67%, and all mechanically ventilated patients were diagnosed with ARDS.4 The pathogenesis of ALI/ARDS is quite complicated and is characterised by destruction of the alveolar septal barrier and severe pulmonary inflammation, leading to alveolar exudation, excessive neutrophil and macrophage cell influx and infiltration, cytokine release, surfactant dysfunction and eventually significant hypoxaemia.3 5 6
In patients with severe pulmonary compromise, venovenous extracorporeal membrane oxygenation (VV ECMO) therapy provides carbon dioxide removal and blood oxygenation, allowing protective lung ventilation and leaving room for lung restoration. The use of VV ECMO in patients with ARDS has increased significantly worldwide over the last decades.7 Although it has been beneficial in treating COVID-19 patients with ARDS by providing effective lung support, the mortality rate reported by Extracorporeal Life Support Organization Registry remains high at 38%.8 What is more, several animal studies have suggested that ECMO may cause damage to the intestinal mucosal barrier and lung tissues and increase the levels of inflammatory cytokines.9,11 Therefore, we hypothesised that adjuvant anti-inflammatory therapy during VV ECMO might further improve its efficiency in ALI/ARDS patients.
Glucocorticoids have been used for many years for the treatment of ALI/ARDS since it could diminish inflammation by inhibiting a variety of cytokines, such as TNF-α, IL-5, IL-8 and IL-6.12 13 Dexamethasone (Dex), as one of these steroids, could protect alveolar epithelial and endothelial cell injury, alleviate pulmonary oedema and decrease the release of inflammatory cytokines.14 Previous randomised controlled trials have investigated the efficacy of Dex for the management ARDS patients,15 16 and a recent meta-analysis found that Dex could significantly reduce all-cause mortality.17 Although both VV ECMO and Dex have been widely used in critically ill ARDS patients with promising results, it remains unknown whether VV ECMO combined with Dex could reduce pulmonary damage in ALI/ARDS patients.
In our previous study, we found that VV ECMO protects against lung injury by activating the Hippo/Yap signalling pathway.18 And Dex could significantly activate Yap expression.19 Therefore, we hypothesised that VV ECMO combined with Dex could reduce lung injury by activating the Hippo/Yap signalling pathway. To validate this hypothesis, we established VV ECMO in oleic acid (OA) induced ALI rats as described in our previous study.20
Materials and methods
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Animal preparation
For in vivo experiments, all animals were purchased from Veterinary Institute, Chinese Academy of Agricultural Sciences (Lanzhou, China). A total of 18 male Sprague-Dawley (SD) rats (350±50 g) were randomly divided into three groups: sham group, ECMO group and ECMO+Dex group. Rats received free access to sterilised water and standard rodent chow in cages before surgery, with the room temperature controlled between 20°C and 22°C with a 12-hour light-dark cycle.
Procedures and drug administration
Rats were anaesthetised with 5% sevoflurane in oxygen for 3–5 min in a plexiglass chamber. Volume-controlled ventilation was provided at a respiratory rate of 70–75 breaths/min, a tidal volume of 6 mL/kg, and positive end-expiratory pressure of 2 cmH2O, with the fractional inspired oxygen (FiO2) in the ventilator circuit set at 40%. Subsequently, anaesthesia was maintained throughout the surgical preparation and the entirety of the experiment with 2.0%–2.5% sevoflurane. The surgical procedure was conducted in accordance with aseptic techniques, and all surgical fields were subsequently infiltrated with 1% lidocaine. To induce ALI and mimic a situation of impaired gas exchange, we used a model of intravenous administration of OA as previously described.21 For sham group, the rats were intubated with cannula without ECMO support and administration of OA was replaced by phosphate buffer saline. For ECMO+Dex group, the rats were given Dex (1 mg/kg) at the beginning of VV ECMO to explore whether it can improve the effectiveness of VV ECMO. The ECMO parameters including flow rate and sweep gas flow were identical in the ECMO and ECMO+Dex group. At the end of the experiment, arterial blood was collected for blood gas analysis (eg,7+, iStar, Abbott) then all rats were euthanatised using an overdose of sevoflurane. The establishment of VV ECMO in rats was well described in our previous studies.20
H&E staining
The lung tissues were cut into 4 µm thick sections after paraffin embedding. The Hematoxylin (#H8070, Solarbio) & Eosin (G1100, Solarbio) staining experiment followed the standard H&E protocol. Histological changes were observed using light microscopy. A double-blinded pathologist determined the ALI score by assessing the alveolar congestion/oedema, pulmonary tissue haemorrhage and thickening, inflammatory cell infiltration in the alveolar and vascular lumen, and alveolar wall congestion, oedema, thickening and hyaline membrane formation.22 The scores were assigned to assess the degree of lung injury in a single visual field: 0=no pathological changes, 1=mild pathological changes, 2=moderate pathological changes, 3=severe pathological changes and 4=very severe pathological changes. Each injury was scored in 10 randomly selected fields (400×) of each slide. The final injury score (the Smith score) was calculated as the mean of all individual injury scores.
ELISA arrays
Relative levels of IL-6, IL-8, IL-10 and TNF-α in bronchoalveolar lavage (BAL) and lung were determined by ELISA Kit (Mlbio, Shanghai, China) according to the manufacturer’s recommendations. Serum levels of soluble receptor for advanced glycation end products (sRAGE) and surfactant protein-D (SP-D) were also detected by ELISA Kit (Mlbio, Shanghai, China) according to the manufacturer’s recommendations. The range limited to the measured cytokines including TNF-α, IL-6, IL-8 and IL-10 was 10–320 pg/mL, 5–160 pg/mL, 7.5–240 pg/mL and 2–64 pg/mL, respectively. The sensitivity of the test is less than 1 pg/mL for TNF-α, IL-6, IL-8 and less than 0.1 pg/mL for IL-10. For lung tissues, we took 50 mg tissue into 1 mL solution, and cytokine dosage was expressed as pg/mg tissue.
Immunohistochemistry and immunofluorescence
Immunohistochemical (IHC) staining was performed based on mouse/rabbit streptomycin ovalbumin-biotin assay system (#SP-9000, ZSGB-BIO, China) and performed following the standard procedure. The following primary antibodies were used: rabbit anti-MPO (1:1000, #ab78486, Abcam). The image was visualised using Zeiss microscope (Zeiss, Germany) and processed with Zeiss software. The process of immunofluorescence (IF) was performed under the standard procedure. The following primary antibodies were used: mouse anti-YAP (1:200, #66900-1-Ig, Proteintech), mouse anti-SPC (1:100, #sc-518029, Santa Cruz), rabbit anti-Claudin 4 (1:200, #16195-1-AP, Proteintech), rabbit anti-Cytokeratin 8 (1:200, #17514-1-AP, Proteintech), rabbit anti-AQP5 (1:200, #ab78486Abcam). Secondary antibodies included goat anti-mouse Alexa Fluor 488 (1:1000; #ab150113, Abcam), goat anti-rabbit Alexa Fluor 594 (1:1000, #ab150080, Abcam) and DAPI (1:10,000; Sigma). Confocal images were obtained using a Zeiss LSM 880 laser microscope. The image processing was not used in our confocal images, and we did not used Z-stacks, the slices were 4 µm thick.
Western Blot
The RIPA buffer (50 mM Tris (pH 7.4), 1% Triton X-100, 1% sodium deoxycholate, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), sodium orthovanadate) containing Protease Inhibitor Cocktail tablets (Roche) and phosSTOP Phosphatase Inhibitor Cocktail tablets (Roche) was used to extract proteins from lung tissues and A549 cells. Bicinchoninic acid assay kit was used to determine protein concentrations following the manufacture’s instruction. The extracted proteins were separated on SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to PVDF membrane. The membranes were blocked with 5% skim milk for 2 hours, then incubated with primary antibody against YAP (1:5000, 66900-1-Ig, Proteintech), SP-C (1:1000, 10774-1-AP, Proteintech), Claudin-4 (1:1000, 16195-1-AP, Proteintech), Cytokeratin 8 (1:2000, 17514-1-AP, Proteintech), AQP5 (1:1000, ab78486, Abcam) at 4℃ overnight. Tanon image system (Tanon 5200 Multi, Shanghai, China) was used to scan the strips on the PVDF membrane, and Image J software was used for quantitative analysis.
RNA isolation and quantitation
TRIzol reagent (MAN0001271, Invitrogen) was used to extract total RNA from lung tissues and A549 cells. A nanodrop (Thermo Fisher Nanodrop2000, Massachusetts, USA) was used to determine RNA concentration. Equivalent amounts of RNA were reversely transcribed into cDNA using PrimeScript RT reagent Kit (RR047A, TaKaRa) following the manufacturer’s instruction. The designed primers and SYBR Green Master Mix (RR820A, TaKaRa) were used for quantitative real-time fluorescence quantitative PCR (qRT-PCR) on the real-time PCR system. The expression levels of target genes were analysed via the 2−△△ Ct method with β-actin as the internal reference. The primers of the design are shown below: rat Yap: 5’ - GAGCAAGCCATGACTCAGGA - 3’, 5’ - TGGTTCATGGCAAAACGAGG – 3; rat: Ctgf: 5’ - AGAACTGTGTACGGAGCGTG - 3’, 5’ - GTGCACCATCTTTGGCAGTG - 3’; rat Cyr61: 5’ - AGAGGCTTCCTGTCTTTGGC - 3’, 5’ - CTCGTGTGGAGATGCCAGTT - 3’; rat β-actin: 5’ - AGATCCTGACCGAGCGTGGC - 3’, 5’ - CCAGGGAGGAAGAGGATGCG - 3’; human Yap: 5’ - CCCTCGTTTTGCCATGAACC - 3’, 5’ - GTTGCTGCTGGTTGGAGTTG - 3’; human Ankrd: 5’ - AGAACTGTGCTGGGAAGACG - 3’, 5’ - GCCATGCCTTCAAAATGCCA - 3’; human Ctgf: 5’ - GTTTGGCCCAGACCCAACTA - 3’, 5’ - GGCTCTGCTTCTCTAGCCTG - 3’; human Cyr61: 5’ - CAGGACTGTGAAGATGCGGT - 3’, 5’-GCCTGTAGAAGGGAAACGCT-3’; human: β-actin: 5’-TGACGTGGACATCCGCAAAG - 3’, 5’ - TCTTCATTGTGCTGGGTGCC - 3’.
Cell culture
The A549 cells were cultured in a DMEM medium (containing 10% fetal bovine serum, 50kU/L penicillin and 50 mg/L streptomycin) at 5% CO2. The Logarithmic phase cells were used for subsequent experiments. The A549 cells were treated with OA and Dex (300 µm and 100 µm, respectively) then cultured for 24 hours. For siRNA transfection, (1) the cells were inoculated in an antibiotic-free medium in six-well plates 1 day before transfection. (2) Transfection solution was then prepared as follows: 5 µL Yap siRNA or NC siRNA was dissolved in 125 µL Opti-MEM medium, then incubated at room temperature for 5 min. Lipo6000 Transfection Reagent (5 µL) (#C0526, Beyotime, China) was dissolved in 125 µL Opti -MEM medium, mixed gently, then incubated at room temperature for 5 min. The siRNA solution was mixed with Lipo6000 Transfection Reagent solution and the final concentration of Yap siRNA and NC siRNA was 50 nM, then incubated at room temperature for 20 min. The mixture was added to the cells and incubated for 6 hours before being replaced with a fresh medium. The intervention was performed 3 days after transfection following the experimental requirements. The cells were collected for subsequent experiments.
Statistical analysis
All data were collected and reported as mean±SD. Comparisons between different groups were conducted by using the Student’s t-test, or the non-parametric Kolmogorov-Smirnov test. When comparing multiple groups, data were analysed by analysis of variance with Bonferroni post-test for multiple comparison of parametric data. P values of less than 0.05 were considered statistically significant. Statistics and graphical representations were performed using GraphPad Prism V.8.0 (Graph Pad Software, San Diego, California, USA).
Results
VV ECMO combined with Dex alleviate OA-induced lung injury
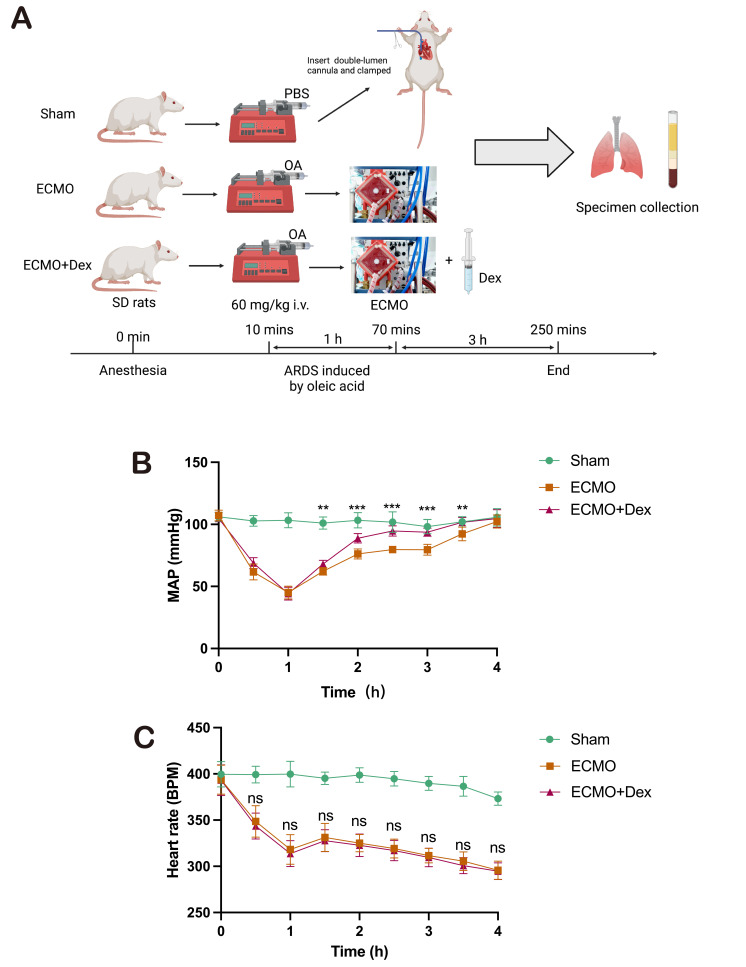
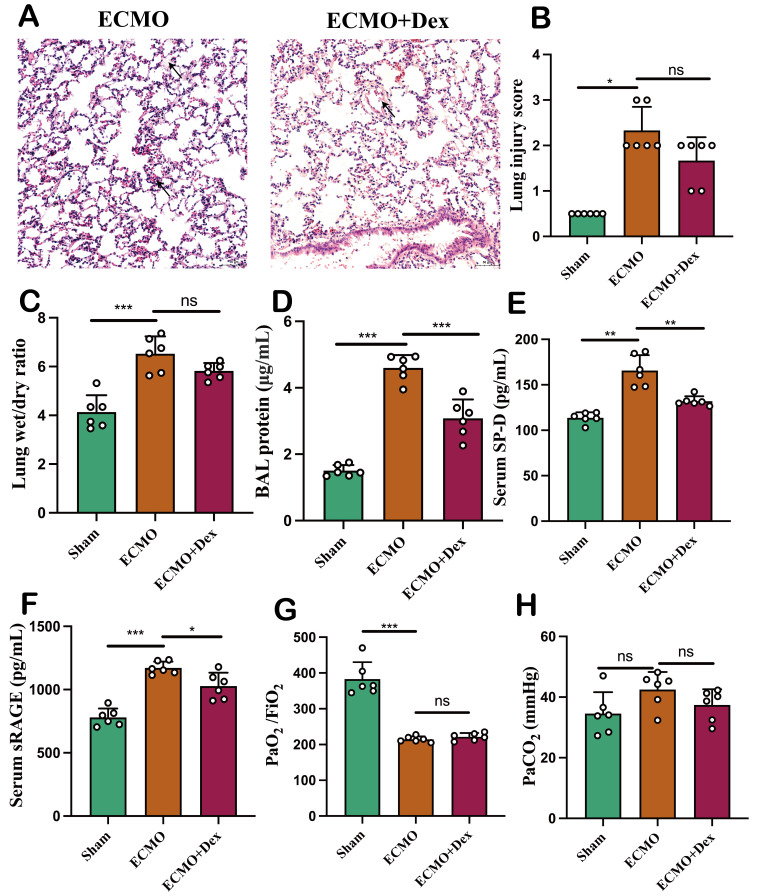
We chose injection of OA in rats to mimics the case of ALI as previously described.21 Animals developed severe hypoxaemia 1 hour following OA injection and were given ECMO treatment (ECMO group) and ECMO combined with Dex treatment (ECMO+Dex group). The Serum, BAL fluid and lung tissue were collected after 3 hours. The study protocol is illustrated in figure 1A. Mean arterial pressure (MAP) and heart rate were recorded to evaluate the haemodynamic state every 30 min (figure 1B,C). The MAP is significantly higher in the ECMO+Dex group compared with ECMO group when ECMO support started. However, there is no significant difference in heart rate. Pathological examination showed that the integrity of alveolar exudate, hyaline alveolar membrane and alveolar septum was better in the ECMO+Dex group than in the ECMO group (figure 2A). However, there is no significant difference in lung injury score between ECMO and ECMO+Dex group (figure 2B). The lung wet/dry ratio indicated that ECMO+Dex had similar pulmonary oedema as well (figure 2C). The disruption of the alveolar septal barrier has been demonstrated to increase protein exudation, we detected protein content of BAL and found that protein content was significantly lower in the ECMO+Dex group than ECMO group (figure 2D). Both sRAGE and SP-D are biomarkers of alveolar epithelial injury and endothelial dysfunction.23 In our study, we found the serum level of sRAGE and SP-D were significantly higher in the ECMO group (figure 2E,F), indicating lung injury is more severe in the ECMO group. However, there is no significant difference between ECMO and ECMO+Dex group in arterial oxygen tension/FiO2 and arterial carbon dioxide tension (figure 2G,H). In general, these results demonstrate that ECMO combined with Dex could alleviate OA-induced lung injury to some extent.
Figure 1. Schematic overview of the study workflow and experimental grouping (A). Created by Biorender. Mean arterial pressure (B). Heart rate (C). P value stands for ECMO versus ECMO+Dex during the experimental. The data shown are the mean±SD. Normality was analysed by Kolmogorov-Smirnov test. If the data were normally distributed, statistical significance was analysed by Student’s t-test, otherwise a non-parametric Kolmogorov-Smirnov test was applied. **p<0.01, ***p<0.001. ARDS, acute respiratory distress syndrome; Dex, dexamethasone; ECMO, extracorporeal membrane oxygenation; ns, not significant; SD, Sprague-Dawley; PBS, phosphate buffer saline.
Figure 2. VV ECMO combined with Dex alleviate oleic acid-induced lung injury. Representative HE staining image (A). Lung injury scores in the three subject groups based on HE staining (B). Evaluation of oedema (C) by lung wet/dry ratio and exudation (D) by BAL protein concentration. The level of alveolar injury biomarker SP-D (E) and sRAGE (F) in the serum. Arterial blood PaO2 and PaCO2 (G, H). The data shown are the mean±SD. Normality was analysed by Kolmogorov-Smirnov test. If the data were normally distributed, statistical significance was analysed by Student’s t-test, otherwise, a non-parametric Kolmogorov-Smirnov test was applied; ns=not significant, *p<0.05, **p<0.01, ***p<0.001. BAL, bronchoalveolar lavage; Dex, dexamethasone; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; SP-D, surfactant protein-D; sRAGE, soluble receptor for advanced glycation end products; VV ECMO, venovenous extracorporeal membrane oxygenation.
VV ECMO combined with Dex reduced pulmonary inflammation
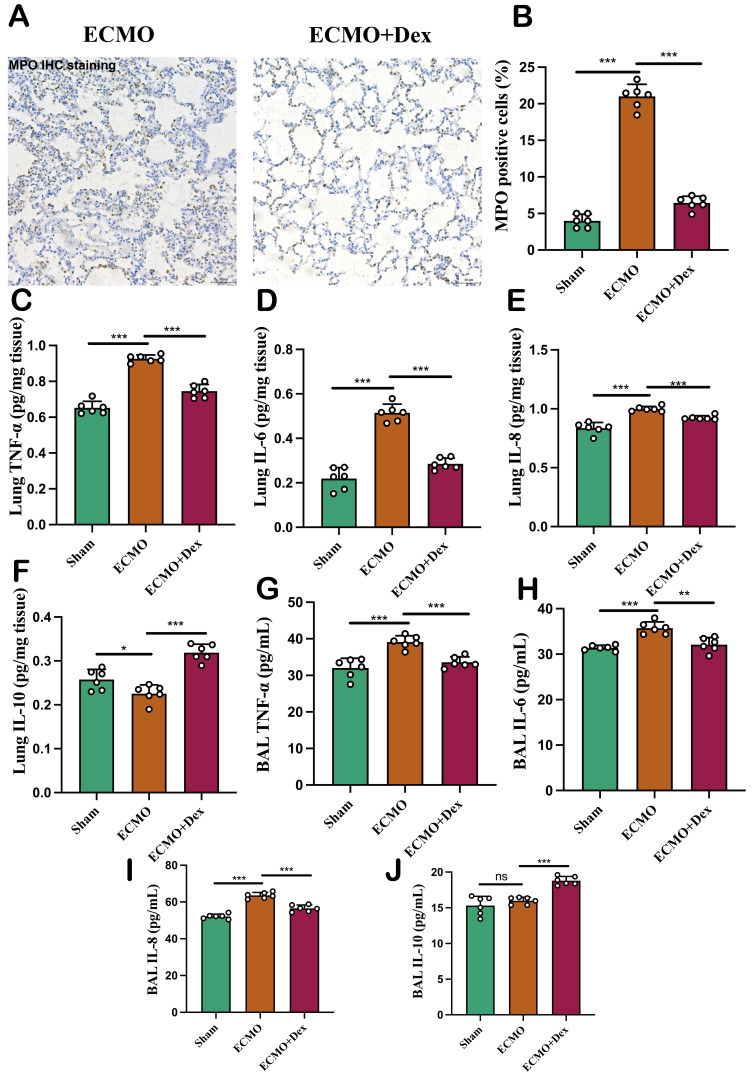
The pathogenesis of ALI is associated with severe inflammation within the lung which results in excessive neutrophil infiltration and the release of inflammatory cytokines. IHC staining revealed a greater number of MPO-positive neutrophil infiltrations in the ECMO group compared with ECMO+Dex group (figure 3A,B). Furthermore, the levels of proinflammatory cytokines including tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8) in the lung were significantly reduced in the ECMO+Dex group (figure 3C–E). However, the level of anti-inflammatory cytokine IL-10 was significantly higher in the ECMO+Dex group than ECMO group (figure 3F). We further measured the levels of TNF-α, IL-6, IL-8 and IL-10 in the BAL. And we found similar results that Dex reduced TNF-α, IL-6, IL-8 and raised IL-10 levels in the BALF (figure 3G–J). These findings indicate that VV ECMO in conjunction with Dex markedly diminished pulmonary inflammation associated with ALI and that Dex exerts a potent anti-inflammatory effect.
Figure 3. VV ECMO combined with Dex reduced pulmonary inflammation. Representative MPO immunohistochemical staining image (A) and comparison of MPO positive cells in the three subject groups (B). The level of TNF-α, IL-6, IL-8, and IL-10 in the lung (C–F). The level of TNF-α, IL-6, IL-8, and IL-10 in the BAL (G–J). The data shown are the mean±SD. Normality was analysed by Kolmogorov-Smirnov test. If the data were normally distributed, statistical significance was analysed by Student’s t-test, otherwise, a non-parametric Kolmogorov-Smirnov test was applied; ns=not significant, *p<0.05, **p<0.01, ***p<0.001. BAL, bronchoalveolar lavage; Dex, dexamethasone; VV ECMO, venovenous extracorporeal membrane oxygenation.
VV ECMO combined with Dex protects alveolar epithelial cells by activation of Hippo/YAP signalling
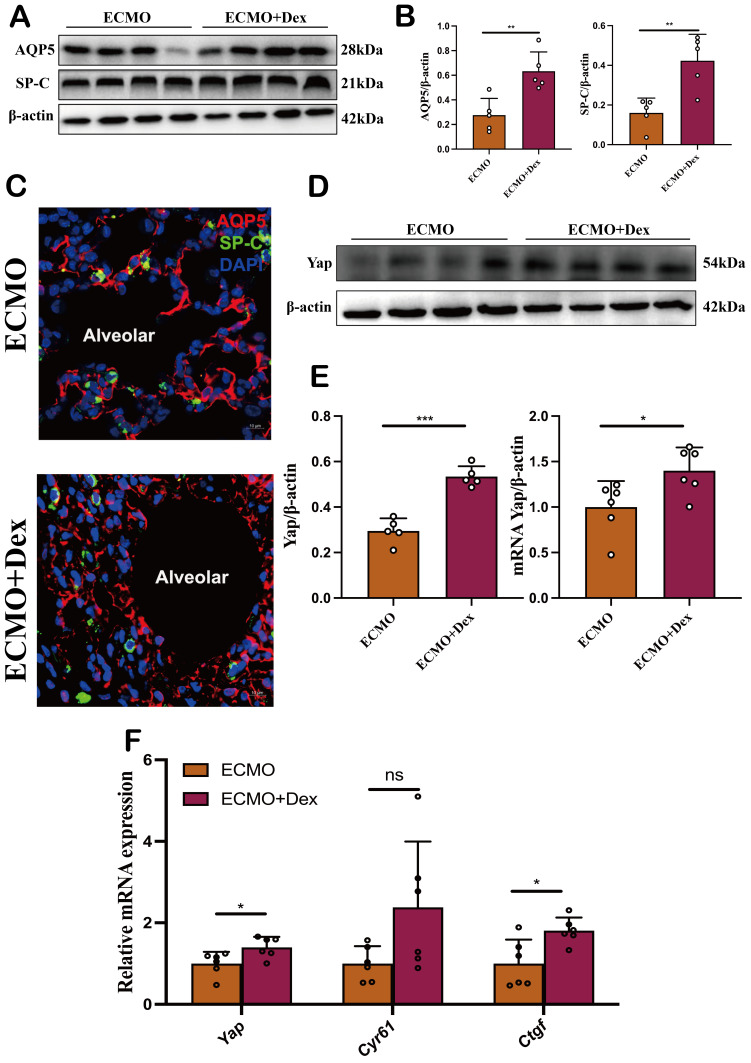
In our previous study, we found VV ECMO protects against lung injury by activating the Hippo/Yap signalling pathway.18 Previous study showed that Dex can significantly activate Yap expression.19 Therefore, we hypothesis VV ECMO combined with Dex could protect alveolar epithelial cells by activation of Hippo/YAP signalling. Alveolar epithelial injury is caused by lung damage and recovery requires alveolar epithelial repair. First, we examined the expression of AQP5 which is an alveolar epithelial type I (AT1) cell-specific marker and SP-C, an alveolar epithelial type II (AT2) cell-specific marker by western blots (figure 4A). We found the relative expression of AQP5 and SP-C was significantly higher in the ECMO+Dex group than in the ECMO group (figure 4B). In addition, immunofluorescent staining also confirmed ECMO combined with Dex enhanced the number and structure of AT1 and AT2 (figure 4C). YAP was a critical regulator in the Hippo/YAP signalling pathway for cell proliferation and differentiation. We examined the expression of YAP by western blots and qPCR, and we found that the relative expression of AQP5 and SP-C was significantly higher in the ECMO+Dex group (figure 4D,E). Further, qPCR analysis revealed that the relative expression of Yap and its downstream gene Ctgf were significantly upregulated in the ECMO+Dex group (figure 4F). These results demonstrate that VV ECMO combined with Dex protects alveolar epithelial cells by activating Hippo/Yap signalling.
Figure 4. VV ECMO combined with Dex protects alveolar epithelial cells by activation of Hippo/YAP signalling. Representative image of western blot bands (A) and relative expression of AQP5 and SP-C (B). Representative confocal images of AT1 and AT2 in two subject groups (C). Representative image of western blot bands (D) and relative expression of Yap by western blot and qPCR (E). Downstream Yap expression including Yap, Cyr61 and Ctgf in the ECMO and ECMO+Dex groups detected via qPCR (F). The data shown are the mean±SD. Normality was analysed by Kolmogorov-Smirnov test. If the data were normally distributed, statistical significance was analysed by Student’s t-test, otherwise, a non-parametric Kolmogorov-Smirnov test was applied; ns=not significant, *p<0.05, **p<0.01, ***p<0.001. Dex, dexamethasone; VV ECMO, venovenous extracorporeal membrane oxygenation.
VV ECMO combined with Dex promote Yap-mediated AT2 differentiation
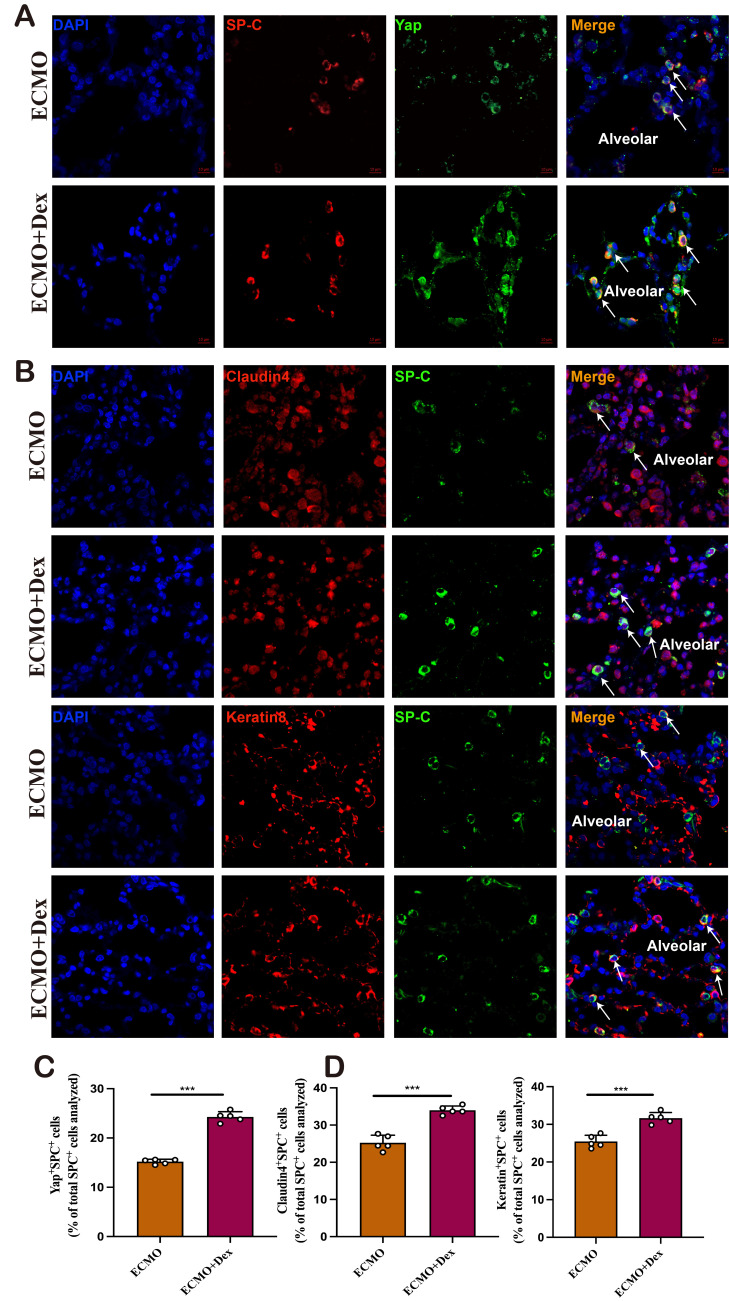
The repair of alveolar epithelium is mainly achieved by the differentiation of AT2 cells into AT1 cells. YAP is a critical downstream effector in the Hippo signalling cascade and plays a crucial role in alveolar epithelial healing. By IF colocalisation, we found the number of Yap+SPC+ cells was significantly higher in the ECMO+Dex group (figure 5A,B). What is more, we measured the AT2 intermediate cell state in sectioned lungs by two markers with transitory expression patterns, Claudin 4 and Keratin 8, which are known to characterise this transitional AT2 cell state in particular. We found both Claudin 4+ SPC+ cells and Keratin 8+ SPC+ cells were more frequent in the ECMO+Dex group (figure 5C,D). These results indicated that Dex could promote Yap-mediated AT2 differentiation, which might associated with alveolar epithelium regeneration and recovery. To further confirm the role of Yap in the process of AT2 differentiation. We conducted an in vitro experiment with A549 and found that Dex could significantly increase the expression levels of Yap, Claudin-4 and Keratin-8. And, Yap siRNA significantly decreased the protein expression of Yap, Claudin-4, Keratin-8 (online supplemental figure 1A-C), the mRNA level of Cyr61, Ctgf, and Ankrd in A549 cells also statistically decreased (online supplemental figure 1D). These results indicate that VV ECMO combined with Dex could promote Yap expression in AT2 cells, which might be associated with alveolar regeneration and recovery.
Figure 5. VV ECMO combined with Dex promote Yap-mediated AT2 differentiation. Representative confocal images of Yap+AT2 cell (A) and quantification of Yap+SPC+ cell in the ECMO and ECMO+Dex group (B). Representative confocal images of Claudin4+AT2 and Keratin8+AT2 cell (C), and quantification of Claudin4+SPC+ cell and Keratin8+ SPC+ in the ECMO and ECMO+Dex group (D). The data shown are the mean±SD. Normality was analysed by Kolmogorov-Smirnov test. If the data were normally distributed, statistical significance was analysed by Student’s t-test, otherwise, a non-parametric Kolmogorov-Smirnov test was applied; ns=not significant,.***p<0.001. Dex, dexamethasone; ns, not significant; VV ECMO, venovenous extracorporeal membrane oxygenation.
Discussion
Although both VV ECMO and Dex are effective solutions for patients with ALI/ARDS, whether adjuvant Dex administration during ECMO support could reduce lung injury remains unknown. In this present study, we aimed to investigate the combined effects of VV ECMO and Dex on lung injury, inflammation, alveolar regeneration, and its underlying mechanism in ALI rats induced by OA. We found that adjuvant Dex administration significantly reduced lung injury and pulmonary inflammation during VV ECMO. Both inflammatory cytokines and neutrophil infiltrations were significantly decreased in the ECMO+Dex group. We further explored the mechanism behind this and discovered Dex combined with VV ECMO activate Hippo/Yap signalling pathway in AT2 cells which increased the ability of AT2 cells to proliferate and differentiate into AT1 cells.
Various clinical conditions, including trauma, aspiration, sepsis and pneumonia, could cause ALI and its critical condition ARDS.2 3 24 The pathophysiology of ALI/ARDS is complex, and our understanding of it is incomplete. However, one thing is certain: proinflammatory cytokines and interconnected inflammatory cascades are important for the inflammatory response.6 As a result of this inflammatory response, injuries on the alveolar epithelial cells and capillary endothelial cells, resulting in the permeability of alveolar capillaries increases, diffuse pulmonary interstitial and alveolar oedema, eventually causing acute hypoxic respiratory insufficiency.25 26 Although VV ECMO improves oxygenation by efficiently supplying more oxygen with minimal mechanical ventilation, enabling the lung to rest and avoid cyclic stretching, thereby facilitating structural reparation and functional restoration of the lungs.27 The ECMO circuit and membrane oxygenator need to be primed with blood products. The contact between the blood products and the biomaterials of the circuit initiates a blood–surface interaction, resulting in the release of inflammatory mediators such as interleukins and tumour necrosis factor.11 28 These circulating inflammatory mediators are known to cause endothelial cell injury and subsequent capillary leak.29 30 Therefore, adjuvant anti-inflammatory therapy during VV ECMO might further enhance its efficiency.
For the study of molecular changes, it is necessary to establish a small animal model that is more portable, efficient and economical. Rats have multiple advantages as animal models of human conditions, including anatomical structures almost identical to their human counterparts, small volume, low cost and relatively easy handling compared with large animal models. Lipopolysaccharide and OA were commonly used to induce ARDS in animal models, which differs highly from the common causes in humans such as infection and trauma. However, we have chosen an OA model in our study. Several studies reported that the blood level of OA was significantly elevated, and the proportion of OA incorporated into surfactant phospholipids was also increased.31 32 So, to simulate the clinical pathological state of ARDS to the greatest extent, we used intravenous OA model to induce ARDS.
In our experimental study, the protein content of BAL was significantly decreased in rats with adjuvant Dex therapy during VV ECMO. What is more, the serum levels of sRAGE and SP-D which indicate lung injury were also reduced significantly. Previous studies have already confirmed VV ECMO could alleviate lung injury.33 This implies that adjuvant Dex could improve the efficiency of VV ECMO to minimise lung injury. Dex has an anti-inflammatory effect, stabilises endothelial cell membranes and increases surfactant production.34 35 All of these effects could potentially improve pulmonary function. The study by Lester et al found that Dex reduces the severity of ARDS and improves patient prognosis with its potential to reduce inflammation in the lungs.36 What is more, previous animal studies found serum TNF-α, IL-6 and VEGF levels of rats treated with OA and Dex were significantly lower than those of rats receiving only OA.37 Similarly, in our experiment, we found the lung tissue and BAL levels of TNF-α, IL-6 and IL-8 were significant decreased in rats with adjuvant Dex administration. Previous research has found that high levels of neutrophil entrapment in the alveolar region are substantially linked to high mortality in ALI cases.38 In our study, the neutrophil infiltrations significantly decreased when rats received Dex at a dose of 1 mg/kg, this indicates Dex could improve the efficiency of VV ECMO by reducing pulmonary inflammation.
In our previous study, we found that VV ECMO protects against lung injury by activating the Hippo/Yap signalling pathway.18 The Hippo/Yap signalling pathway plays a crucial role in alveolar regeneration. It is activated by the YAP transcriptional coactivator, whose biological activity is mediated by the conserved transcriptional enhanced associate domain (TEAD) family transcription factors.39 Hippo pathway-responsive genes require the regulated interaction of YAP and TEAD. Previous studies have reported Dex could activate the expression of Yap.19 40 Therefore, adjuvant Dex may activate the Hippo/YAP signalling pathway to promote alveolar regeneration and reduce lung injury. The dosage of Dex is 1 mg/kg, when compared with standard clinical practice, this seems like a very high dose. However, for patients with ARDS, the main purpose of Dex management was to alleviate inflammatory response. In this study, we focused on Dex as a YAP agonist, and a study by Sorrentino et al reported Dex was given 20 mg/kg to investigate YAP activation and breast cancer19; Gong et al found Dex activated YAP and TAZ and led to improved neurologic function at a dosage of 50 mg/kg.40 Therefore, we chose a 1 mg/kg dosage based on literature and preliminary experiment results. Our study found the Hippo/YAP signalling pathway was activated, and the expression of Yap was significantly upregulated. In the alveolar epithelium, there are only two types of cells: AT1 and AT2.41 Previous studies have shown that OA damages epithelial cells.42 Alveolar regeneration occurs in many mammals after ALI, and AT2 could promptly proliferate and transdifferentiate into AT1 to replace damaged AT1.43 44 YAP was discovered to be a critical regulator in AT2 cell proliferation and differentiation, with the Hippo/YAP signalling pathway playing a critical role.45 46 We found adjuvant Dex during VV ECMO enhanced the number and structure of AT1 and AT2 in our study. As previous studies reported the presence of alveolar regeneration in ARDS patients with ECMO support only.47 48 VV ECMO combined with Dex may enhance the process of lung regeneration in ALI/ARDS patients.
The repair of alveolar epithelium is mainly achieved by the differentiation of AT2 cells into AT1 cells. YAP is also required for AT2 cell proliferation and differentiation in response to mechanical strain in the lungs, according to research on pneumonectomy-induced alveolar regeneration.33 46 Similarly, we found the upregulated Yap was mainly in AT2 cells, and the AT2 intermediate cell state was more frequent in ECMO+Dex group. In vitro, we found Dex could significantly increase the expression levels of Yap, Claudin 4 and Keratin 8. However, the expression of Yap, Claudin-4, Keratin-8, Cyr61, Ctgf and Ankrd was significantly decreased after we knock down Yap by siRNA. These results indicate Dex could activate Hippo/Yap signalling pathway and YAP plays a crucial role in AT2 differentiation. A previous clinical study also found that the Dex given during the first 3 days of ECMO results in significant improvement in lung injury scores in infants.48 Therefore, the findings of this study may provide some guidance for future clinical trials.
The essential function of the lung is oxygen exchange. Meanwhile, constant exposure to the external environment makes the lungs more vulnerable, leading to injury and diseases. Alveolar regeneration is critical for maintaining the integrity of the alveolar epithelial barrier and a sufficient gas exchange surface. Lineage tracing studies have found that AT2 cells can self-renew and differentiate into AT1 cells in the alveolar epithelium. However, the time scale of the alveolar regeneration process remains to be determined, and how long it takes to regenerate the alveoli and restore the lung remains unclear. The time point of euthanasia was relatively early, with 3 hours of experiment in our study, which is a significant limitation of this study. Nevertheless, we found adjuvant Dex during VV ECMO might promote alveolar regeneration and AT2 differentiation. This indicates the process of alveolar regeneration could be started at an early stage. For clinical practice, the duration of VV ECMO often lasts much longer, further investigations of longer experiment time are warranted to draw meaningful conclusions about alveolar regeneration.
This study has some limitations as well. First, we used OA-induced lung damage, which is not the same as the mechanisms seen in various human insults. Second, only male animals were used in our study, the results and their implications for females are still unknown. Finally, Dex was given by single dose with VV ECMO support was only last for 3 hours to avoid pump-related blood damage.
Conclusions
In this study, we investigated the efficiency of adjuvant Dex during VV ECMO in ALI rats induced by OA. Dex alleviates OA-induced lung injury and pulmonary inflammation, and the underlying mechanism could be the activation of Hippo/Yap signalling pathway to promote alveolar regeneration and AT2 differentiation. Dex could improve the efficiency of VV ECMO and reduce lung injury in rats to some extent.
supplementary material
Footnotes
Funding: This study was funded by National Key R&D Program of China (NO.2021YFC2701703, 2021YFC2701700); Shenzhen Key Medical Discipline Construction Fund (No.SZXK00036); the Science and Technology Planning Project of Chengguan District (2022RCCX0023); Joint scientific research project (23JRRA1502); the Talent Introduction Plan of the Lanzhou University Second Hospital (No. YJRCKYQDJ-2021-02).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: All animal experiments were approved by the Lanzhou Second Hospital, Lanzhou University, Gansu (No. P2021-019). All animals received humane care in compliance with the ‘Principles of laboratory animal care’ formulated by the National Society for Medical Research and the ‘Guide for the care and use of laboratory animal resources’ published by the US National Institute of Health (NIH publication No. 85–23, revised 1996). The current research performed on animals meets the most recent ARRIVE guidelines.
Contributor Information
Shi-Lin Wei, Email: weishilinv@qq.com.
Jun-Zhe Du, Email: djzlfia@163.com.
Ke-Rong Zhai, Email: 867293057@qq.com.
Jian-Bao Yang, Email: 13669395545@163.com.
Ran Zhang, Email: zhangran@301hospital.com.cn.
Xiang-Yang Wu, Email: wuxyok@163.com.
Yongnan Li, Email: lyngyq2006@foxmail.com.
Bin Li, Email: dr.leebin@outlook.com.
Data availability statement
Data are available on reasonable request.
References
- 1.Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 2.Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med. 2017;195:725–36. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- 3.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398:622–37. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400:1145–56. doi: 10.1016/S0140-6736(22)01485-4. [DOI] [PubMed] [Google Scholar]
- 6.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodie D, Slutsky AS, Combes A. Extracorporeal Life Support for Adults With Respiratory Failure and Related Indications: A Review. JAMA. 2019;322:557–68. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 8.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–8. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Yu W, Shi J, et al. The effect of venovenous extra-corporeal membrane oxygenation (ECMO) therapy on immune inflammatory response of cerebral tissues in porcine model. J Cardiothorac Surg. 2013;8:186. doi: 10.1186/1749-8090-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurundkar AR, Killingsworth CR, McIlwain RB, et al. Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr Res. 2010;68:128–33. doi: 10.1203/PDR.0b013e3181e4c9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McILwain RB, Timpa JG, Kurundkar AR, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90:128–39. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiegers GJ, Reul JM. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci. 1998;19:317–21. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen X-Y, Wang S-M, Li N, et al. Creation of lung-targeted dexamethasone immunoliposome and its therapeutic effect on bleomycin-induced lung injury in rats. PLoS One. 2013;8:e58275. doi: 10.1371/journal.pone.0058275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B, Wang DX, Deng W. Protective effects of dexamethasone on early acute lung injury induced by oleic acid in rats. Int J Clin Exp Med. 2014;7:4698–709. [PMC free article] [PubMed] [Google Scholar]
- 15.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307–16. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–76. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 17.Feng L-H, Li X-D, Zhang X-Y, et al. Dexamethasone for the treatment of acute respiratory distress syndrome: A systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e30195. doi: 10.1097/MD.0000000000030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Zhang R, Zhai K, et al. Venovenous extracorporeal membrane oxygenation promotes alveolar epithelial recovery by activating Hippo/YAP signaling after lung injury. J Heart Lung Transplant. 2022;41:1391–400. doi: 10.1016/j.healun.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Sorrentino G, Ruggeri N, Zannini A, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Huang J, Zhang R, et al. Establishment of a venovenous extracorporeal membrane oxygenation in a rat model of acute respiratory distress syndrome. Perfusion. 2023;38:85–91. doi: 10.1177/02676591211031468. [DOI] [PubMed] [Google Scholar]
- 21.Chen HI, Hsieh N-K, Kao SJ, et al. Protective effects of propofol on acute lung injury induced by oleic acid in conscious rats. Crit Care Med. 2008;36:1214–21. doi: 10.1097/CCM.0b013e31816a0607. [DOI] [PubMed] [Google Scholar]
- 22.Mrozek JD, Smith KM, Bing DR, et al. Exogenous surfactant and partial liquid ventilation: physiologic and pathologic effects. Am J Respir Crit Care Med. 1997;156:1058–65. doi: 10.1164/ajrccm.156.4.9610104. [DOI] [PubMed] [Google Scholar]
- 23.Atmowihardjo LN, Heijnen NFL, Smit MR, et al. Biomarkers of alveolar epithelial injury and endothelial dysfunction are associated with scores of pulmonary edema in invasively ventilated patients. Am J Physiol Lung Cell Mol Physiol. 2023;324:L38–47. doi: 10.1152/ajplung.00185.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front Immunol. 2020;11:1722. doi: 10.3389/fimmu.2020.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26:160116. doi: 10.1183/16000617.0116-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crimi E, Slutsky AS. Inflammation and the acute respiratory distress syndrome. Best Pract Res Clin Anaesthesiol. 2004;18:477–92. doi: 10.1016/j.bpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Combes A, Schmidt M, Hodgson CL, et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020;46:2464–76. doi: 10.1007/s00134-020-06290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plötz FB, van Oeveren W, Bartlett RH, et al. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993;105:823–32. [PubMed] [Google Scholar]
- 29.Burke-Gaffney A, Keenan AK. Modulation of human endothelial cell permeability by combinations of the cytokines interleukin-1 alpha/beta, tumor necrosis factor-alpha and interferon-gamma. Immunopharmacology. 1993;25:1–9. doi: 10.1016/0162-3109(93)90025-l. [DOI] [PubMed] [Google Scholar]
- 30.Anderson HL, III, Coran AG, Drongowski RA, et al. Extracellular fluid and total body water changes in neonates undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27:1003–8. doi: 10.1016/0022-3468(92)90547-K. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan GJ, Lamb NJ, Evans TW, et al. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med. 1996;24:241–6. doi: 10.1097/00003246-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt R, Meier U, Yabut-Perez M, et al. Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am J Respir Crit Care Med. 2001;163:95–100. doi: 10.1164/ajrccm.163.1.9903029. [DOI] [PubMed] [Google Scholar]
- 33.Araos J, Alegría L, García P, et al. Extracorporeal membrane oxygenation improves survival in a novel 24-hour pig model of severe acute respiratory distress syndrome. Am J Transl Res. 2016;8:2826–37. [PMC free article] [PubMed] [Google Scholar]
- 34.Schleimer RP. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45 Suppl 1:S3–7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- 35.Noreen S, Maqbool I, Madni A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894:173854. doi: 10.1016/j.ejphar.2021.173854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lester M, Sahin A, Pasyar A. The use of dexamethasone in the treatment of COVID-19. Ann Med Surg (Lond) 2020;56:218–9. doi: 10.1016/j.amsu.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin M, Qiu Z. Changes in TNF-α, IL-6, IL-10 and VEGF in rats with ARDS and the effects of dexamethasone. Exp Ther Med. 2019;17:383–7. doi: 10.3892/etm.2018.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma X. TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect. 2001;3:121–9. doi: 10.1016/s1286-4579(00)01359-9. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves-de-Albuquerque CF, Silva AR, Burth P, et al. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediators Inflamm. 2015;2015:260465. doi: 10.1155/2015/260465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong P, Zhang Z, Zou C, et al. Hippo/YAP signaling pathway mitigates blood-brain barrier disruption after cerebral ischemia/reperfusion injury. Behav Brain Res. 2019;356:8–17. doi: 10.1016/j.bbr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paris AJ, Hayer KE, Oved JH, et al. STAT3-BDNF-TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat Cell Biol. 2020;22:1197–210. doi: 10.1038/s41556-020-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi J, Park J-E, Tsagkogeorga G, et al. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell. 2020;27:366–82. doi: 10.1016/j.stem.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaCanna R, Liccardo D, Zhang P, et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest. 2019;129:2107–22.:125014. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Wu H, Jiang K, et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Rep. 2016;16:1810–9. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Hu C, Sun J, Du J, et al. The Hippo-YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury. Cell Biol Int. 2019;43:1174–83. doi: 10.1002/cbin.11098. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Wu H, Yu Y, et al. Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res. 2020;30:708–10. doi: 10.1038/s41422-020-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochs M, Timm S, Elezkurtaj S, et al. Collapse induration of alveoli is an ultrastructural finding in a COVID-19 patient. Eur Respir J. 2021;57:2004165. doi: 10.1183/13993003.04165-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin MP, Wooldridge P, Alford BA, et al. Dexamethasone therapy in neonates treated with extracorporeal membrane oxygenation. J Pediatr. 2004;144:296–300. doi: 10.1016/j.jpeds.2003.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.