Abstract
Abstract
Objectives
Increased aortic stiffness measured with carotid-femoral pulse wave velocity (cf-PWV) has been associated with adverse cardiovascular outcomes. Some studies have reported increased cf-PWV in living kidney donors after nephrectomy. This review aimed to determine the effects of living kidney donation on cf-PWV, glomerular filtration rate (GFR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and their differences versus non-nephrectomised healthy individuals.
Design
Systematic review and meta-analysis.
Data sources
Electronic databases (MEDLINE, EMBASE, Cochrane Central databases, Cochrane Register of Controlled Trials, Cochrane Methodology Register, Health Technology Database, Technologies in Health, EBM Reviews, ProQuest and ‘Grey Matters Light’). Databases were searched from inception to December 2022.
Eligibility criteria
We searched for studies that measured cf-PWV in living kidney donors before and/or after nephrectomy. Non-nephrectomised healthy individuals included as controls were the comparators. Studies that provided age-adjusted cf-PWV reference values in normotensive healthy individuals were also included.
Outcome measures
We evaluated the mean differences in cf-PWV, GFR and BP before-and-after nephrectomy and their mean differences versus non-nephrectomised healthy comparators. We also explored differences in yearly adjusted cf-PWV changes between donors and normotensive healthy individuals.
Data extraction/synthesis
Two independent reviewers extracted data and assessed risk of bias (Risk of Bias tool for non-Randomised studies: ROBINS-I) and quality of evidence (GRADE). Pooled effect estimates were calculated using the inverse variance method and analysed with random effect models.
Results
Nine interventional (652 donors; 602 controls) and 6 reference studies (6278 individuals) were included. cf-PWV increased at 1-year postdonation (p=0.03) and was on average 0.4 m/s (95% CI 0.07; 0.60) higher than in healthy controls (p=0.01). These differences were non-significant 5 years postnephrectomy (p=0.54). GFR decreased after nephrectomy (p<0.001) and remained reduced compared with healthy controls (p<0.001), but SBP and DBP were not significantly different (p≥0.14). Yearly changes in cf-PWV postnephrectomy were similar to age-adjusted reference values in healthy normotensive individuals (p=0.76).
Conclusions
Aortic stiffness increases independent of BP 1 year after kidney donation, but the long-term effects seem minimal. These findings may impact future consent of prospective living kidney donors.
PROSPERO registration number
CRD42020185551.
Keywords: Cardiovascular Disease, Renal transplantation, TRANSPLANT MEDICINE, End stage renal failure
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Comprehensive systematic review using multiple electronic databases and rigorous assessment of study quality and certainty of the evidence.
The Risk of Bias tool for non-Randomised studies was used to independently evaluate risk of bias and study quality.
The inclusion of two comparator groups of non-nephrectomised healthy controls was used to assess changes in carotid-femoral pulse wave velocity in living kidney donors after donation.
The inclusion of blood pressure as outcome permitted to assess if changes in carotid femoral pulse wave velocity were dependent on changes in blood pressure.
The study was limited by the small number of studies and the paucity of well-designed cohort studies with long-term follow-ups.
Introduction
Living kidney donors (LKDs) are exposed to perioperative and long-term risks, including potential adverse effects on kidney health.1 Although kidney hypertrophy is a recognised physiological response to unilateral nephrectomy, LKDs ultimately lose on average 30% of their predonation total glomerular filtration rate (GFR).1 2 Although this reduction in GFR may be of concern to donors and clinicians,3 the absolute risk increase for kidney failure, cardiovascular disease or death after donation is small and even lower than in the general population.2 4 5
Carotid-femoral pulse wave velocity (cf-PWV) is a surrogate of the intrinsic stiffness of the arterial wall and has been reported as highly predictive of cardiovascular events in high-risk populations.6 7 The prognostic value of cf-PWV has been associated with the integrated measure of the impact of cardiovascular risk factors on the arterial wall and the adverse haemodynamic effect of aortic stiffness.6,8 Recently, several prospective studies involving measurements of cf-PWV have documented that LKDs have increased aortic stiffness after nephrectomy when compared with healthy controls of similar age.9,15 Although most of these investigations involved small samples and limited follow-up times,16 17 these findings are relevant since increased cf-PWV is associated with adverse cardiovascular outcomes and all-cause mortality in the general population.18 Since most of these studies did not detect increases in systemic blood pressure (BP) postnephrectomy,17 a reduction in GFR may be an independent graded risk factor for cardiovascular remodelling in LKDs.19 Moreover, this phenomenon may be particularly important for young LKDs who have the longest risk exposure to the effects of reduced kidney mass.
To determine the effects of living kidney donation on aortic stiffness and their differences relative to non-nephrectomised healthy individuals, we conducted a systematic review and meta-analysis to evaluate the progression of cf-PWV, changes in arterial BP and GFR in LKDs before-and-after nephrectomy. We also gathered data on differences in cf-PWV, BP and GFR between LKDs and their non-nephrectomised healthy comparators. Finally, we explored whether yearly changes in aortic stiffness in LKDs determined by cf-PWV, differed from age-adjusted reference values in normotensive healthy individuals. We hypothesised that living kidney donation would decrease kidney function and increase aortic stiffness and arterial BP compared with non-nephrectomised healthy individuals.
Materials and methods
The review was conducted in accordance with the Cochrane Collaboration Methods, Systematic Reviews standards and reported according to Preferred Reporting items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.20 The study protocol has been published21 and registered in PROSPERO (CRD42020185551) (www.crd.york.ac.uk/prospero).22 The study protocol is uploaded as online supplemental file 2. The PRISMA guidelines were followed and a checklist file is included.23
Data sources, search criteria and eligibility
We conducted a comprehensive search (online supplemental appendix 1) to retrieve all observational studies published to December 2022 that included healthy individuals participating in a kidney donation programme who underwent measurements of cf-PWV before and/or after nephrectomy. Our initial search during protocol registration was undertaken until December 2020 and it was subsequently updated until March 2021 at the time of protocol publication.21 The broad nature of our original search captured studies with additional metrics of arterial stiffness.21 However, these secondary outcomes were not considered in this review as we focused on cf-PWV. The search was applied to several electronic databases including MEDLINE, EMBASE, Cochrane Central databases, Cochrane Register of Controlled Trials, Cochrane Methodology Register, Health Technology Database, Technologies in Health and EBM Reviews. EMBASE, MEDLINE, EBM reviews were searched through the OVID platform and the Cochrane Register was searched via EBM. We searched for grey literature through the “Grey Matters Light” platform from the Canadian Agency for Drugs and Technology in Health and the ProQuest website for dissertations and theses. We also searched for studies that included cf-PWV in healthy individuals from the general population that evaluated age effects and aortic stiffness. Population-based studies were searched using the following keywords and filters: aortic stiffness, arterial stiffness, cf-PWV, PWV, age, adults, humans, reference or normal values, healthy participants or subjects and normal volunteers. There were no language restrictions in the initial search although during screening only studies published in English, French, Spanish, Portuguese and Italian were included. We also identified data sources from manual searches of references in some relevant citations. All search results were downloaded into an Excel spreadsheet and screened by title and authors to remove duplicates.
Study inclusion and exclusion criteria
Our target population included healthy adult individuals (>18 years of age) who met standard institutional kidney donation criteria and had aortic stiffness evaluated with cf-PWV before and/or after nephrectomy. Non-nephrectomised healthy individuals included as healthy controls within the same study were used as comparators. Since prospective randomised clinical trials of kidney donation would never be possible for ethical reasons, we included prospective non-randomised (cohort, case–control, case series, before and after) and retrospective studies, provided that ≥10 subjects per study were enrolled.
Outcomes
The primary outcomes were the mean differences in cf-PWV before and after nephrectomy in LKDs, and the mean differences versus their non-nephrectomised healthy comparators. Secondary outcomes were the predonation and postdonation mean differences in systolic BP (SBP) and diastolic BP (DBP) and GFRs in LKDs and the mean differences versus their non-nephrectomised healthy comparators. Exploratory outcomes were the differences in the yearly adjusted changes in cf-PWV between LKDs and a group of normotensive healthy individuals who participated in population-based studies of aortic stiffness.
Screening and study selection
Two independent reviewers screened abstracts and titles. We excluded non-human, in vitro or modelling studies, narrative/systematic reviews, paediatric investigations and letters to the editor. After screening was completed, reviewers examined the study methods to confirm that cf-PWV measurements were performed with validated automatic devices. The selected studies underwent full-text review by two independent reviewers according to predefined inclusion and exclusion criteria (online supplemental appendix 2). In case of disagreement, a third reviewer was available to achieve consensus by discussion. We also screened for studies that included healthy individuals from the general population where age-adjusted values for cf-PWV were reported (reference studies). The two reviewers selected those studies that explicitly included healthy normotensive individuals (>18 years) with no history of cancer, cardiovascular, neurologic, inflammatory or kidney disease. To clarify missing information, we contacted study authors by electronic mail. We declared a null response if no reply was obtained after three email attempts within a 4-month period.
Data extraction
A data extraction form was prepared a priori from consensus among investigators and piloted for optimisation. Two reviewers independently performed full data extraction (online supplemental appendix 3). Published secondary analyses associated with an original study were considered part of a single study.
Study quality
The risk of bias was assessed using the Risk of Bias tool in non-Randomised studies (ROBINS-I) and each study was independently evaluated by two reviewers according to seven domains including confounding, selection, classification of the intervention, deviation from intended intervention, missing data, outcome measurement and reporting.24 Each reviewer classified the risk of bias for each domain as low, moderate, serious, critical or no information available. A final consensus produced an overall risk of bias for each study. Since the purpose of including reference studies was to provide normative values, their study quality was not assessed.
Certainty of the evidence
Quality of the certainty of the evidence was evaluated according to the five domains of the Grades of Recommendation, Assessment, Development and Evaluations (GRADE), and the overall assessment was reported as very low, low, moderate or high.25
Statistical analyses
Meta-analysis
The weighted mean differences and their 95% CIs were calculated using the reported means and SDs from each study. In cases where different measures of central tendency (ie, median) and distribution (ie, IQR) were reported, means and SD were estimated according to the algorithms described by Luo et al.26 For studies9 13 that did not include predonation values, postdonation differences between LKDs and healthy controls were estimated using the mean absolute cf-PWV. To determine the level of skewness in small sample size studies (n<35), we subtracted the extreme value of the reported range or quartile distribution from the estimated means calculated by the Luo et al’s method26 and divided by the estimated SD according to Altman and Bland.27 Only cases with a ratio less than 1 (suggesting severe skewness) were log transformed. To explore statistical heterogeneity between studies, the Q test and the I2 statistic were used (with a value of I2>65 considered to be a highly important heterogeneity). To find potential sources of heterogeneity, we stratified studies by subgroups according to the duration of follow-up and study design. Sensitivity analyses included examination of effect model, parameter estimates and methodological quality. If suitable, the pooled effect estimates were calculated using the method of the inverse variance and data was modelled according to the DerSimonian-Laird Method (random effects model) (p<0.05). To minimise the risk of artificially increasing the precision of the effect estimates due to counting the same patient twice in before-and-after studies (‘double-counting’ error),28 we reduced by 50% the number of study participants for each measurement.29 To determine the strength of this approach, a sensitivity analysis between the models with and without adjustment was performed. Intergroup differences were analysed using the Cochrane Q test with p<0.10. Publication bias was investigated by Funnel plots, and asymmetry was evaluated if the number of studies in the meta-analysis was greater than 10. All meta-analyses used RevMan V.5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Reference studies
Yearly changes in cf-PWV (m/s/year) for kidney donors and healthy controls were estimated using the mean differences between predonation and postdonation values divided by the number of years of observation. In reference studies, the yearly changes in cf-PWV (m/s/year) were estimated according to the age-decade average differences reported at the 90–97.5th percentile of the distribution. This cut-off would ensure that the area under the normal curve would fall within 1.282–1.960 SD from the mean cf-PWV for each decade. If these data were not available, we used the beta coefficient of the age and cf-PWV regression function. The significance of between-group comparisons was assessed by independent t-tests (two tailed) (p<0.05). The differences in cf-PWV are reported as the means and their 95% CI (or their SD, if noted), while for absolute cf-PWV values, medians and quartiles are described. Quantitative analyses used IBM SPSS statistics, V.29.
Results
Study characteristics
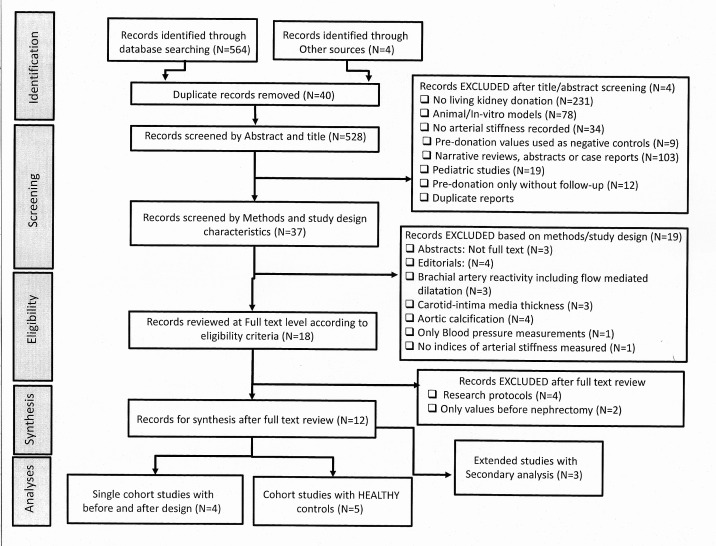
The search strategy found 568 citations. After screening and full-text review, nine studies met the final eligibility criteria (figure 1). Five studies9,15 compared LKDs and healthy controls, but only three of those had measurements before-and-after donation.11 12 14 15 Four additional studies included single cohorts of LKDs with measurements predonation and postdonation.30,33 We identified three reports based on secondary analyses10 11 34 that were considered part of their original publication9 12 (figure 1). Three of our included studies12 14 15 that were published by the same research group (UK) had participants evaluated at different time periods and some degree of overlap was assumed. In the absence of confirmation, these studies were analysed independently. Online supplemental table S1 and online supplemental appendix 4 summarise the characteristics of studies, participants and country of origin.
Figure 1. PRISMA flow chart. PRISMA, Preferred Reporting items for Systematic Reviews and Meta-Analysis.
Population characteristics
Living kidney donors
A total of 652 LKDs had measurements of cf-PWV after kidney donation, but only 438 LKDs (in 7 studies)1112 14 15 30,33 had examinations before and after nephrectomy. The remaining 214 LKDs (in 2 additional studies)9 10 13 did not have predonation assessment. The cf-PWV was measured in two studies at 6 months after donation,11 12 33 in six studies at 12 months1112 14 15 30,32 and in three studies at 5 years or longer (5, 6 and 9 years)9 13 15 (online supplemental table S-1). Among all studies, average age at donation was 48.0 years (± 5.0 years) (range: 41.0–54.1 years) with most organs donated by females with an average proportion of 63.4% (range: 54%–87%) per study. Only three studies11,1315 reported the ethnic composition of LKDs. Donors were predominantly white Caucasian (range: 90%–94.6%) with a minority of Asian (range: 6%–7%) and black heritage (range: 0%–3%). Only two studies9 31 reported a detailed definition of hypertension characterised as SBP>140 mm Hg and/or DBP>90 mm Hg; or by the use of antihypertensive therapy due to previously diagnosed hypertension. In seven of the nine studies, an average of 12.5% (range: 0%–32%) of LKDs were hypertensive at the time of donation and this rate increased to an average of 17.2 .% (range: 4%–32%; 4 studies) and 12.8% (range: 5.4%–18.8%; 4 studies) at 12 months and 5–9 years after donation, respectively. Moreover, an average of 32.9% of donors (range: 28%–44%) were current smokers and/or individuals with a history of previous smoking, although the duration of exposure was not reported. The most common medications prescribed for LKDs prior to organ donation were antihypertensives and lipid-reducing drugs (eg, statins). The most common antihypertensive medications were angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) (range: 0%–19% in 5 studies), calcium channel blockers (range: 2%–5%; in 6 studies) and beta blockers (2%; in 3 studies). Statins were reported with an average rate between 0% and 12% in 6 studies. There was no information on cardiovascular risk assessment predonation and hypertension management with diuretics.
Healthy controls
A total of 602 healthy individuals were included as comparators in 5 studies (online supplemental table S1). Two studies had comparative assessments at 12 months after nephrectomy,11 12 14 one at 12 and 60 months,15 and two at 5 years or longer (5, 6 and 9 years).9 10 13 The average age of healthy controls in these studies was 46 years (range: 43–49 years) compared with 49 years (range: 46–51 years) in kidney donors. The incidence of hypertension, history of cardiovascular disease and diabetes mellitus was higher in kidney donors postdonation, relative to controls. The average proportion of hypertension, history of cardiovascular disease and diabetes mellitus was 6.3% (range: 0%–9% in 5 studies), 16.7% (range: 0%–28% in 3 studies) and 0.5% (range: 0%–2% in 4 studies) in healthy controls compared with 11.0% (range: 5%–18.8% in 5 studies), 19.6% (range: 4.9%–34% in 3 studies) and 1.6% (range: 0%–5.9% in 4 studies), respectively in LKDs. Only three studies11 12 14 15 documented the proportions of current and previous smokers between these two subpopulations ranging between 2% and 28% in controls vs 6% and 44% in donors. The most frequent medications prescribed to healthy controls as reported in two studies14 15 were ACE inhibitors/ARBs, statins and calcium channel blockers. Their proportions at the time of initial recruitment ranged from 3% to 7%, 7% to 13% and 2% to 3%, respectively. Ethnicity in healthy controls was only reported in three studies.11,1315 The ethnic distribution of these participants was white Caucasian (range: 84%–95%), Asian (9%) and black heritage (6%–7%). None of the studies reported cardiovascular risk in healthy controls. Additional baseline characteristics were either part of the exclusion criteria or were not sufficiently reported.
Outcome measures
Aortic stiffness
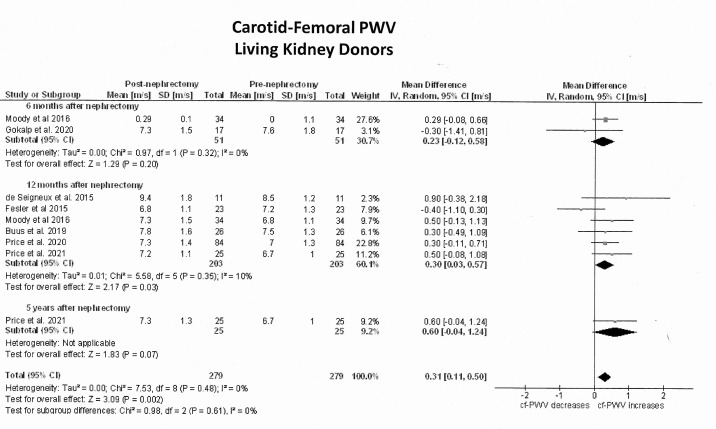
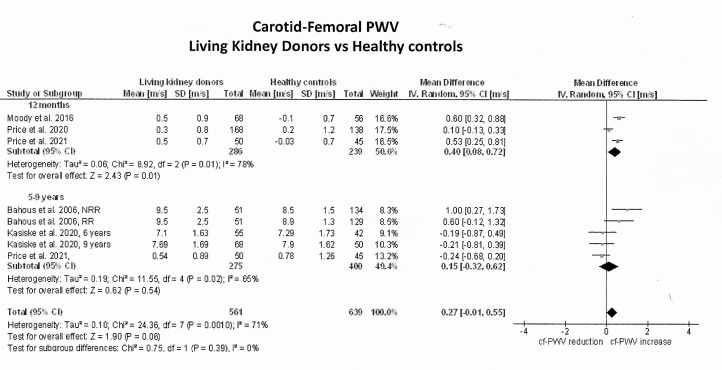
The primary outcome analysis included seven studies1112 30,33 with non-adjusted cf-PWV values plus 2 studies14 15 whose values were adjusted according to mean BP and heart rate. Due to limited information in these two studies, their adjusted values were not transformed. Online supplemental tables S2 and S3 summarise the unadjusted cf-PWV values in donors and controls, respectively. The median unadjusted cf-PWV prior to nephrectomy was 7.10 m/s (quartiles: 6.80, 7.52) and this value increased to a median of 7.21 m/s (quartiles: 7.14, 7.27) at 6 months, 7.30 m/s (quartiles: 7.22, 7.68) at 12 months and 7.69 m/s (quartiles: 7.50, 8.60) at 5 years. Figure 2 shows the forest plots of the effect estimates on the unadjusted cf-PWV in LKDs before-and-after nephrectomy and figure 3 illustrates their differences against healthy comparators. The unadjusted cf-PWV in LKDs increased with time after nephrectomy (Z=3.1, p=0.002; I2=0%). While these effects were statistically significant at 12 months after nephrectomy (Z=2.2, p=0.03; I2=10%; 6 studies), they were not significant at 6 months (Z=1.3, p=0.20; I2=0%; 2 studies) or 5 years and longer (Z=1.8; p=0.07; 1 study). The mean difference in the unadjusted cf-PWV before and after donation was 0.23 m/s (95% CI −0.12; 0.58) at 6 months, 0.30 m/s at 12 months (95% CI 0.03; 0.57) and 0.60 m/s at 5 years (95% CI −0.04; 1.24). At 12 months postdonation, unadjusted cf-PWV values in LKDs were on average 0.4 m/s (95% CI 0.08; 0.72) higher than in healthy controls (Z=2.43; p=0.01; 3 studies), but this difference became non-significant (mean: 0.15 m/s; 95% CI −0.32; 0.62) at 5 years or longer after donation (Z=0.62; p=0.54). Statistical heterogeneity between studies was high at 12 months (I2=78%; p=0.01) and at 5 years (I2=65%; p=0.02).
Figure 2. Pooled effect estimates on the carotid-femoral pulse wave velocity (cf-PWV) (m/s) in living kidney donors from before to after nephrectomy. In single cohort studies with before-and-after design, the number of living kidney donors allocated to each measurement was reduced by 50% to decrease ‘double-counting’ errors during the analysis.
Figure 3. Pooled effect estimates on the differences in carotid-femoral pulse wave velocity (cf-PWV) (m/s) between living kidney donors and their respective healthy comparators. Because predonation values for Bahous et al9 and Kasiske et al13 were not provided, mean differences between living kidney donors and controls were calculated using their mean absolute cf-PWV values. In the study by Bahous et al, the number of living kidney donors allocated to each measurement was reduced by 50% to decrease ‘double-counting errors’ during the analysis. NRR, non-recipient related; RR, recipient related.
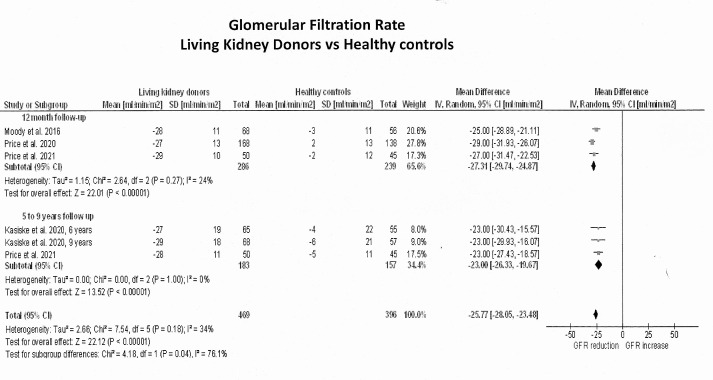
Kidney function
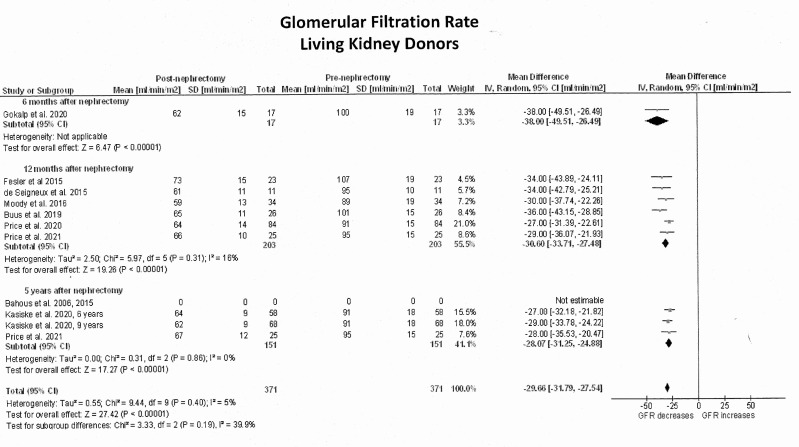
GFR in LKDs was measured in one study at 6 months postnephrectomy,33 in six studies at 12 months1112 14 15 30,32 and in three studies at 5 years or longer.9 10 13 15 Six studies estimated GFR using the chronic kidney disease (CKD) Epidemiology Collaboration equation (CKD-EPI) based on the Cr51EDTA clearance,1112 14 15 30,32 one study33 estimated GFR from 24-hour urine creatinine clearance, and two additional studies9 10 13 used both the modification of Diet in renal disease and CKD-EPI from Iohexol clearance. Figure 4 shows the forest plots of the effects estimates on GFR in LKDs and figure 5 exhibits their differences against healthy controls. Relative to before nephrectomy, GFR decreased by an average of 30 mL/min/1.73 m2 (95% CI −32; −28) throughout the 5-year follow-up period (Z=27.4; p<0.001). In particular, GFR decreased by 38 mL/min/1.73 m2 (95% CI −49; −26) within the first 6 months after nephrectomy (1 study; Z=6.5; p<0.001), by 31 mL/min/1.73 m2 (95% CI −34; −27) at 12 months (6 studies; Z=19.3; p<0.0001) and by 28 mL/min/1.73 m2 (95% CI −31; −25) at 5 years or longer (3 studies; Z=17.3; p<0.0001). When these values were compared with healthy controls, LKDs had significantly lower GFRs (mean differences: −26 mL/min/1.73 m2; 95% CI −28; −23; Z=22.1; p<0.001).
Figure 4. Pooled effect estimates on the glomerular filtration rate (mL/min/1.73 m2) in living kidney donors from before to after nephrectomy. In all single-cohort studies with before-and-after design, the number of living kidney donors allocated to each measurement was reduced by 50% to decrease ‘double-counting’ errors during the analysis.
Figure 5. Pooled effect estimates on the differences in glomerular filtration rate (mL/min/1.73 m2) between living kidney donors and their healthy comparators. In the study by Bahous et al,9 the number of living kidney donors allocated to each measurement was reduced by 50% to decrease ‘double-counting errors’ during the analysis.
Systemic BP
In LKDs, SBP and DBP were measured non-invasively at 6 months postdonation in one study,32 at 12 months in four studies,11 12 14 31 32 at 1 and 5 years in another,15 and longer than 5 years in two studies.9 10 13 A single study30 did not report BP postnephrectomy. Five studies11,1532 reported the daily average BP derived from 24-hour BP monitoring, while four studies9 10 30 31 33 reported BP values from the average of three measurements taken at the time of the office visit. Most studies except one9 10 measured BP in controls at initial recruitment and follow-up. The forest plots of the effect estimates on the SBP are represented in online supplemental figures S1, S2 and on the diastolic BP are presented in online supplemental figures S3, S4, respectively. Diastolic BP (Z=2.6; p=0.009) but not SBP (Z=0.8; p=0.44) increased with time after donation. This effect was only significant at 5 years postnephrectomy when diastolic BP increased by an average of 5 mm Hg (95% CI 2.1, 8.8; I2=63%; Z=3.2; p=0.001) relative to predonation values. When these time-related changes were compared with healthy controls, differences in systolic (mean differences: 0.8 mm Hg 95% CI −1.2; 2.7) and diastolic BP (mean differences: 1.1 mm Hg; 95% CI −0.4; 2.6) at 5 years or longer were non-significant (systolic: Z=0.8; p=0.43; diastolic: Z=1.48, p=0.14). Overall, statistical heterogeneity was moderate for systolic (I2=44%; χ2=12.5; p=0.08) and marginal for diastolic BP (I2=53%; χ2=14.1; p=0.04).
Comparison with reference values
Online supplemental table S4 shows the yearly changes in cf-PWV for 6 reference studies that included 6278 normotensive healthy participants (>18 and <70 years).35,40 Online supplemental table S5 shows the estimated yearly changes in non-adjusted cf-PWV for LKDs and healthy controls. The non-adjusted cf-PWV increased by an average of 0.174 m/s per year (±0.720) in LKDs (eight studies) and 0.090 m/s per year (±0.951) in healthy controls (four studies). The yearly increases in LKDs and their controls were comparable to the 0.1203 m/s per year (±0.1486) average increase from normotensive healthy individuals (>18 to <70 years) (donors: t=0.20; p=0.84; controls: t=0.078; p=0.93). Since previous studies have indicated a larger yearly increase in cf-PWV for older age groups, we performed a subgroup analysis for individuals ≤60 years and >60 years. The average yearly increase in cf-PWV in reference studies for individuals ≤60 years was 0.0751 m/s (±0.061) compared with 0.158 m/s (±0.143) in those >60 years (online supplemental table S4). Our analysis showed that there was no difference in the average yearly change in cf-PWV between LKDs (t=−0.301; p=0.76) or healthy controls (t=−0.026; p=0.97) against normotensive healthy individuals ≤60 years.
Sensitivity analyses
The effect of overlapping on the effect estimates between LKDs and healthy controls was tested by sequential exclusion/inclusion of the involved studies.11 12 15 Exclusion decreased the mean cf-PWV difference at 12 months (full model: 0.40 m/s, partial models: 0.34 m/s, 0.31 m/s) and increased statistical heterogeneity (full model: 78%; partial models: 81%, 86%), but there was no effect on the overall estimates (χ2=0.32; df=1, p=0.57). Our assessment of parameter estimates, quality and effect model did not change the final analysis.
We evaluated the impact of adjusting our model for ‘double-counting’ errors on the effect estimates in studies with before-and-after design1112 14 15 30,33 by investigating the differences in the model with and without adjustment. The forest plots for the non-adjusted analyses (primary and secondary outcomes) are illustrated in online supplemental figures S5,S6,S7 and S8 . The mean differences and statistical heterogeneity for the model with and without adjustment are summarised in online supplemental table S6. The pooled mean differences and their precision were not significantly different between the two quantitative models. Although the standard error (SE) in the non-adjusted model increased only by 3% (quartiles: −5.1% to 7.4%), its statistical heterogeneity (I2 value) notably increased by 35% (range: 22%–47%) compared with the adjusted model.
Risk of bias
Online supplemental table S-7 summarises the assessment of the risk of bias with the ROBINS-I tool. Four of the five studies that included a control group11,15 had moderate risk of bias (80%) and one serious risk of bias.9 10 Three single cohort studies30 31 33 had serious risk of bias (75%) and one moderate risk of bias.32 No study was classified as low risk or critical risk of bias. Risk of bias was associated with the presence of confounding bias, selection bias due to relaxation of inclusion criteria for donors and controls, missing data and selective reporting.
Funnel plots of asymmetry
The small number of studies (<10) in the meta-analysis and the likelihood that any test on asymmetry would be underpowered precluded using any test for reporting bias. Online supplemental figure S9 shows effect estimates and sample sizes for studies with cf-PWV between LKDs and controls. A large asymmetry for both small and large sample size studies was evident and suggested potential risk for publication bias.
Certainty of the evidence
Online supplemental table S-8 summarises certainty of the evidence for all outcomes according to the GRADE methodology. Confidence in the effect estimates was low to moderate for the cf-PWV, low for systemic BP and moderate to high for GFR.
Discussion
In this systematic review, we pooled data from 652 LKDs, 602 healthy controls and 6278 normotensive healthy participants with standard cf-PWV measurements to evaluate the effects of nephrectomy on aortic stiffness after living kidney donation. Based on low to moderate quality of evidence, our findings suggest that the impact of nephrectomy on aortic stiffness at 5 years postdonation or longer is minimal, despite a reduction in kidney function. On the other hand, cf-PWV increases within the first year after nephrectomy, exceeding values observed in selected groups of non-nephrectomised healthy individuals (average difference: 0.4 m/s), although these differences are negligible at 5 years postdonation (average difference: 0.15 m/s). Additionally, the yearly changes in cf-PWV after donation were similar to those in healthy normotensive individuals from the general population. Our review also suggests that 5 years after donation, SBP and DBP increased by an average of 3 and 5 mm Hg, respectively, but these changes were similar to those identified in healthy control groups. Thus, we hypothesise that vascular remodelling occurs within the first-year postnephrectomy, leading to discrete elevation of aortic stiffness with no changes in systemic BP. Five years after nephrectomy, however, progression of aortic stiffness in LKDs is similar to the age-dependent effects observed in a healthy normotensive population.
Compared with values before donation, GFR in LKDs decreases by an average of 30 mL/min/1.73 m2 between 6 months and 5 years after nephrectomy. These results are comparable to previous studies that reported reductions in kidney function between 30% and 50% after kidney donation.1 2 14 15 41 Our current analysis supports that a reduction in kidney function of such magnitude after donation is insufficient alone to cause significant effects on aortic stiffness at least 5 years postdonation. In contrast, similar reductions in kidney function in early-stage CKD are associated with increased aortic stiffness and reduced vascular distensibility.42,45 Inflammation-mediated endothelial injury,15 42 increased upregulation of matrix metallo-proteinase-2,46 abnormal calcium/phosphorous mineral balance47 and extracellular fluid excess42 are mechanisms of vascular injury more likely found in CKD patients, which may play a role in the increased aortic stiffness in CKD, but not after kidney donation.9 41 46 48 49
Studies on the progression of aortic stiffness after kidney donation have had contradictory results. While some studies have shown an increase in aortic stiffness9,1532 others have documented a negligible effect.30 31 33 Varying study designs, small sample sizes, short-term follow-ups and differences between BP-adjusted and non-adjusted cf-PWV values may have contributed to the heterogeneity in the results. Our findings confirm that there is a paucity of well-designed cohort studies with large sample sizes and long-term follow-ups. In addition, although our meta-analysis increased the robustness of the comparisons between donors and controls, this analysis may have been underpowered to detect small differences. A difference of 0.4 m/s (SD: 3) in cf-PWV between donors and controls would have required at least 883 participants per group with 80% power and level of significance of 5%. Although our analysis was adjusted for duration of follow-up and study quality, heterogeneity between studies was still present. We speculate that relaxation of study inclusion criteria may have led to unbalanced distribution of risk determinants (ie, hypertension, smoking, diabetes, dyslipidaemia) between the two cohorts. Because these confounders may decrease comparability, baseline differences should be minimised in future studies.
The effect of reduced kidney function, independent of increased BP, on aortic stiffness in LKDs is controversial.17 44 50 In partially nephrectomised rats, reduced kidney function modified the viscoelastic properties of large arteries independent of the effects of age and BP.51 However, since serum creatinine increased more than double compared with control animals, the magnitude of reduction in GFR may have not been similar to what is observed in LKDs. Our review suggests that except for a small increase in cf-PWV within the first-year postdonation, there were no differences in BP between healthy donors and controls. These findings support previous studies that have reported a reduction in the MRI-detected aortic distensibility in LKDs but not in healthy controls at 1-year postdonation,12 with these differences becoming negligible at 5 years postnephrectomy.15 Furthermore, these changes in donor’s aortic stiffness may be associated with an increase in left ventricular mass 1-year postnephrectomy,12 33 which is no longer noticeable at 5 years.15 52
Several risk factors (eg, African American or Hispanic ethnicity, obesity, age and diabetes) may increase the risk for elevated BP and aortic stiffness postdonation.1731 53,55 However, few studies have documented the role of genetics or ethnicity factors in the development of CKD and increased aortic stiffness.9 10 56 57 Kidney donors of African ancestry with mutations in the Apolipoprotein L1 gene (APOL1) are at higher risk for developing CKD, imposing new challenges to the process of donor selection and consent.58 59 Bahous et al9 10 who explored differences in cf-PWV between recipient and non-recipient-related healthy volunteers of Lebanese ancestry, found a significantly higher rate of elevated aortic stiffness in recipient-related healthy controls. Moreover, Muzaale et al57 and Wu et al60 reported marked differences in the risk for kidney failure across different types of donor-recipient and ethnicity relationships, suggesting genetic factors. Consequently, the role of genetic determinants in modifying risk of aortic stiffness postdonation cannot be ruled out.
Beyond biological effects of reduced kidney function, nephrectomy may also result in alterations of the arterial network that are associated with changes in haemodynamics and functional stiffness of the arterial tree including those associated with the effect of the different types of yuxta-aortic vascular surgeries.61 Although few studies have documented that compensatory growth of the remaining kidney is commonly seen after unilateral nephrectomy,62 the relationship of this phenomenon with cardiovascular remodelling and vascular stiffness remains elusive. Interestingly, several circulating growth factors released during compensatory kidney hypertrophy63 have been associated with myocardial and central vascular remodelling.64 In particular, growth hormone (GH) and its main mediator insulin growth factor-1 (IGF-1) are implicated in the early stages of compensatory renal hypertrophy65 and increase aortic wall thickness in transgenic mice models without any significant change in arterial BP.66 Thus, we speculate that these circulating growth factors may be linked to the cardiovascular remodelling process and transient increase in aortic stiffness early after nephrectomy.
Limitations
The strength of this review includes a rigorous systematic methodology and assessment of study quality and certainty of the evidence. Nevertheless, our conclusions may be limited by the small number of studies and participants, and the restricted access to information for data standardisation.11 12 14 15 In particular, over-representation of the Caucasian population in these studies prevents the applicability of our conclusions to other ethnicity groups. Furthermore, our sensitivity analysis on studies where overlapping was suspected11 12 14 16 suggested a reduced mean difference in cf-PWV at 12 months postdonation. Thus, the likelihood that overlapping might have influenced our effect estimates cannot be completely excluded. Since cf-PWV is an operator-dependent technique,67 important issues in the interpretation of these results are the comparability between medical devices,68 the variation due to the different calculating algorithms68 69 and the technical reproducibility of these measurements.67 All selected studies used standard devices (online supplemental table S-5), although no information was given on their reproducibility.54 67 Despite our efforts to detect potential sources of heterogeneity, residual confounding was still present, and this may have impacted comparability between cohorts. Additionally, we recognise that the different techniques used in the measurement of GFR (estimated vs direct measurement), and BP (24-hour monitoring vs office) may have contributed to the variability of these outcomes.70 71 Moreover, the confounding effects of antihypertensive therapy on the control of BP after donation and the limitations for adjusting the effects of gender and age72 73 in our analysis cannot be ignored. Age, in particular, may have a differential effect on arterial stiffness for males and females.72 Although both sexes experience an increase in arterial stiffness with ageing, the increase seems to be steeper in males than females.72 73 We believe that an individual participant data meta-analysis would have been a more appropriate way to synthesise our data and adjust aortic stiffness according to the different risk factors. Finally, the risk of publication and selection bias cannot be entirely ruled out.
Conclusions
Our systematic review and meta-analysis documented that reduced kidney function after living kidney donation is associated with a small elevation in aortic stiffness within the first year, independent of changes in systemic BP. These effects, however, become negligible 5 years postdonation. The data suggest that vascular remodelling occurs within the first year postnephrectomy but is no longer detected after 5 years. In the absence of other critical cardiovascular risk factors, the effects of nephrectomy on aortic stiffness in LKDs at least 5 years after donation is insignificant. These results may have implications for the future evaluation and consent of prospective LKDs.
supplementary material
Acknowledgements
We acknowledge the assistance and support of Ms. Risa Shorr from the Learning services at the Ottawa Hospital during the development of our search strategy. We express our appreciation for 'all individuals that donate a kidney for the benefit of another human being' and recognise the inspiring motivation of Adolfo M. Rodriguez-Trejo.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-082725).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: Ethical approval was not required since our study did not involve participation of human subjects.
Contributor Information
Rosendo A Rodriguez, Email: rrodriguez1847@gmail.com.
Kylie McNeill, Email: kmacneill@toh.ca.
Mohsen Agharazii, Email: Mohsen.Agharazii@crchudequebec.ulaval.ca.
Ann Bugeja, Email: abugeja@toh.ca.
Edward G Clark, Email: edclark@toh.ca.
Kevin D Burns, Email: KBURNS@toh.ca.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Kasiske BL, Anderson-Haag T, Israni AK, et al. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 2015;66:114–24. doi: 10.1053/j.ajkd.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lentine KL, Lam NN, Segev DL. Risks of Living Kidney Donation: Current State of Knowledge on Outcomes Important to Donors. Clin J Am Soc Nephrol. 2019;14:597–608. doi: 10.2215/CJN.11220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 4.Muzaale AD, Massie AB, Wang M-C, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matas AJ, Rule AD. Long-term Medical Outcomes of Living Kidney Donors. Mayo Clin Proc. 2022;97:2107–22. doi: 10.1016/j.mayocp.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angoff R, Mosarla RC, Tsao CW. Aortic Stiffness: Epidemiology, Risk Factors, and Relevant Biomarkers. Front Cardiovasc Med. 2021;8:709396. doi: 10.3389/fcvm.2021.709396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 8.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 9.Bahous SA, Stephan A, Blacher J, et al. Aortic stiffness, living donors, and renal transplantation. Hypertension. 2006;47:216–21. doi: 10.1161/01.HYP.0000201234.35551.2e. [DOI] [PubMed] [Google Scholar]
- 10.Bahous SA, Khairallah M, Al Danaf J, et al. Renal function decline in recipients and donors of kidney grafts: role of aortic stiffness. Am J Nephrol. 2015;41:57–65. doi: 10.1159/000371858. [DOI] [PubMed] [Google Scholar]
- 11.Moody WE, Ferro C, Edwards N, et al. EFFECTS OF NEPHRECTOMY ON CARDIOVASCULAR STRUCTURE AND FUNCTION IN LIVING KIDNEY DONORS. J Am Coll Cardiol. 2015;65:A2150. doi: 10.1016/S0735-1097(15)62150-7. [DOI] [Google Scholar]
- 12.Moody WE, Ferro CJ, Edwards NC, et al. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors. Hypertension. 2016;67:368–77. doi: 10.1161/HYPERTENSIONAHA.115.06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasiske BL, Anderson-Haag TL, Duprez DA, et al. A prospective controlled study of metabolic and physiologic effects of kidney donation suggests that donors retain stable kidney function over the first nine years. Kidney Int. 2020;98:168–75. doi: 10.1016/j.kint.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Price AM, Greenhall GHB, Moody WE, et al. Changes in Blood Pressure and Arterial Hemodynamics following Living Kidney Donation. Clin J Am Soc Nephrol. 2020;15:1330–9. doi: 10.2215/CJN.15651219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price AM, Moody WE, Stoll VM, et al. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors at 5 Years. Hypertension. 2021;77:1273–84. doi: 10.1161/HYPERTENSIONAHA.120.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin J Am Soc Nephrol. 2006;1:885–95. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 17.Ferro CJ, Townend JN. Risk for subsequent hypertension and cardiovascular disease after living kidney donation: is it clinically relevant? Clin Kidney J. 2022;15:644–56. doi: 10.1093/ckj/sfab271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 19.Blom KB, Bergo KK, Espe EKS, et al. Cardiovascular rEmodelling in living kidNey donorS with reduced glomerular filtration rate: rationale and design of the CENS study. Blood Press. 2020;29:123–34. doi: 10.1080/08037051.2019.1684817. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez RA, Trentin Sonoda M, Agharazii M, et al. Effects of living kidney donation on arterial stiffness: a systematic review protocol. BMJ Open. 2021;11:e045518. doi: 10.1136/bmjopen-2020-045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute for Health Research Prospero international prospective register of systematic reviews. 2020. https:// www.crd.york.ac.uk/prospero/ Available.
- 23.Beller EM, Glasziou PP, Altman DG, et al. PRISMA for Abstracts: reporting systematic reviews in. J Conf Abstr PLoS Med. 2013;10:e1001419. doi: 10.1371/journal.pmed.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senn SJ. Overstating the evidence: double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;9:10. doi: 10.1186/1471-2288-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung MWL. A Guide to Conducting a Meta-Analysis with Non-Independent Effect Sizes. Neuropsychol Rev. 2019;29:387–96. doi: 10.1007/s11065-019-09415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Seigneux S, Ponte B, Berchtold L, et al. Living kidney donation does not adversely affect serum calcification propensity and markers of vascular stiffness. Transpl Int. 2015;28:1074–80. doi: 10.1111/tri.12595. [DOI] [PubMed] [Google Scholar]
- 31.Fesler P, Mourad G, du Cailar G, et al. Arterial stiffness: an independent determinant of adaptive glomerular hyperfiltration after kidney donation. Am J Physiol Renal Physiol. 2015;308:F567–71. doi: 10.1152/ajprenal.00524.2014. [DOI] [PubMed] [Google Scholar]
- 32.Buus NH, Carlsen RK, Hughes AD, et al. Influence of Renal Transplantation and Living Kidney Donation on Large Artery Stiffness and Peripheral Vascular Resistance. Am J Hypertens. 2020;33:234–42. doi: 10.1093/ajh/hpz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gokalp C, Guner Oytun M, Gunay E, et al. Increase in interventricular septum thickness may be the first sign of cardiovascular change in kidney donors. Echocardiography. 2020;37:276–82. doi: 10.1111/echo.14593. [DOI] [PubMed] [Google Scholar]
- 34.Bahous SA, Stephan A, Blacher J, et al. Cardiovascular and renal outcome in recipients of kidney grafts from living donors: role of aortic stiffness. Nephrol Dial Transplant. 2012;27:2095–100. doi: 10.1093/ndt/gfr578. [DOI] [PubMed] [Google Scholar]
- 35.Reference Values for Arterial Stiffness’ Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31:2338–50. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farro I, Bia D, Zócalo Y, et al. Pulse wave velocity as marker of preclinical arterial disease: reference levels in a uruguayan population considering wave detection algorithms, path lengths, aging, and blood pressure. Int J Hypertens. 2012;2012:169359. doi: 10.1155/2012/169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baier D, Teren A, Wirkner K, et al. Parameters of pulse wave velocity: determinants and reference values assessed in the population-based study LIFE-Adult. Clin Res Cardiol. 2018;107:1050–61. doi: 10.1007/s00392-018-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias MF, Dore GA, Davey A. Norms and reference values for pulse wave velocity: one size does not fit all. JBM. 2011;1:1–10. doi: 10.5780/jbm2011.4. [DOI] [Google Scholar]
- 39.Kozakova M, Morizzo C, Guarino D, et al. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J Hypertens. 2015;33:1446–51. doi: 10.1097/HJH.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Sánchez M, Patino-Alonso MC, Gómez-Sánchez L, et al. Reference values of arterial stiffness parameters and their association with cardiovascular risk factors in the Spanish population. The EVA Study. Rev Esp Cardiol (Engl Ed) 2020;73:43–52. doi: 10.1016/j.rec.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Rossi M, Campbell KL, Johnson DW, et al. Uremic toxin development in living kidney donors: a longitudinal study. Transplantation. 2014;97:548–54. doi: 10.1097/01.tp.0000436906.48802.c4. [DOI] [PubMed] [Google Scholar]
- 42.Essig M, Escoubet B, de Zuttere D, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant. 2008;23:239–48. doi: 10.1093/ndt/gfm542. [DOI] [PubMed] [Google Scholar]
- 43.Chue CD, Edwards NC, Ferro CJ, et al. Effects of age and chronic kidney disease on regional aortic distensibility: a cardiovascular magnetic resonance study. Int J Cardiol. 2013;168:4249–54. doi: 10.1016/j.ijcard.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 44.London GM. Arterial Stiffness in Chronic Kidney Disease and End-Stage Renal Disease. Blood Purif. 2018;45:154–8. doi: 10.1159/000485146. [DOI] [PubMed] [Google Scholar]
- 45.Elias MF, Davey A, Dore GA, et al. Deterioration in renal function is associated with increased arterial stiffness. Am J Hypertens. 2014;27:207–14. doi: 10.1093/ajh/hpt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung AWY, Yang HHC, Kim JM, et al. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120:792–801. doi: 10.1161/CIRCULATIONAHA.109.862565. [DOI] [PubMed] [Google Scholar]
- 47.Keung L, Perwad F. Vitamin D and kidney disease. Bone Rep. 2018;9:93–100. doi: 10.1016/j.bonr.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubota Y, Hatakeyama S, Narita I, et al. Clinical impact of glomerular basement membrane thickness on post-donation renal function in living donors. Int J Urol. 2019;26:309–11. doi: 10.1111/iju.13850. [DOI] [PubMed] [Google Scholar]
- 49.Madero M, Wassel CL, Peralta CA, et al. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009;20:1086–93. doi: 10.1681/ASN.2008030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawamoto R, Kohara K, Tabara Y, et al. An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med. 2008;47:593–8. doi: 10.2169/internalmedicine.47.0825. [DOI] [PubMed] [Google Scholar]
- 51.Amann K, Neusüss R, Ritz E, et al. Changes of Vascular Architecture Independent of Blood Pressure in Experimental Uremia. Am J Hypertens. 1995;8:409–17. doi: 10.1016/0895-7061(94)00248-a. [DOI] [PubMed] [Google Scholar]
- 52.Bellavia D, Cataliotti A, Clemenza F, et al. Long-Term Structural and Functional Myocardial Adaptations in Healthy Living Kidney Donors: A Pilot Study. PLoS ONE. 2015;10:e0142103. doi: 10.1371/journal.pone.0142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rastogi A, Yuan S, Arman F, et al. Blood Pressure and Living Kidney Donors: A Clinical Perspective. Transplant Direct. 2019;5:e488. doi: 10.1097/TXD.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLoach SS, Meyers KEC, Townsend RR. Living donor kidney donation: another form of white coat effect. Am J Nephrol. 2012;35:75–9. doi: 10.1159/000335070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xagas E, Sarafidis P, Iatridi F, et al. Kidney transplantation and kidney donation do not affect short-term blood pressure variability. Blood Press. 2023;32:2181640. doi: 10.1080/08037051.2023.2181640. [DOI] [PubMed] [Google Scholar]
- 56.Freedman BI, Langefeld CD, Turner J, et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int. 2012;82:805–11. doi: 10.1038/ki.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muzaale AD, Massie AB, Al Ammary F, et al. Donor-Recipient Relationship and Risk of ESKD in Live Kidney Donors of Varied Racial Groups. Am J Kidney Dis. 2020;75:333–41. doi: 10.1053/j.ajkd.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruzel-Davila E, Wasser WG, Aviram S, et al. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant. 2016;31:349–58. doi: 10.1093/ndt/gfu391. [DOI] [PubMed] [Google Scholar]
- 59.Kalil RS, Smith RJ, Rastogi P, et al. Late Reoccurrence of Collapsing FSGS After Transplantation of a Living-Related Kidney Bearing APOL 1 Risk Variants Without Disease Evident in Donor Supports the Second Hit Hypothesis. Transplant Direct. 2017;3:e185. doi: 10.1097/TXD.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu HH, Kuo CF, Li IJ, et al. Family Aggregation and Heritability of ESRD in Taiwan: A Population-Based Study. Am J Kidney Dis. 2017;70:619–26. doi: 10.1053/j.ajkd.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Obeid H, Bikia V, Fortier C, et al. Assessment of Stiffness of Large to Small Arteries in Multistage Renal Disease Model: A Numerical Study. Front Physiol. 2022;13:832858. doi: 10.3389/fphys.2022.832858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cleper R. Mechanisms of compensatory renal growth. Pediatr Endocrinol Rev. 2012;10:152–63. [PubMed] [Google Scholar]
- 63.Rojas-Canales DM, Li JY, Makuei L, et al. Compensatory renal hypertrophy following nephrectomy: When and how? Nephrology (Carlton) 2019;24:1225–32. doi: 10.1111/nep.13578. [DOI] [PubMed] [Google Scholar]
- 64.Castro-Diehl C, Song RJ, Sawyer DB, et al. Circulating growth factors and cardiac remodeling in the community: The Framingham Heart Study. Int J Cardiol. 2021;329:217–24. doi: 10.1016/j.ijcard.2020.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurevich E, Segev Y, Landau D. Growth Hormone and IGF1 Actions in Kidney Development and Function. Cells. 2021;10:3371. doi: 10.3390/cells10123371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dilley RJ, Schwartz SM. Vascular remodeling in the growth hormone transgenic mouse. Circ Res. 1989;65:1233–40. doi: 10.1161/01.res.65.5.1233. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez RA, Cronin V, Ramsay T, et al. Reproducibility of carotid-femoral pulse wave velocity in end-stage renal disease patients: methodological considerations. Can J Kidney Health Dis. 2016;3:20. doi: 10.1186/s40697-016-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milan A, Zocaro G, Leone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37:1547–57. doi: 10.1097/HJH.0000000000002081. [DOI] [PubMed] [Google Scholar]
- 69.Millasseau SC, Stewart AD, Patel SJ, et al. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension. 2005;45:222–6. doi: 10.1161/01.HYP.0000154229.97341.d2. [DOI] [PubMed] [Google Scholar]
- 70.Giron-Luque F, Garcia-Lopez A, Baez-Suarez Y, et al. Comparison of Three Glomerular Filtration Rate Estimating Equations with 24-Hour Urine Creatinine Clearance Measurement in Potential Living Kidney Donors. Int J Nephrol. 2023;2023:2022641. doi: 10.1155/2023/2022641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miladinović A, Ajčević M, Siveri G, et al. Ambulatory blood pressure monitoring versus office blood pressure measurement: Are there sex differences? Procedia Comput Sci. 2021;192:2912–8. doi: 10.1016/j.procs.2021.09.062. [DOI] [Google Scholar]
- 72.AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–41. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y, Kiechl S, Wang J, et al. Global Pulse Wave Velocity Study Group. EBioMedicine. 2023;92:104619. doi: 10.1016/j.ebiom.2023.104619. [DOI] [PMC free article] [PubMed] [Google Scholar]