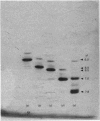
Abstract
The sequence of three alcohol dehydrogenase alleloenzymes from the fruitfly Drosophila melanogaster has been determined by the sequencing of peptides produced by trypsin, chymotrypsin, thermolysin, pepsin and Staphylococcus aureus-V8-proteinase digestion. The amino acid sequence shows no obvious homology with the published sequences of the horse liver and yeast enzymes, and secondary structure prediction suggests that the nucleotide-binding domain is located in the N-terminal half of the molecule. The amino acid substitutions between AdhN-11 (a point mutation of AdhF), AdhS and AdhUF alleloenzymes were identified. AdhN-11 alcohol dehydrogenase differed from the other two by a glycine-14-(AdhS and AdhUF)-to-aspartic acid substitution, the AdhS enzyme from AdhN-11 and AdhUF enzymes by a threonine-192-(AdhN-11 and AdhUF)-to-lysine (AdhS) substitution and the AdhUF enzyme was found to differ by an alanine-45-(AdhS and AdhN-11)-to-aspartic acid (AdhUF) charge substitution and a 'silent' asparagine-8-(AdhS and AdhN-11)-to-alanine (AdhUF) substitution. Detailed sequence evidence has been deposited as Supplementary Publication SUP 50107 (36 pages) at the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978) 169, 5.
Full text
PDF

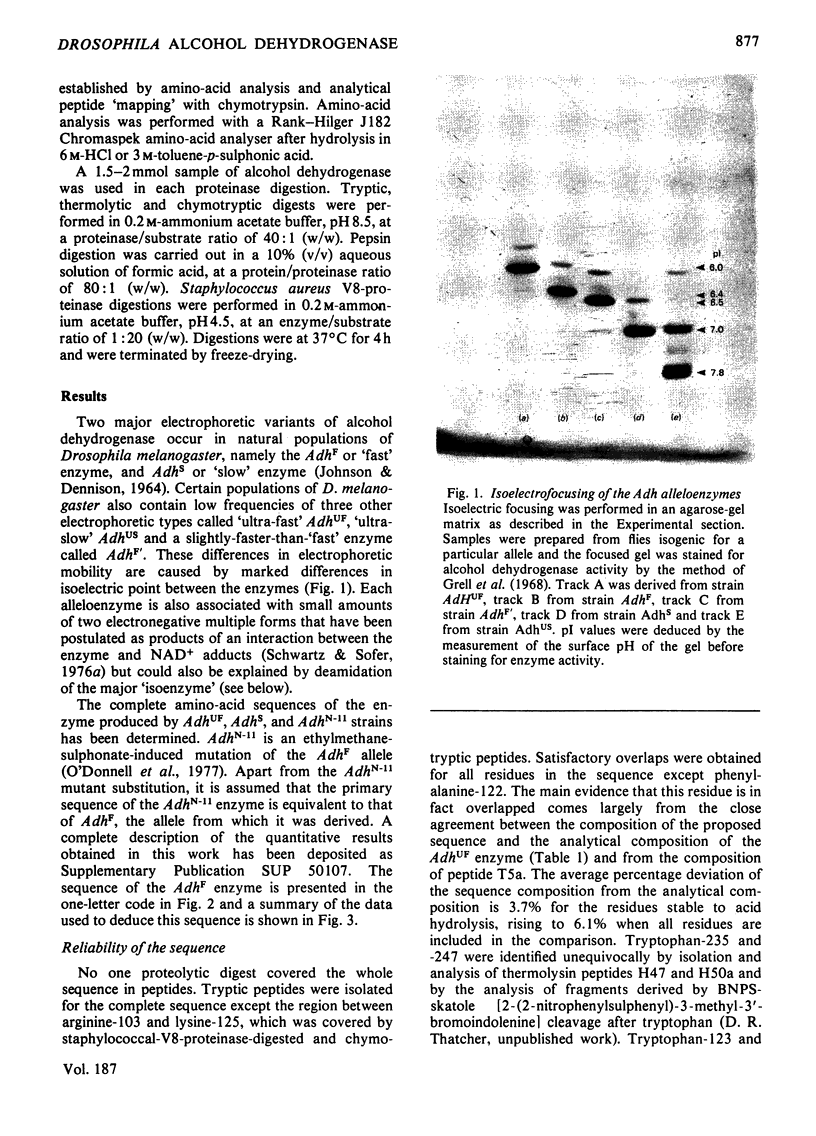
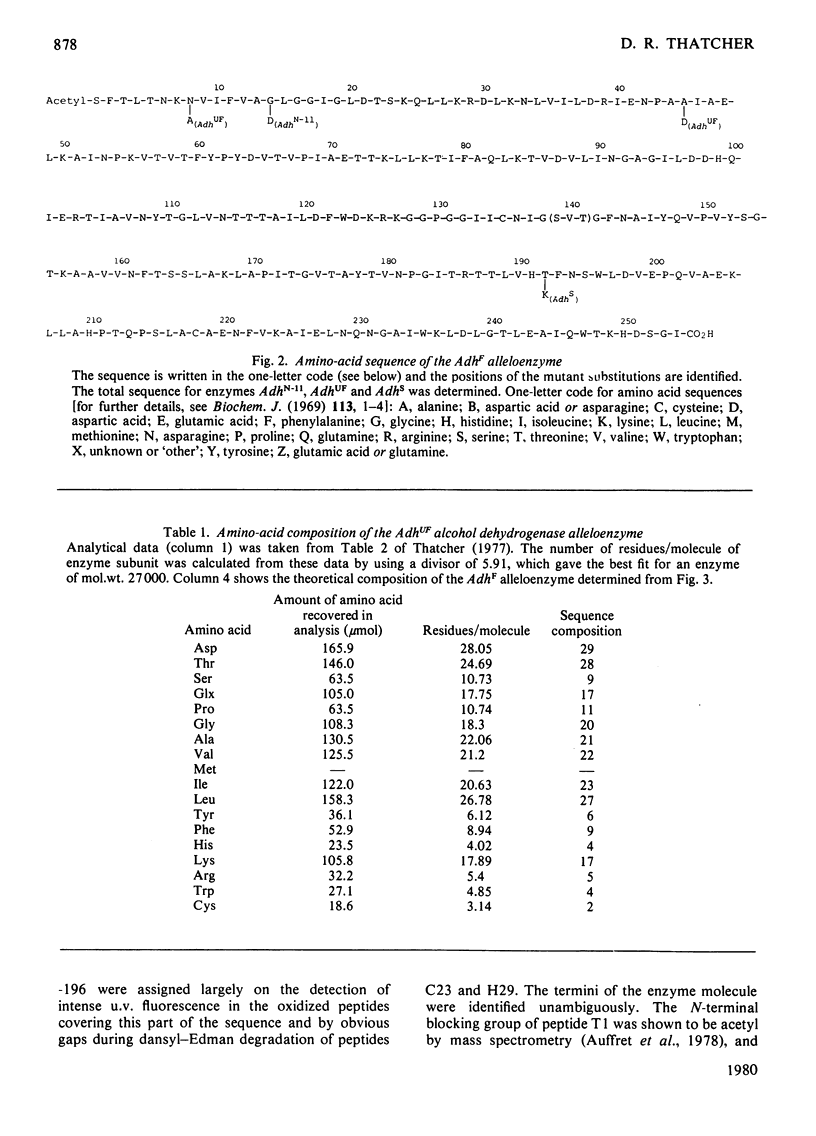





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The amino acid sequence of cytochrome c' from Alcaligenes sp. N.C.I.B. 11015. Biochem J. 1973 Dec;135(4):751–758. doi: 10.1042/bj1350751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffret A. D., Williams D. H. Identification of the blocked N-terminus of an alcohol dehydrogenase from Drosophila melanogaster N-11. FEBS Lett. 1978 Jun 15;90(2):324–326. doi: 10.1016/0014-5793(78)80396-2. [DOI] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Structure of proteins: packing of alpha-helices and pleated sheets. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4130–4134. doi: 10.1073/pnas.74.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. The contribution of ecological genetics to evolutionary theory: detecting the direct effects of natural selection on particular polymorphic loci. Genetics. 1975 Jun;79 (Suppl):101–113. [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Fletcher T. S., Ayala F. J., Thatcher D. R., Chambers G. K. Structural analysis of the ADHS electromorph of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5609–5612. doi: 10.1073/pnas.75.11.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell E. H., Jacobson K. B., Murphy J. B. Alterations of genetics material for analysis of alcohol dehydrogenase isozymes of Drosophila melanogaster. Ann N Y Acad Sci. 1968 Jun 14;151(1):441–455. doi: 10.1111/j.1749-6632.1968.tb11907.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. M., DENNISTON C. GENETIC VARIATION OF ALCOHOL DEHYDROGENASE IN DROSOPHILIA MELANOGASTER. Nature. 1964 Nov 28;204:906–907. doi: 10.1038/204906a0. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- O'Donnell J., Mandel H. C., Krauss M., Sofer W. Genetic and cytogenetic analysis of the Adh region in Drosophila melanogaster. Genetics. 1977 Jul;86(3):553–566. doi: 10.1093/genetics/86.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzios A. D., Thatcher D. R. Chemical basis of the electrophoretic variation observed at the alcohol dehydrogenase locus of Drosophila melanogaster. Biochimie. 1979;61(5-6):701–704. doi: 10.1016/s0300-9084(79)80169-8. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Rydén A. C., Rydén L., Philipson L. Isolation and properties of a staphylococcal protease, preferentially cleaving glutamoyl-peptide bonds. Eur J Biochem. 1974 May 2;44(1):105–114. doi: 10.1111/j.1432-1033.1974.tb03462.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. F., Jörnvall H. Structural analyses of mutant and wild-type alcohol dehydrogenases from drosophila melanogaster. Eur J Biochem. 1976 Sep;68(1):159–168. doi: 10.1111/j.1432-1033.1976.tb10774.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Sofer W. Alcohol dehydrogenase-negative mutants in Drosophila: defects at the structural locus? Genetics. 1976 May;83(1):125–136. doi: 10.1093/genetics/83.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Sofer W. Diet-induced alterations in distribution of multiple forms of alcohol dehydrogenase in Drosophila. Nature. 1976 Sep 9;263(5573):129–131. doi: 10.1038/263129a0. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Camfield R. Chemical basis of the electrophoretic variation between two naturally occurring alcohol dehydrogenase alloenzymes from Drosophila melanogaster. Biochem Soc Trans. 1977;5(1):271–272. doi: 10.1042/bst0050271. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. Enzyme instability and proteolysis during the purification of an alcohol dehydrogenase from Drosophila melanogaster. Biochem J. 1977 May 1;163(2):317–323. doi: 10.1042/bj1630317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R. The partial amino acid sequence of the extracellular beta-lactamase I of Bacillus cereus 569/H. Biochem J. 1975 May;147(2):313–326. doi: 10.1042/bj1470313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigue C. L., Johnson F. M. Isozyme variability in species of the genus Drosophila. VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem Genet. 1973 Jul;9(3):213–227. doi: 10.1007/BF00485735. [DOI] [PubMed] [Google Scholar]
- Woodruff R. C., Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. I. Characterization of deficiencies and mapping of ADH and visible mutations. Genetics. 1979 May;92(1):117–132. doi: 10.1093/genetics/92.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. C., Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. II. Lethal mutations in the region. Genetics. 1979 May;92(1):133–149. doi: 10.1093/genetics/92.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]