Abstract
Abstract
Purpose
Coronary CT angiography (CCTA) is well established for the diagnostic evaluation and prognostication of coronary artery disease (CAD). The growing burden of CAD in Asia and the emergence of novel CT-based risk markers highlight the need for an automated platform that integrates patient data with CCTA findings to provide tailored, accurate cardiovascular risk assessments. This study aims to develop an artificial intelligence (AI)-driven platform for CAD assessment using CCTA in Singapore’s multiethnic population. We will conduct a hybrid retrospective-prospective recruitment of patients who have undergone CCTA as part of the diagnostic workup for CAD, along with prospective follow-up for clinical endpoints. CCTA images will be analysed locally and by a core lab for coronary stenosis grading, Agatston scoring, epicardial adipose tissue evaluation and plaque analysis. The images and analyses will also be uploaded to an AI platform for deidentification, integration and automated reporting, generating precision AI toolkits for each parameter.
Participants
CCTA images and baseline characteristics have been collected and verified for 4196 recruited patients, comprising 75% Chinese, 6% Malay, 10% Indian and 9% from other ethnic groups. Among the participants, 41% are female, with a mean age of 55±11 years. Additionally, 41% have hypertension, 51% have dyslipidaemia, 15% have diabetes and 22% have a history of smoking.
Findings to date
The cohort data have been used to develop four AI modules for training, testing and validation. During the development process, data preprocessing standardised the format, resolution and other relevant attributes of the images.
Future plans
We will conduct prospective follow-up on the cohort to track clinical endpoints, including cardiovascular events, hospitalisations and mortality. Additionally, we will monitor the long-term impact of the AI-driven platform on patient outcomes and healthcare delivery.
Trial registration number
Keywords: Coronary heart disease, Computed tomography, Artificial Intelligence
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study uses a large, multiethnic Asian cohort, allowing for diverse population sampling.
A hybrid retrospective-prospective design allows for comprehensive clinical follow-up and outcome validation.
A standardised coronary CT angiography protocol ensures consistency across multiple healthcare centres.
The use of advanced artificial intelligence models facilitates automated analysis of calcium score, epicardial adipose tissue and plaque characteristics.
The study population is exclusively Asian, limiting the generalisability of the findings to other ethnic groups.
Introduction
As in the rest of the world, coronary artery disease (CAD) is a leading cause of death in Asia, and its increasing prevalence signals a significant healthcare and economic burden.1 2 Coronary CT angiography (CCTA) has become firmly established as an essential modality for the early detection, clinical evaluation and risk stratification of CAD. This is reflected in guidelines from the National Institute for Health and Care Excellence,3 the European Society of Cardiology4 and the American Heart Association.5 These recommendations are based on robust evidence demonstrating that early use of CCTA improves event-free survival by facilitating the timely initiation of guideline-directed medical therapy,6 reduces unnecessary cardiac catheterisation7 and enables faster discharge of patients presenting with possible acute coronary syndrome in emergency settings.8
CCTA is not only the preferred modality for anatomical assessment of the coronary vasculature but also an indispensable tool for disease characterisation and risk stratification. CT-derived parameters, including the Agatston score,9 epicardial adipose tissue (EAT)10 and plaque characteristics,11 offer incremental value in these assessments. However, the routine clinical adoption of these measurements is limited by the laborious and time-intensive nature of manual quantification. Additionally, manual assessment introduces significant interobserver variability—reportedly as high as 20%, even among expert readers.12 Thus, there is an unmet need for automated solutions to streamline these processes and fully harness the diagnostic and prognostic potential of CCTA in CAD management.
Moreover, existing cardiovascular risk prediction models have shown limited accuracy in Asian populations. For example, the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry revealed that Asian sites reported a threefold lower-than-expected CAD prevalence.13 Similarly, an observational study by Villadsen et al identified ethnic differences in coronary plaque composition, with South Asian patients exhibiting a higher proportion of non-calcified plaque compared with Caucasians.14 Our group previously assessed the performance of the CAD Consortium (CAD2) model in a mixed Asian population in Singapore and found suboptimal predictive accuracy, though it improved significantly after local calibration.15 More recently, we evaluated the prognostic utility of pooled cohort equations (PCEs) and Agatston scores in a symptomatic, mixed Asian cohort. PCEs alone showed no discriminative value beyond random chance, and the addition of Agatston scores did not provide meaningful improvement.16 These findings highlight the need for automated models that account for ethnic variations to accurately predict cardiovascular risk in Singapore’s multiethnic population. To achieve this, a contemporary study of CAD prevalence and characteristics in Asia is essential.
Cohort description
Patient and public involvement
Patients and/or the public were not directly involved in the design, or conduct, or reporting, or dissemination plans of this research. The study outcomes will be disseminated through publication in peer-reviewed biomedical, cardiac imaging and clinical journals, as well as presentations at scientific conferences. This will pave the way for a range of clinical, population health, research and commercial applications.
Cohort objectives
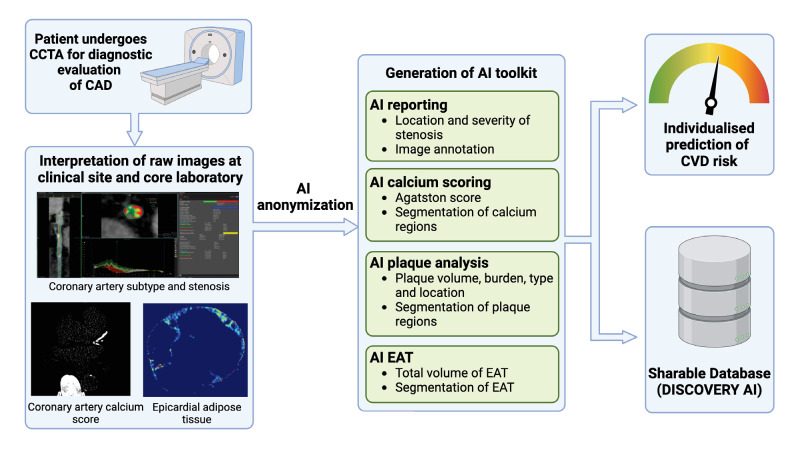
We aim to build a first-in-Asia, AI-driven national platform for CCTA for clinical, and industrial applications (APOLLO), creating a mixed-ethnic phenotypic registry of CAD in Singapore (online supplemental graphical abstract). APOLLO will serve a range of clinical, research and industrial purposes. First, as a large registry, APOLLO will offer valuable insights into patient demographics and disease patterns within a highly characterised multiethnic Asian population. Second, the development of precision artificial intelligence (AI) toolkits will enable the automation of anonymisation, reporting, Agatston scoring, EAT and plaque quantification, facilitating the integration of these tasks into routine clinical practice. Third, as a deidentified and sharable database, APOLLO will support the calibration and development of Asian-based prediction models and accelerate the introduction of novel medical and device therapies in the Asian context (figure 1).
Figure 1. Workflow of the APOLLO platform. CT images are anonymised and uploaded into the APOLLO database. The images are then processed using AI engines, and the results are re-uploaded into the database. A summary report is generated and presented to the end user. AI, artificial intelligence; CAD, coronary artery disease; CVD, cardiovascular disease; EAT, epicardial adipose tissue.
Study type
APOLLO is a hybrid, retrospective-prospective, open-label, observational, multicentre study. It includes retrospective recruitment of patients who underwent CCTA as part of the diagnostic workup for CAD, along with prospective follow-up to monitor several clinical endpoints. Additionally, prospective patient recruitment will involve identifying and enrolling eligible patients from multiple participating centres. These patients will undergo CCTA as part of their clinical care and will be followed over time to track key outcomes, including cardiovascular events, hospitalisations and mortality. Details of the study design are also available in ClinicalTrials.gov (Identifier: NCT05509010).
Study population
There are three hospital sites participating in the creation of APOLLO: National Heart Centre Singapore (NHCS), National University Hospital and Tan Tock Seng Hospital. These institutions represent the three largest cardiac healthcare systems in the country. The study uses a hybrid recruitment approach, both retrospective and prospective, targeting a total of 5000 CAD patients. For the retrospective arm, patients who underwent CT scans between 2007 and 2017 will be screened and included if they meet the inclusion criteria. Outcomes will be obtained through a review of medical records and national registries, with follow-up continuing until 31 December 2025. For the prospective arm, patient recruitment occurred from October 2021 to July 2024. Clinical events and outcomes will be tracked for a period of 5 years following enrolment, with data collected from hospital medical records and national databases. The inclusion and exclusion criteria are listed in box 1. Patients aged 21 and above were included. Exclusion criteria include acute coronary syndrome, a body mass index exceeding 40 kg/m2 and a history of percutaneous or surgical intervention for CAD. Baseline demographic and clinical characteristics will be obtained from the electronic medical records and case notes, including but not limited to (1) age, gender, race, socioeconomic status, marital status, (2) comorbidities, (3) laboratory tests, (4) radiological tests, (5) cardiac investigations, (6) cardiac procedures, (7) medication use and (8) chest pain characteristics.
Box 1. Inclusion and exclusion criteria for prospective patient recruitment.
Inclusion criteria.
Age ≥21 years old.
Clinically indicated for evaluation by CCTA.
Exclusion criteria
Known complex congenital heart disease.
Planned invasive angiography for reasons other than coronary artery disease.
Non-cardiac illness with life expectancy <2 years.
Pregnancy.
Concomitant participation in another clinical trial in which subject is subject to investigational drug or device.
Cardiac event and/or coronary revascularisation (percutaneous coronary intervention and/or coronary artery bypass grafting) and/or valvular repair/replacement prior to CCTA.
Glomerular filtration rate ≤30 mL/min.
Known allergy to iodinated contrast agent.
Contraindications to beta blockers or nitroglycerin or adenosine.
CCTA, coronary computed tomographyCT angiography.
CCTA protocol
CCTA acquisition will be performed using six state-of-the-art CT scanners (Canon, Siemens, GE, Philips) with ≥256-detector rows, following image acquisition protocols outlined in the Society of Cardiovascular Computed Tomography (SCCT) guidelines.17 Medication, as per guidelines, may be administered to control heart rate in patients with a heart rate above 60 bpm. Sublingual glyceryl trinitrate will be administered prior to scanning. The CCTA scans will use a prospective ECG-triggered scanning mode. A tri-phasic injection protocol will be applied, consisting of contrast injections of approximately 50 mL at 5 mL/s and 20 mL at 3.5 mL/s sequentially, followed by a third injection of 30 mL saline at 3 mL/s.
Cardiac CT analysis
The CT images will be analysed and interpreted both at the clinical site level and at the core laboratory. At the clinical site, CT interpretation will follow the SCCT guidelines.17 Core lab analysis will be performed at the CardioVascular Systems Imaging and AI research core of NHCS, blinded to the clinical site interpretation. Core lab analyses will include:
Coronary stenosis grading: This assessment focuses on the severity and precise localisation of stenosis within the coronary circulation. A key aspect involves visually estimating luminal narrowing caused by plaque. Following the SCCT guidelines,17 stenosis is graded across a spectrum from minimal narrowing to total occlusion. Additionally, distinctions are made between obstructive and non-obstructive stenosis, with the SCCT model guiding accurate localisation.
Agatston scoring analysis: Calcified plaque is evaluated using Agatston scoring programmes, aligned with SCCT clinical guidelines.17 Pixels exceeding 130 Hounsfield units (HU) are identified as indicative of calcium in non-contrast studies.18 Lesions within each coronary vessel distribution are identified, and the scoring programme generates a summed score for each vessel based on area-density (Agatston score) measurements. The total coronary Agatston score aggregates all calcified lesions across the coronary tree.
EAT analysis: This analysis quantifies the total volume of EAT and pericardial adipose tissue, both metabolically active fats associated with increased cardiovascular disease risk.19 Quantification on non-contrast CT scans requires manual annotation of the pericardium. EAT is identified using adipose tissue attenuation values between −190 and −30 HU.20 Due to potential variations in HU values from scan noise or attenuation changes, the final EAT region is verified by an experienced radiologist or cardiologist.
Plaque analysis: This analysis focuses on plaque volume, burden, type and anatomical locations. Coronary segmentation is performed for segments with a diameter ≥1.5 mm, with the SCCT model17 aiding in precise plaque localisation. For each plaque, detailed assessments are conducted, including start and end points, area, volume, burden and type (non-calcified, calcified or mixed).21 Non-calcified plaque is further subclassified as low attenuation plaque (LAP) if HU<30 or non-LAP if HU>30.
Patient outcomes
Patients whose CCTA data are used for this study will be followed for several outcome measures. This follow-up will enable the prognostic validation of AI-derived measurements. The patient outcomes to be monitored are as follows:
Mortality (all-cause and cardiovascular): Throughout the follow-up period, the study will track mortality rates, including both all-cause mortality and deaths specifically attributable to cardiovascular events.
Major adverse cardiovascular events (MACE): In addition to mortality, the study will assess MACE, which includes acute myocardial infarction (AMI), stroke, heart failure and percutaneous or surgical revascularisation.
Rehospitalisation: A key aspect of the outcome measures will involve evaluating the incidence of rehospitalisation. This parameter serves as a valuable indicator of the long-term impact of AI interventions on the need for repeated hospital admissions, providing insights into sustained health outcomes and healthcare resource utilisation.
These data will be collected from hospital medical records and national registries in accordance with institutional, ministry and national-level regulations. At the institutional level, tracking and extraction of outcomes will be performed by the respective IT teams under the supervision of the principal investigator/study team. The clinical research coordinator at each institution will also track and match outcomes using electronic medical records. At the national level, the matching and tracking of outcome data from national registries will be handled by staff from the National Registry of Diseases Office (NRDO). One of the registries analysed through the NRDO will be the Singapore Myocardial Infarction Registry (SMIR) for aggregate data.
AI-based parameters
The images, data and analyses will be uploaded to an AI platform for deidentification, analysis, integration and automated reporting. A container will encapsulate all AI solutions developed during the study, allowing for seamless deployment across third-party environments, including laptops, cloud platforms and both Windows and Linux operating systems. The toolkits can also be integrated with commercial third-party software platforms.
-
AI anonymisation: The anonymisation of pixel data follows a pipeline consisting of the following steps:
Extracting personal data from the DICOM metadata that may be present in the pixel image. This information defines a set of words the pipeline searches for within the image.
Preprocessing the image to enhance contrast and reduce noise.
Deploying a convolutional neural network (CNN) for alphabet recognition, which identifies characters within the image.
Matching and removing personal data by cross-referencing identified words with those found in the DICOM metadata.
-
AI stenosis grading:
Coronary artery tree detection: Error-tolerant graph neural network technology22 is integrated into the platform. Building on prior work by Huang et al,23 we use an enhanced three-dimensional (3D) U-Net model to identify coronary arteries. A graph U-Net model further filters these candidates based on topological, positional and image features (online supplemental figure 1). Non-coronary segments and discontinuous arteries are either removed or reconnected as necessary. The result is a coronary artery tree that is easily mapped due to its graph structure.
Joint stenosis grading and plaque quantification on 3D images: Stenosis grading and plaque quantification are performed simultaneously by an algorithm combining a 3D U-Net model and a 3D image classifier (online supplemental figure 2). The U-Net generates segmentation masks for the lumen, calcified plaque and non-calcified plaque. These are then used as inputs for the image classifier, which outputs stenosis grades and plaque types
AI Agatston score analysis: AI-based Agatston scoring begins with the segmentation of calcified plaque on non-contrast CT scans (online supplemental figure 3), leveraging CNNs.24 25 A novel approach in our platform involves combining non-contrast and contrast CT scans, aligned through multimodal image registration. A deep learning multitask network analyses both plaque and calcification. This interpretable multitask learning algorithm provides a more accurate analysis.
AI EAT: AI-based EAT quantification uses 2D axial slices (online supplemental figure 4), with segmentation achieved through fully convolutional networks (eg, U-Net) or fully annotated CTs.
-
AI reporting: The AI-generated reports include Agatston scoring and stenosis grading. Automated tasks include:
CCTA image quality evaluation.
Heart segmentation.
EAT segmentation and analysis.
Aorta segmentation.
Detection and registration of the coronary artery tree.
Agatston scoring and stenosis grading.
Data sharing
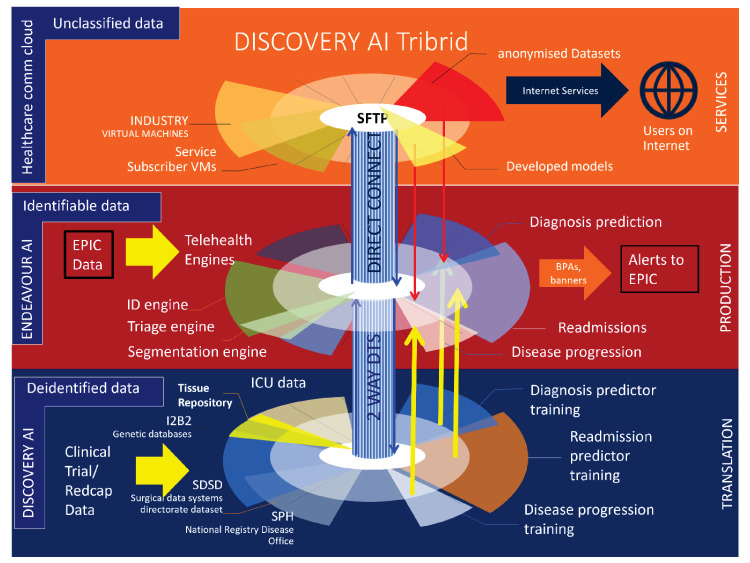
Data sharing will be facilitated through the National University Health System’s DISCOVERY AI platform, a production system that provides centralised anonymisation, equitable data access and differential data linkage capabilities.26 The processes of the DISCOVERY AI platform are summarised in figure 2, with oversight resting with the custodian of each database. DISCOVERY AI incorporates centralised anonymisation and data handling measures in compliance with the Singapore Personal Data Protection Act (PDPA) 2012, the Human Biomedical Research Act 2015 and the Human Biomedical Research Regulations 2017. In accordance with PDPA guidelines, all data managed within DISCOVERY AI are anonymised by removing protected health identifiers, such as names, addresses and identification numbers. The platform also features proprietary security measures, including data obfuscation and ledger-based access logs.
Figure 2. DISCOVERY AI tribrid platform processes. Clinical and research data are processed by various production AI modules to generate predictive clinical warnings within the electronic health record system. AI, artificial intelligence; EPIC, an American privately held healthcare software company; I2B2, Informatics for Integrating Biology & the Bedside; ICU, intensive care unit; SDSD, Surgical Data Systems Directorate dataset; SPH, School of Public Health.
Sample size calculation
Conventional sample size calculations rely on a predefined margin of error; however, in AI, estimating the required sample size prior to experimentation is not always feasible because this margin of error cannot be established until the deep learning model development process has begun. Instead, during deep learning analysis, we will use cross-validation techniques, such as k-fold cross-validation and hold-out test sets, to ensure robust statistical confidence in our model. These methods allow us to derive confidence intervals and assess model performance iteratively.
To further ensure statistical rigour, we will also perform a post hoc power analysis to evaluate the actual power achieved by the analysis and report these findings. Additionally, previous studies provide support for the adequacy of our dataset size. For example, a coronary artery calcium deep learning project using 377 subjects achieved over 90% accuracy.27 Similarly, Commandeur et al demonstrated improved predictive performance using AI on cardiac CT in 1912 asymptomatic subjects, achieving a higher area under the curve (0.82 vs 0.77, p<0.05) compared with conventional methods like coronary artery calcium scoring.28 These results suggest that the dataset collected in our current study should be sufficient to train the deep learning models effectively and achieve high performance for each specific aim.
In previous studies, the 5-year rate of the primary endpoint in the CTA group was reported as 2.3% in one study,6 while another study observed a primary endpoint event in 164 out of 4996 patients (3.3%) over a median follow-up of 25 months.29 Based on these findings, we predict the event rate in our current study to be approximately 3% over the 5-year follow-up period. With a predicted event rate of 3%, we aim to capture at least 100 events to ensure robust model estimation. Following the general rule of 10 events per predictor variable,30 we anticipate including 10 predictors in our models, resulting in a required sample size of approximately 3333 patients.
Clinical risk model development
This study aims to build a clinical risk model that incorporates parameters derived from AI calcium score, AI EAT, AI stenosis and AI plaque characteristics to predict patient outcomes or progression of atherosclerosis. In addition to the AI-derived features, traditional clinical and demographic data will also be included. The risk model will be developed using logistic regression, Cox proportional hazards models or machine learning algorithms. By combining the AI-derived features with traditional risk factors, patients will be categorised into risk groups (eg, low, intermediate and high risk) based on output probabilities or risk scores. After model construction, a post hoc power analysis will be conducted to ensure that the sample size used is sufficient to detect meaningful associations and that the model possesses adequate statistical power.
Dissemination
Project outcomes will be disseminated through publications in peer-reviewed biomedical, cardiac imaging and clinical journals, as well as presentations at scientific conferences. Patient confidentiality will be maintained by ensuring that no individually identifiable information is included in publications.
Findings to date
CCTA images and baseline characteristics have been collected and verified for 4196 recruited patients. Demographic data indicate that the study population consists of 75% Chinese, 6% Malay, 10% Indian and 9% from other ethnic groups. Among the participants, 41% are female, with a mean age of 55±11 years. Additionally, 41% have hypertension, 51% have dyslipidaemia, 15% have diabetes and 22% have a history of smoking (table 1). All collected data have been anonymised and stored in the DISCOVERY AI platform. Furthermore, four AI modules are in development using 2983 CT imaging studies for training, testing and validation. During the development of each AI module, data preprocessing standardised the format, resolution and other relevant attributes of the images.
Table 1. Baseline characteristics.
| All(n=4196) | Retrospective(n=1929) | Prospective(n=2267) | |
| Age, years | 55±11 | 54±11 | 55±11 |
| Gender, male/female | 2487/1709 | 1177/752 | 1310/957 |
| Height, cm | 165±9 | 165±9 | 166±9 |
| Weight, kg | 71±15 | 71±15 | 71±15 |
| Body mass index, kg/m2 | 26±5 | 26±5 | 26±5 |
| Race | |||
| Chinese, n (%) | 3143 (75) | 1381 (72) | 1762 (78) |
| Malay, n (%) | 253 (6) | 122 (6) | 131 (6) |
| Indian, n (%) | 407 (10) | 197 (10) | 210 (9) |
| Others, n (%) | 393 (9) | 229 (12) | 164 (7) |
| Cardiac risk factors | |||
| Hypertension, n (%) | 1731 (41) | 864 (45) | 867 (38) |
| Diabetes, n (%) | 638 (15) | 295 (15) | 343 (15) |
| Dyslipidaemia, n (%) | 2153 (51) | 1013 (53) | 1140 (50) |
| Family history, n (%) | 1115 (27) | 412 (21) | 703 (31) |
| Smoking, n (%) | 921 (22) | 373 (19) | 548 (24) |
| Peripheral artery disease, n (%) | 7 (0.2) | 5/ (0.3) | 2 (0.1) |
| Medication | |||
| Aspirin, n (%) | 511 (12) | 307 (16) | 204 (9) |
| Thienopyridine, n (%) | 156 (4) | 111 (6) | 45 (2) |
| Ticagrelor, n (%) | 8 (0.2) | 5 (0.3) | 3 (0.1) |
| Statin, n (%) | 1531 (36) | 746 (39) | 785 (35) |
| Beta blocker, n (%) | 610 (15) | 376 (19) | 234 (10) |
| Calcium channel blocker, n (%) | 708 (17) | 321 (17) | 387 (17) |
| ACE-inhibitor, n (%) | 224 (5) | 130 (7) | 94 (4) |
| Angiotensin II antagonist, n (%) | 527 (13) | 249 (13) | 278 (12) |
| Mineralocorticoid antagonist, n (%) | 28 (1) | 19 (1) | 9 (0.4) |
| Oral hypoglycaemics, n (%) | 413 (10) | 193 (10) | 220 (10) |
| Insulin, n (%) | 55 (1) | 27 (1) | 28 (1) |
| Blood test | |||
| Glomerular filtration rate, mL/min | 96 (86, 104) | 96 (86, 105) | 95 (86, 103) |
| Total cholesterol, mmol/L | 5.0 (4.3, 5.7) | 5.0 (4.3, 5.7) | 5.0 (4.3, 5.8) |
| High-density cholesterol, mmol/L | 1.3 (1.1, 1.6) | 1.3 (1.0, 1.5) | 1.4 (1.1, 1.6) |
| Triglycerides, mmol/L | 1.3 (1.0, 1.9) | 1.3 (1.0, 1.9) | 1.3 (1.0, 1.9) |
| Low-density cholesterol, mmol/L | 3.0 (2.3, 3.7) | 3.1 (2.4, 3.7) | 2.9 (2.3, 3.6) |
| Haemoglobin, g/dL | 14 (13, 15) | 14 (13, 15) | 14 (13, 15) |
| Haemoglobin A1c, % | 5.8 (5.5, 6.5) | 5.8 (5.4, 6.4) | 5.8 (5.5, 6.6) |
Discussion
The APOLLO study targets a cohort that reflects the current real-world Asian population undergoing CAD assessment. Given the growing clinical burden of CAD, a national AI platform to facilitate CCTA analysis is desirable for several reasons. First, as the largest CCTA registry of patients with suspected CAD, APOLLO will provide much-needed, highly representative insights into the current and emerging states of diagnostic testing in the region. Second, APOLLO aims to enhance the efficiency of CCTA reporting, including the analysis of labour-intensive parameters such as plaque characterisation and EAT quantification. Third, through robust validation against patient outcomes, APOLLO may enable cardiovascular risk prediction, thus improving triage and clinical workflows. Finally, this large, deidentified and well-characterised patient registry will have extensive clinical, research and industrial applications. Recruitment is progressing ahead of schedule, reflecting comprehensive and efficient collaboration across Singapore’s largest cardiac healthcare institutions.
A forecast analysis based on data from the SMIR31 (2007–2018) predicts that from 2025 to 2050, the incidence of AMI in Singapore will rise by 194.4%, from 482 to 1418 cases per 100 000 population.32 The largest projected increases in metabolic risk factors among AMI patients include overweight/obesity (880.0% increase), followed by hypertension (248.7%), diabetes (215.7%), hyperlipidaemia (205.0%) and active/previous smoking (164.8%).32 The number of AMI-related deaths is expected to increase by 294.7% among individuals with overweight/obesity, while mortality is predicted to decline by 11.7% for hyperlipidaemia, 29.9% for hypertension, 32.7% for diabetes and 49.6% for smoking from 2025 to 2050.
Similar trends are expected across Asia, as the ethnic distribution in Singapore (comprising Chinese, Malay and Indian populations) broadly reflects that of many Asian regions.33 The baseline characteristics of the APOLLO cohort suggest notable differences in cardiovascular risk factors compared with other study populations. For example, compared with the Danish cohort studied by Winther et al, APOLLO participants exhibit a higher prevalence of dyslipidaemia (51% vs 30%) and diabetes mellitus (15% vs 7%).34 Likewise, while over half of the participants in the SCOT-HEART study were current or former smokers,6 only one in five participants in the APOLLO cohort reported smoking. Given the predominantly Chinese composition of APOLLO’s cohort, the prevalence of cardiovascular risk factors aligns most closely with that observed in Chinese populations studied by Zhou et al35 and Tay et al,36 although with a notably higher prevalence of dyslipidaemia in the overall cohort. This discrepancy may reflect dietary and lifestyle differences rather than ethnic variations, as previous studies in Singapore found no significant differences in dyslipidaemia prevalence across ethnic subgroups.37 38 Additionally, compared with the MASALA study,39 which analysed coronary artery calcium progression among South Asians in the USA, the APOLLO cohort shows half the prevalence of diabetes mellitus. These variations in baseline characteristics underscore the need for a tailored approach to developing an AI platform for cardiovascular risk prediction in Asia.
APOLLO will be the largest CCTA-based risk prediction study in Asia. The most comparable study to date, conducted by Zhou et al in China, involved 4207 participants and demonstrated that combining coronary artery calcium with clinical risk factors effectively identified the lowest-risk patients with stable chest pain.35 Other Chinese cohort studies40,47 have generally included fewer than 2000 participants and have focused on functionally significant coronary lesions rather than comprehensive CT-based risk markers, as APOLLO does. In Japan, the ADVANCE registry by Shiono et al studied 1829 subjects,48 while Yang et al in Korea examined 1100 lesions across 643 patients, focusing on CCTA markers for functionally obstructive CAD and poor vessel outcomes.49 50 APOLLO not only exceeds these studies in sample size but also offers a more comprehensive evaluation of CCTA-based information for risk stratification.
Data sharing presents challenges for all healthcare institutions, with concerns surrounding privacy, consent, ethics and scalability. Platform solutions, such as deidentification, anonymisation, secure data instances, common data models and federated analysis, have been implemented across multiple institutions in Singapore. Notable examples include the DISCOVERY AI26 and Odyssey51 platforms, which federate data under a shared governance framework to allow secure data usage. These platforms demonstrate that on-premise cloud infrastructure can support multiple clinical and research teams while providing safety, scalability and cost-effective supercomputing resources.
Building on these successes, Singapore’s Ministry of Health has developed a national platform called TRUST,52 which enables secure, cloud-based data sharing at a national level. TRUST facilitates cross-institution data sharing through a multistakeholder access committee, ensuring equitable access to datasets generated by public entities. The platform also offers remote access, a wider range of online tools and pay-per-use pricing, enhancing data-sharing efficiency.
This study has limitations. First, although APOLLO uses a national, multicentre platform, there may still be inherent limitations regarding the completeness and consistency of data across different centres—an issue commonly encountered in multicentre studies. Second, challenges may arise with the scalability of data sharing and the federated use of data across various healthcare entities. Third, the study focuses exclusively on a multiethnic Asian population, which may limit the generalisability of the findings to Western populations. Differences in genetic, environmental and lifestyle factors indicate that further validation studies are needed to ensure the broader applicability of the developed AI models.
A planned substudy could focus on comparing CAD characteristics across different Asian ethnic groups within the APOLLO study cohort. The aim would be to investigate potential ethnic-specific variations in CAD presentation, severity and plaque characteristics. This substudy would leverage CCTA combined with AI-driven analyses to (1) assess the distribution and extent of coronary artery plaques across various ethnic groups (eg, Chinese, Malay and Indian); (2) examine differences in plaque composition (eg, calcified, non-calcified or mixed plaques) and their association with cardiovascular risk factors (eg, hypertension and diabetes) and (3) explore how genetic, environmental and lifestyle factors contribute to CAD risk and progression among different ethnicities, potentially revealing unique risk profiles or protective factors in specific groups.
In summary, APOLLO is a first-of-its-kind national AI platform for the diagnosis, collection, analysis and interpretation of CAD using CCTA. The development of an integrated AI toolkit may revolutionise the use of CCTA for CAD detection by incorporating a diverse range of patient demographics and CT-based risk markers to produce accurate, individualised cardiovascular risk estimates. This platform will undergo longitudinal validation in a multiethnic Asian population, with the potential for scalability and secure data-sharing capabilities. APOLLO is poised to deliver transformative impact across clinical, research, public health and commercial domains in Singapore.
supplementary material
Acknowledgements
The authors would like to thank Malay Singh, Arvind Srinivasa and Tan Ruphing from the Bioinformatics Institute, Agency for Science, Technology and Research, Singapore, for their contributions to the development of the APOLLO software. Special thanks also to Xiaomeng Wang from Duke-NUS Medical School, Singapore, for his assistance with manuscript editing.
Footnotes
Funding: This study received funding support from the Industry Alignment Fund—Pre-positioning Programme (H20c6a0035). LB was supported by the National Medical Research Council (NMRC) of Singapore Centre Grant [Program for Transforming and Evaluating Outcomes in Cardiometabolic disease (PROTECT), Grant number: (CG21APR1006) and the National Medical Research Council (NMRC) of Singapore Transitional Award Grant (Improving Obstructive Coronary Artery Disease and Cardiovascular Risk Prediction Using Deep Learning Analysis on Coronary Artery Calcium Imaging, Grant number: TA21nov-0001.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-089047).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by SingHealth Centralised Institutional Review Board; the reference number for the approval letter was 2021/2363 for the prospective study and 2021/2288 for the retrospective study. Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Collaboration: The APOLLO team invites researchers to contact the corresponding author to request collaboration of any kind.
Contributor Information
Lohendran Baskaran, Email: lohendran.baskaran@singhealth.com.sg.
Shuang Leng, Email: leng.shuang@nhcs.com.sg.
Utkarsh Dutta, Email: utkarsh.dutta@kcl.ac.uk.
Lynette Teo, Email: lynette_ls_teo@nuhs.edu.sg.
Min Sen Yew, Email: min_sen_yew@ttsh.com.sg.
Ching-Hui Sia, Email: ching_hui_sia@nuhs.edu.sg.
Nicholas WS Chew, Email: nicholas_ws_chew@nuhs.edu.sg.
Weimin Huang, Email: wmhuang@i2r.a-star.edu.sg.
Hwee Kuan Lee, Email: leehk@bii.a-star.edu.sg.
Roger Vaughan, Email: rogerv@duke-nus.edu.sg.
Kee Yuan Ngiam, Email: kee_yuan_ngiam@nuhs.edu.sg.
Zhongkang Lu, Email: zklu@i2r.a-star.edu.sg.
Xiaohong Wang, Email: Wang_Xiaohong@i2r.a-star.edu.sg.
Eddy Wei Ping Tan, Email: eddy_tan@bii.a-star.edu.sg.
Nicholas Zi Yi Cheng, Email: Nicholas_Cheng@bii.a-star.edu.sg.
Swee Yaw Tan, Email: tan.swee.yaw@singhealth.com.sg.
Mark Y Chan, Email: mark_chan@nuhs.edu.sg.
Liang Zhong, Email: gmszl@nus.edu.sg.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Singapore M. Principal causes of death. 2023. [20-Apr-2024]. https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death Available. Accessed.
- 2.Narang A, Sinha SS, Rajagopalan B, et al. The Supply and Demand of the Cardiovascular Workforce: Striking the Right Balance. J Am Coll Cardiol. 2016;68:1680–9. doi: 10.1016/j.jacc.2016.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skinner JS, Smeeth L, Kendall JM, et al. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974–8. doi: 10.1136/hrt.2009.190066. [DOI] [PubMed] [Google Scholar]
- 4.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 5.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 6.Investigators S-H, Newby DE, Adamson PD, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924–33. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 7.Chang H-J, Lin FY, Gebow D, et al. Selective Referral Using CCTA Versus Direct Referral for Individuals Referred to Invasive Coronary Angiography for Suspected CAD: A Randomized, Controlled, Open-Label Trial. JACC Cardiovasc Imaging. 2019;12:1303–12. doi: 10.1016/j.jcmg.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 9.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 10.Nalliah CJ, Bell JR, Raaijmakers AJA, et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J Am Coll Cardiol. 2020;76:1197–211. doi: 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) J Am Coll Cardiol. 2013;62:468–76. doi: 10.1016/j.jacc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 12.Jonas RA, Weerakoon S, Fisher R, et al. Interobserver variability among expert readers quantifying plaque volume and plaque characteristics on coronary CT angiography: a CLARIFY trial sub-study. Clin Imaging. 2022;91:19–25. doi: 10.1016/j.clinimag.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Villadsen PR, Petersen SE, Dey D, et al. Coronary atherosclerotic plaque burden and composition by CT angiography in Caucasian and South Asian patients with stable chest pain. Eur Heart J Cardiovasc Imaging. 2017;18:556–67. doi: 10.1093/ehjci/jew085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskaran L, Neo YP, Lee JK, et al. Evaluating the Coronary Artery Disease Consortium Model and the Coronary Artery Calcium Score in Predicting Obstructive Coronary Artery Disease in a Symptomatic Mixed Asian Cohort. J Am Heart Assoc. 2022;11:e022697. doi: 10.1161/JAHA.121.022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskaran L, Lee JK, Ko MSM, et al. Comparing the pooled cohort equations and coronary artery calcium scores in a symptomatic mixed Asian cohort. Front Cardiovasc Med. 2023;10:1059839. doi: 10.3389/fcvm.2023.1059839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2016;10:435–49. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Villasante Fricke AC, Iacobellis G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int J Mol Sci. 2019;20:5989. doi: 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–39. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 22.Gao HY, Ji SW. Graph u-nets. 2019. https://arxiv.org/abs/1905.05178 Available.
- 23.Huang W, Huang L, Lin Z, et al. Coronary Artery Segmentation by Deep Learning Neural Networks on Computed Tomographic Coronary Angiographic Images. Annu Int Conf IEEE Eng Med Biol Soc . 2018;2018:608–11. doi: 10.1109/EMBC.2018.8512328. [DOI] [PubMed] [Google Scholar]
- 24.Lessmann N, van Ginneken B, Zreik M, et al. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks With Dilated Convolutions. IEEE Trans Med Imaging. 2018;37:615–25. doi: 10.1109/TMI.2017.2769839. [DOI] [PubMed] [Google Scholar]
- 25.Wolterink JM, Leiner T, de Vos BD, et al. Automatic coronary artery calcium scoring in cardiac CT angiography using paired convolutional neural networks. Med Image Anal. 2016;34:123–36. doi: 10.1016/j.media.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262–73. doi: 10.1016/S1470-2045(19)30149-4. [DOI] [PubMed] [Google Scholar]
- 27.Singh G, Al’Aref SJ, Lee BC, et al. End-to-End, Pixel-Wise Vessel-Specific Coronary and Aortic Calcium Detection and Scoring Using Deep Learning. Diagnostics (Basel) 2021;11:215. doi: 10.3390/diagnostics11020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commandeur F, Slomka PJ, Goeller M, et al. Machine learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res. 2020;116:2216–25. doi: 10.1093/cvr/cvz321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 31.Office N. Singapore myocardial infarction registry annual report. 2021 https://www.nrdo.gov.sg/publications/ami Available.
- 32.Chew NWS, Chong B, Kuo SM, et al. Trends and predictions of metabolic risk factors for acute myocardial infarction: findings from a multiethnic nationwide cohort. Lancet Reg Health West Pac . 2023;37:100803. doi: 10.1016/j.lanwpc.2023.100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mensah GA, Fuster V, Murray CJL, et al. Global Burden of Cardiovascular Diseases and Risks Collaborators. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol. 2023;82:2350–473. doi: 10.1016/j.jacc.2023.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winther S, Schmidt SE, Mayrhofer T, et al. Incorporating Coronary Calcification Into Pre-Test Assessment of the Likelihood of Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2421–32. doi: 10.1016/j.jacc.2020.09.585. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Li C, Cong H, et al. Comparison of Different Investigation Strategies to Defer Cardiac Testing in Patients With Stable Chest Pain. JACC Cardiovasc Imaging. 2022;15:91–104. doi: 10.1016/j.jcmg.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Tay SY, Chang PY, Lao WT, et al. The proper use of coronary calcium score and coronary computed tomography angiography for screening asymptomatic patients with cardiovascular risk factors. Sci Rep. 2017;7:17653. doi: 10.1038/s41598-017-17655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes K, Lee BL, Feng X, et al. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Free Radic Biol Med. 2002;32:132–8. doi: 10.1016/s0891-5849(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 38.Hughes K, Yeo PP, Lun KC, et al. Cardiovascular diseases in Chinese, Malays, and Indians in Singapore. II. Differences in risk factor levels. J Epidemiol Community Health. 1990;44:29–35. doi: 10.1136/jech.44.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanaya AM, Vittinghoff E, Lin F, et al. Incidence and Progression of Coronary Artery Calcium in South Asians Compared With 4 Race/Ethnic Groups. J Am Heart Assoc. 2019;8:e011053. doi: 10.1161/JAHA.118.011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Feng Y, Sun J, et al. Fully automated artificial intelligence-based coronary CT angiography image processing: efficiency, diagnostic capability, and risk stratification. Eur Radiol. 2024;34:4909–19. doi: 10.1007/s00330-023-10494-6. [DOI] [PubMed] [Google Scholar]
- 41.Lyu L, Pan J, Li D, et al. A stepwise strategy integrating dynamic stress CT myocardial perfusion and deep learning-based FFRCT in the work-up of stable coronary artery disease. Eur Radiol. 2024;34:4939–49. doi: 10.1007/s00330-023-10562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y-W, Wu Y-J, Hung Y-C, et al. Natural course of coronary artery calcium progression in Asian population with an initial score of zero. BMC Cardiovasc Disord. 2020;20:212. doi: 10.1186/s12872-020-01498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang CX, Qiao HY, Zhang XL, et al. Functional CAD-RADS using FFRCT on therapeutic management and prognosis in patients with coronary artery disease. Eur Radiol. 2022;32:5210–21. doi: 10.1007/s00330-022-08618-5. [DOI] [PubMed] [Google Scholar]
- 44.Qiao HY, Tang CX, Schoepf UJ, et al. One-year outcomes of CCTA alone versus machine learning-based FFRCT for coronary artery disease: a single-center, prospective study. Eur Radiol. 2022;32:5179–88. doi: 10.1007/s00330-022-08604-x. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Li X, Xu K, et al. Relationship between epicardial fat volume on cardiac CT and atherosclerosis severity in three-vessel coronary artery disease: a single-center cross-sectional study. BMC Cardiovasc Disord. 2022;22:76. doi: 10.1186/s12872-022-02527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen D, Li J, Ren J, et al. Pericoronary adipose tissue CT attenuation and volume: Diagnostic performance for hemodynamically significant stenosis in patients with suspected coronary artery disease. Eur J Radiol. 2021;140:109740. doi: 10.1016/j.ejrad.2021.109740. [DOI] [PubMed] [Google Scholar]
- 47.Zhu XL, Pang ZY, Jiang W, et al. Synergistic prognostic value of coronary distensibility index and fractional flow reserve based cCTA for major adverse cardiac events in patients with Coronary artery disease. BMC Cardiovasc Disord. 2022;22:220. doi: 10.1186/s12872-022-02655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiono Y, Matsuo H, Kawasaki T, et al. Clinical Impact of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve on Japanese Population in the ADVANCE Registry. Circ J. 2019;83:1293–301. doi: 10.1253/circj.CJ-18-1269. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Koo B-K, Hoshino M, et al. CT Angiographic and Plaque Predictors of Functionally Significant Coronary Disease and Outcome Using Machine Learning. JACC Cardiovasc Imaging. 2021;14:629–41. doi: 10.1016/j.jcmg.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 50.Yang S, Koo B-K, Hwang D, et al. High-Risk Morphological and Physiological Coronary Disease Attributes as Outcome Markers After Medical Treatment and Revascularization. JACC: Cardiovascular Imaging. 2021;14:1977–89. doi: 10.1016/j.jcmg.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Narayanan R, Lam SWS, Aw KL, et al. Implementation of on-premise research data science and systems explorer (odyssey) 2023. [20-Apr-2024]. https://www.singaporehealthcaremanagement.sg/Documents/Poster%20Competition%202023/Operations/SHM_OP054%20-%20NARAYANAN%20RAGAVENDRAN%20_SHHQ%20-%20Implementation%20of%20On-premise%20Research%20Data%20Science%20and%20Systems%20Explorer.pdf Available. Accessed.
- 52.TRUST TRUST: improving health outcomes through trusted data exchange. 2023. [20-Apr-2024]. https://trustplatform.sg/ Available. Accessed.