Abstract
ABSTRACT
Background
A novel handheld point-of-care high-sensitivity cardiac troponin I analyser has recently been introduced to the market. Evaluating its diagnostic performance against laboratory standards is imperative, given the variations in cardiac troponin levels across populations. This study compared the diagnostic performance between the point-of-care high-sensitivity cardiac troponin I assay (Siemens Healthineers Atellica VTLi) and a laboratory high-sensitivity cardiac troponin I assay (Abbott ARCHITECT STAT High Sensitive Troponin-I) performed using blood samples from various populations (overall, male, female, younger and older) of Chinese patients with chest pain.
Methods
This cross-sectional study included 585 consecutive Chinese patients (age ≥18 year) who presented to an emergency department with chest pain (lasting >5 min) and were managed following the chest pain protocol between 1 August 2023 and 12 June 2024. For both assays, blood samples were collected at two time points (0 hour (initial) and 3 hour (subsequent)). The primary outcome was the diagnostic performance of the two assays, evaluated with their 99th percentile upper reference limits used as the cut-off values for diagnosing myocardial infarction. The gold standard for comparison was the final diagnoses made by attending physicians.
Results
The point-of-care and laboratory assays exhibited equivalent sensitivity and negative predictive values (both 100%) for blood samples collected at both time points. However, the point-of-care assay outperformed the laboratory assay in terms of specificity (initial: 90.5% to 96.3% vs 79.8% to 94.7%; subsequent: 87.8% to 94.8% vs 77.7% to 92.4%) and positive predictive value (initial: 24.4% to 30.8% vs 11.6% to 23.5%; subsequent: 12.5% to 25.0% vs 5.9% to 18.8%), particularly in older patients.
Conclusion
The point-of-care assay is recommended for rapid clinical decision-making. Future studies should explore the effects of its integration into clinical practice and the feasibility of using sex–race–age-specific 99th percentile upper reference limits to enhance its diagnostic performance.
Keywords: Chest Pain, Acute Coronary Syndrome, Myocardial Infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
A novel handheld point-of-care high-sensitivity cardiac troponin I analyser, which provides results within 8 min, has recently been introduced to the market. Evaluating its diagnostic performance against laboratory standards is imperative, given the variations in cardiac troponin levels across populations.
WHAT THIS STUDY ADDS
Compared with a laboratory high-sensitivity cardiac troponin I assay, the point-of-care high-sensitivity cardiac troponin I assay exhibits similar rule-out efficacy and superior rule-in ability, particularly in older Chinese patients with chest pain.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Implementing the point-of-care high-sensitivity cardiac troponin I may shorten length of stay, expedite treatment, enhance clinical outcomes and reduce healthcare costs. Evaluating the effects of its real-world implementation and the use of sex–race–age-specific 99th percentile upper reference limits in improving its diagnostic performance should be prioritised in the future.
Introduction
Chest pain is one of the most common reports in emergency departments (EDs) and a primary indicator of coronary artery disease, the leading cause of mortality worldwide.1 Identifying the precise cause of chest pain is challenging because of its varied origins, which can range from musculoskeletal conditions to myocardial infarction (MI).2 ED visits for chest pain are associated with high healthcare costs, primarily resulting from an extended length of stay (LOS) in the hospital.3 Early diagnosis of MI and timely initiation of treatments are crucial for minimising myocardial damage and associated mortality.4 5 Patients with harmless chest pain should be discharged promptly, whereas those with life-threatening MI should be treated immediately.
Cardiac troponin (cTn), including cTnI and cTnT, is a standard biomarker used for diagnosing MI.6 The Fourth Universal Definition of Myocardial Infarction indicates the 99th percentile upper reference limit (URL) in a normal reference population as the diagnostic threshold for MI. High-sensitivity (hs) cTn assays can detect cTn levels below the 99th percentile URL in >50% of all individuals in a normal reference population; the corresponding coefficient of variation (CV) is ≤10%. These assays are preferred over conventional cTn assays because of their enhanced precision and low limits of detection (LoD).7
The results of cTn assays should ideally be available within 60 min from blood collection.8 Traditionally, only laboratory analysers have been used for hs-cTn assays. However, most laboratories struggle to obtain assay results within the aforementioned timeframe because of delays in the transport and processing of blood samples.6 9 10 By contrast, point-of-care (POC) cTn analysers have a short turnaround time, and therefore, they can enable clinicians to make more timely decisions, which can shorten LOS in the hospital, expedite treatment, improve clinical outcomes and reduce healthcare costs.310,12 However, the detection sensitivity is lower for traditional POC cTn assays than for laboratory hs-cTn assays, particularly when cTn levels are low—which is a common scenario when blood is collected within 3 hours after symptom onset.611,13
A new handheld POC hs-cTnI analyser has recently been introduced to the market. This device provides cTnI results within 8 min from the addition of a single drop of whole blood to the experimental cartridge.14 15 Despite its rapid processing time, its diagnostic performance remains to be confirmed, particularly because cTn levels vary depending with race and age, which influences the sensitivity and specificity of diagnostic assays.16 17 Thus, evaluating the diagnostic performance of the POC hs-cTnI assay against laboratory standards is crucial for its adoption in clinical practice.
Evidence suggests that the POC hs-cTnI assay is effective in both male and female American patients with chest pain.15 However, limited data are available regarding the diagnostic performance of the POC assay in Chinese patients belonging to different age groups; such data are essential because cTn levels vary across populations in terms of race, and older adults typically have higher cTn levels than do younger adults.16,18 In the present study, we compared the diagnostic performance between the POC hs-cTnI assay and a laboratory hs-cTnI assay by using blood samples from the overall, male, female, younger and older populations of Chinese patients with chest pain.
Methods
This study was approved by the research ethics committee of Queen Elizabeth Hospital (KC/KE-22–0208/ER-4) and conformed to the principles outlined in the Declaration of Helsinki.
Study design
This was a cross-sectional study. The present study adhered to the 2015 Standards for Reporting of Diagnostic Accuracy Studies guidelines.19
Study sample
This study included consecutive patients with chest pain who had visited the ED of Queen Elizabeth Hospital, Hong Kong, between 1 August 2023 and 12 June 2024. Patients were included if they were Chinese (age ≥18 year), presented with chest pain (lasting >5 min) and were managed following the chest pain protocol of the ED (blood collection for the laboratory hs-cTnI assay after a consultation and at 3 hours after the initial blood collection). Patients were excluded if they were pregnant, were critically ill, were mentally incompetent, had a language barrier or had a history of chronic kidney disease, which might have led to false-positive results in cTn assays.20
The required sample size was calculated using the following formulas: nse=Zα/22Se(1−Se)/d2Prev and nsp=Zα/22Sp(1−Sp)/d2(1−Prev), where nse and nsp are the minimum sample sizes based on sensitivity and specificity values, respectively. Zα/2 is 1.96 when α is 0.05. Se, Sp, d and Prev represent the predetermined values of sensitivity, specificity, error margin, and disease prevalence, respectively.21 A study conducted among patients with chest pain reported that the sensitivity and specificity values of the POC hs-cTnI assay were 81.3% and 84.9%, respectively; the prevalence of MI in the aforementioned population was approximately 10%.15 In the present study, the error margin was set at 0.1. The minimum sample sizes were 584 and 55 based on sensitivity and specificity values, respectively. To achieve an accurate estimation of diagnostic performance, this study required a sample size of 584 patients.
Troponin measurement
The POC hs-cTnI assay was performed using the Siemens Healthineers Atellica VTLi Patient-side Immunoassay Analyzer with lithium-heparinized whole blood samples. The LoD is 1.6 ng/L; the 99th percentile URLs for the overall, male and female populations are 22.9, 27.1 and 18.5 ng/L, respectively. The CV ranges from 7.1% to 9.5%. The assay measures cTnI levels at or above the LoD in 84% of all individuals in a normal reference population (87% for men and 80% for women).14 15
The laboratory hs-cTnI assay was performed using the Abbott ARCHITECT STAT High Sensitive Troponin-I kit run on the Abbott ARCHITECT i2000SR analyser. Lithium-heparinised plasma samples were used for the assay. The LoD ranges from 1.1 to 1.9 ng/L; the 99th percentile URLs for the overall, male and female populations are 26.2, 34.2 and 15.6 ng/L, respectively. The CV at the overall 99th percentile URL is 4.0% (men: 3.5%; women: 5.3%). The assay measures cTnI levels at or above the LoD in 85% of all individuals in a normal reference population (92% for men and 78% for women).22
Study outcome
The primary outcome was the diagnostic performance—sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV)—of the POC and laboratory hs-cTnI assays.
Gold standard
The gold standard for comparison was final diagnoses made by attending physicians in the ED or other hospital units. The final diagnoses were made on the basis of the clinical presentations of patients’ conditions and the results of electrocardiography, echocardiography, coronary angiography, laboratory tests and diagnostic imaging examinations. These diagnoses were categorised into MI and non-MI diagnoses.23 MI was diagnosed on the basis of the Fourth Universal Definition of Myocardial Infarction.7
Data collection
Patients received an initial consultation from a physician. A research assistant who worked as a lab technician for >6 years and was experienced in processing blood tests, including cTn assays, assessed the eligibility of the patients and invited them to participate in the present study and sign a consent form. The research assistant collected blood samples (5 mL) in specimen tubes at two time points and sent them to the laboratory for hs-cTnI assay. Additionally, a drop of the same blood sample was used for POC hs-cTnI assay. Both assays were repeated at 3 hours after the initial blood collection. The physicians, emergency nurses and phlebotomists were not informed about the POC cTnI results to avoid any influence on clinical decision-making. Data were retrieved from the computer system of the study hospital by using a self-developed form. The research assistant manually recorded the POC cTnI results as soon as they were available.
Statistical analysis
Data were analysed using SPSS (V.26.0; IBM Corporation, Armonk, New York) for Windows and MedCalc (V.22.026; MedCalc Software, Ostend, Belgium). Descriptive statistics, including frequency, percentage, mean and SD values, were used to describe participant characteristics. Diagnostic performance was evaluated in terms of sensitivity, specificity, NPV and PPV, with 95% CI values. The overall and sex-specific 99th percentile URLs were used as the cut-off values for MI diagnosis. Subgroup analysis by age (<65 and ≥65 year) was performed using sex-specific 99th percentile URLs as the cut-off values for MI diagnosis. The McNemar test was used to compare sensitivity and specificity between the two assays.24 Significance was set at a two-tailed p value of<0.05.
Results
Patient characteristics
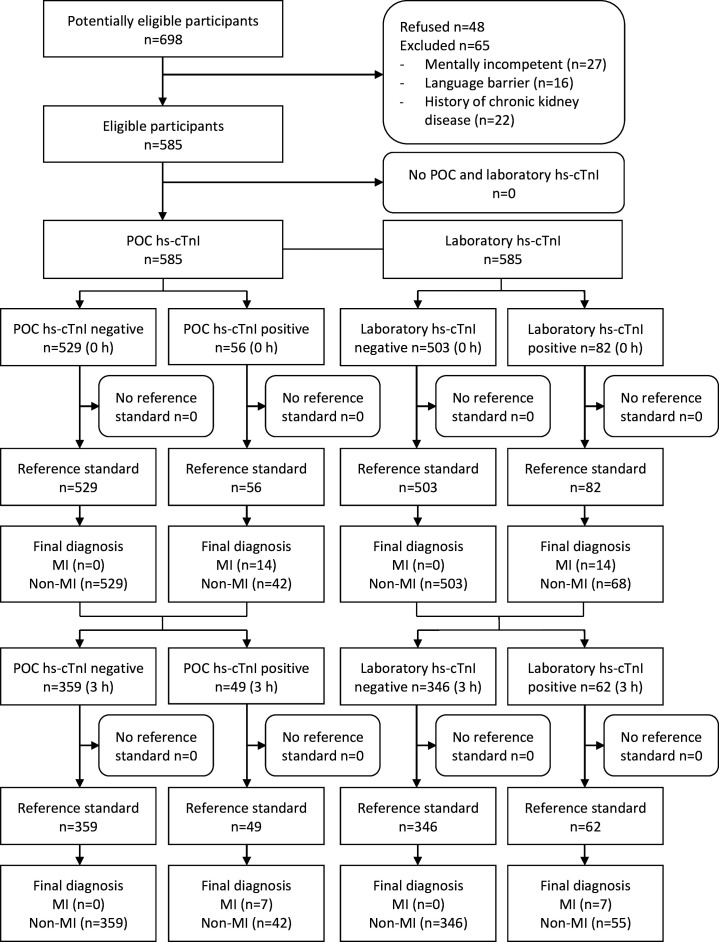
This study included 585 patients. Blood samples were collected from all patients at the initial time point and from approximately 70% of them at the subsequent time point. This discrepancy was due to a second hs-cTnI assay not being performed in the ED for 68 patients who were discharged and 109 patients who were admitted (figure 1).
Figure 1. Flow diagram of the participants through the study. MI, myocardial infarction; POC, point-of-care.
Table 1 presents the personal characteristics of the study cohort. The sex distribution was balanced, with 304 men (52.0%) and 281 women (48.0%). The patients’ overall mean age was 65.9±16.7 year. Among the patients, 326 (55.7%) had hypertension, 272 (46.5%) had hypercholesterolemia, 131 (22.4%) had diabetes and 175 (29.9%) had cardiovascular disease. In addition, 53 patients (9.1%) reported a family history of coronary artery disease. Regarding lifestyle factors, 47 patients (8.0%) were current smokers, 50 (8.5%) were former smokers, 40 (6.8%) were current drinkers and 13 (2.2%) were former drinkers. At the time of the initial blood collection, 513 patients (87.7%) had chest pain for >3 hours. A total of 320 patients (54.7%) were admitted to the hospital. Only 14 patients (2.4%) were given a diagnosis of MI; the remaining 571 patients (97.6%) received non-MI diagnoses, which mainly included chest pain or discomfort, stable or unstable angina, palpitation or arrhythmia, syncope or dizziness, ischaemic heart disease, congestive heart failure, fluid overload and musculoskeletal pain.
Table 1. Personal characteristics of the study cohort (N=585).
| Personal characteristics | N (%) |
| Sex | |
| Male | 304 (52.0) |
| Female | 281 (48.0) |
| Age (years) | |
| Overall | 65.9 (16.7)* |
| Younger (<65 years) | 50.3 (11.8)* |
| Older (≥65 years) | 77.4 (8.4)* |
| Risk factors for coronary artery disease at baseline | |
| Diabetes | 131 (22.4) |
| Hypertension | 326 (55.7) |
| Hypercholesterolemia | 272 (46.5) |
| Cardiovascular disease | 175 (29.9) |
| Family history | 53 (9.1) |
| Current smoker | 47 (8.0) |
| Former smoker | 50 (8.5) |
| Current drinker | 40 (6.8) |
| Former drinker | 13 (2.2) |
| Chest pain onset | |
| ≤3 hours | 72 (12.3) |
| >3 hours | 513 (87.7) |
| Disposition decision at the ED | |
| Discharged | 265 (45.3) |
| Admitted | 320 (54.7) |
| Final diagnosis at discharge from the ED or other hospital units | |
| MI | 14 (2.4) |
| Non-MI | 571 (97.6) |
Data are presented in mean (standard deviationSD).
ED, emergency department; MI, myocardial infarction; N, number
Diagnostic performance measured using initial blood sample
Table 2 presents the diagnostic performance of the POC and laboratory hs-cTnI assays performed using the initial blood samples. The turnaround times for the POC and laboratory hs-cTnI assays were 10.6±0.9 min and 84.6±36.2 min, respectively. The mean ages of the younger (age <65 year) and older (age ≥65 year) patients were 50.3±11.8 year and 77.4±8.4 year, respectively. The sensitivity values for the POC and laboratory assays were 100% in the overall (95% CI 76.8 to 100.0), male (95% CI 66.4 to 100.0), female (95% CI 47.8 to 100.0), younger (95% CI 39.8 to 100.0) and older (95% CI 69.2 to 100.0) populations. The McNemar test could not be used for sensitivity comparisons because both assays detected all cases of MI without any false-negative results.
Table 2. Diagnostic performance of the point-of-care and laboratory high-sensitivity cardiac troponin I assays performed using the initial blood samples.
| Population | Assay | Positive | Negative | MI | Non-MI | Sensitivity(95% CI) | Specificity(95% CI) | P value | PPV(95% CI) | NPV(95% CI) |
| Overall(N=585) | POC | 56 | 529 | 14 | 571 | 100% (76.8 to 100.0) | 92.6% (90.2 to 94.7) | < 0.001 | 25.0% (20.0 to 30.8) | 100% (99.3 to 100.0) |
| Laboratory | 82 | 503 | 14 | 571 | 100% (76.8 to 100.0) | 88.1% (85.2 to 90.6) | 17.1% (14.1 to 20.5) | 100% (99.3 to 100.0) | ||
| Male(N=304) | POC | 35 | 269 | 9 | 295 | 100% (66.4 to 100.0) | 91.2% (87.4 to 94.2) | = 0.001 | 25.7% (19.3 to 33.3) | 100% (98.6 to 100.0) |
| Laboratory | 50 | 254 | 9 | 295 | 100% (66.4 to 100.0) | 86.1% (81.6 to 89.8) | 18.0% (14.2 to 22.6) | 100% (98.6 to 100.0) | ||
| Female(N=281) | POC | 19 | 262 | 5 | 276 | 100% (47.8 to 100.0) | 94.9% (91.6 to 97.2) | < 0.001 | 26.3% (17.7 to 37.3) | 100% (98.6 to 100.0) |
| Laboratory | 43 | 238 | 5 | 276 | 100% (47.8 to 100.0) | 86.2% (81.6 to 90.1) | 11.6% (8.9 to 15.0) | 100% (98.5 to 100.0) | ||
| Younger(<65 years)(N=248) | POC | 13 | 235 | 4 | 244 | 100% (39.8 to 100.0) | 96.3% (93.1 to 98.3) | = 0.289 | 30.8% (20.0 to 45.8) | 100% (98.4 to 100.0) |
| Laboratory | 17 | 231 | 4 | 244 | 100% (39.8 to 100.0) | 94.7% (91.1 to 97.1) | 23.5% (15.4 to 34.3) | 100% (98.4 to 100.0) | ||
| Older(≥65 years)(N=337) | POC | 41 | 296 | 10 | 327 | 100% (69.2 to 100.0) | 90.5% (86.8 to 93.5) | < 0.001 | 24.4% (18.8 to 31.1) | 100% (98.8 to 100.0) |
| Laboratory | 76 | 261 | 10 | 327 | 100% (69.2 to 100.0) | 79.8% (75.1 to 84.0) | 13.2% (10.9 to 15.8) | 100% (98.6 to 100.0) |
The McNemar test was used to produce the P values given.
MI, myocardial infarction; N, number; NPV, negative predictive value; POC, point-of-care; PPV, positive predictive value
The NPVs for the POC and laboratory assays were 100% in the overall (95% CI 99.3 to 100.0), male (95% CI 98.6 to 100.0), female (95% CI 98.6 to 100.0 vs 98.5 to 100.0), younger (95% CI 98.4 to 100.0) and older (95% CI 98.8 to 100.0 vs 98.6 to 100.0) populations.
The specificity values for the POC and laboratory assays were, respectively, 92.6% (95% CI 90.2 to 94.7) and 88.1% (95% CI 85.2 to 90.6) in the overall population (p<0.001); 91.2% (95% CI 87.4 to 94.2) and 86.1% (95% CI 81.6 to 89.8) in the male population (p=0.001); 94.9% (95% CI 91.6 to 97.2) and 86.2% (95% CI 81.6 to 90.1) in the female population (p<0.001); 96.3% (95% CI 93.1 to 98.3) and 94.7% (95% CI 91.1 to 97.1) in the younger population (p=0.289); and 90.5% (95% CI 86.8 to 93.5) and 79.8% (95% CI 75.1 to 84.0) in the older population (p<0.001).
The PPVs for the POC and laboratory assays were, respectively, 25.0% (95% CI 20.0 to 30.8) and 17.1% (95% CI 14.1 to 20.5) in the overall population; 25.7% (95% CI 19.3 to 33.3) and 18.0% (95% CI 14.2 to 22.6) in the male population; 26.3% (95% CI 17.7 to 37.3) and 11.6% (95% CI 8.9 to 15.0) in the female population; 30.8% (95% CI 20.0 to 45.8) and 23.5% (95% CI 15.4 to 34.3) in the younger population; and 24.4% (95% CI 18.8 to 31.1) and 13.2% (95% CI 10.9 to 15.8) in the older population.
Diagnostic performance measured using subsequent blood sample
Table 3 presents the diagnostic performance of the POC and laboratory hs-cTnI assays performed using the subsequent blood samples. The turnaround times for the POC and laboratory hs-cTnI assays were 10.4±0.7 min and 81.4±30.5 min, respectively. The mean ages of the younger (age <65 year) and older (age ≥65 year) patients were 51.0±11.5 year and 77.8±8.6 year, respectively. The POC and laboratory assays exhibited sensitivity values of 100% in the overall (95% CI 59.0 to 100.0), male (95% CI 47.8 to 100.0), female (95% CI 15.8 to 100.0), younger (95% CI 29.4 to 100.0 vs 29.2 to 100.0) and older (95% CI 39.8 to 100.0) populations. The McNemar test could not be used for sensitivity comparisons because both assays detected all cases of MI cases without any false-negative results.
Table 3. Diagnostic performance of the point-of-care and laboratory high-sensitivity cardiac troponin I assays performed using the subsequent blood samples.
| Population | Assay | Positive | Negative | MI | Non-MI | Sensitivity(95% CI) | Specificity(95% CI) | P value | PPV(95% CI) | NPV(95% CI) |
| Overall(N=408) | POC | 49 | 359 | 7 | 401 | 100% (59.0 to 100.0) | 89.5% (86.1 to 92.4) | = 0.011 | 14.3% (11.1 to 18.2) | 100% (99.0 to 100.0) |
| Laboratory | 62 | 346 | 7 | 401 | 100% (59.0 to 100.0) | 86.3% (82.5 to 89.5) | 11.3% (9.1 to 14.0) | 100% (98.9 to 100.0) | ||
| Male(N=218) | POC | 28 | 190 | 5 | 213 | 100% (47.8 to 100.0) | 89.2% (84.2 to 93.0) | = 0.035 | 17.9% (12.9 to 24.2) | 100% (98.0 to 100.0) |
| Laboratory | 37 | 181 | 5 | 213 | 100% (47.8 to 100.0) | 85.0% (79.5 to 89.5) | 13.5% (10.2 to 17.7) | 100% (98.0 to 100.0) | ||
| Female(N=190) | POC | 16 | 174 | 2 | 188 | 100% (15.8 to 100.0) | 92.6% (87.8 to 95.9) | < 0.001 | 12.5% (7.9 to 19.1) | 100% (97.9 to 100.0) |
| Laboratory | 34 | 156 | 2 | 188 | 100% (15.8 to 100.0) | 83.0% (76.8 to 88.0) | 5.9% (4.4 to 7.9) | 100% (97.7 to 100.0) | ||
| Younger(<65 years)(N=175) | POC | 12 | 163 | 3 | 172 | 100% (29.4 to 100.0) | 94.8% (90.3 to 97.6) | = 0.219 | 25.0% (15.0 to 38.6) | 100% (97.8 to 100.0) |
| Laboratory | 16 | 159 | 3 | 172 | 100% (29.2 to 100.0) | 92.4% (87.4 to 95.9) | 18.8% (12.0 to 28.0) | 100% (97.7 to 100.0) | ||
| Older(≥65 years)(N=233) | POC | 32 | 201 | 4 | 229 | 100% (39.8 to 100.0) | 87.8% (82.8 to 91.7) | < 0.001 | 12.5% (9.2 to 16.8) | 100% (98.2 to 100.0) |
| Laboratory | 55 | 178 | 4 | 229 | 100% (39.8 to 100.0) | 77.7% (71.8 to 83.0) | 7.3% (5.8 to 9.1) | 100% (98.0 to 100.0) |
The McNemar test was used to produce the P values given.
MI, myocardial infarction; N, number; NPV, negative predictive value; POC, point-of-care; PPV, positive predictive value
The NPVs for the POC and laboratory assays were 100% in the overall (95% CI 99.0 to 100.0 vs 98.9 to 100.0), male (95% CI 98.0 to 100.0), female (95% CI 97.9 to 100.0 vs 97.7 to 100.0), younger (95% CI 97.8 to 100.0 vs 97.7 to 100.0) and older (95% CI 98.2 to 100.0 vs 98.0 to 100.0) populations.
The specificity values for the POC and laboratory assays were, respectively, 89.5% (95% CI 86.1 to 92.4) and 86.3% (95% CI 82.5 to 89.5) in the overall population (p=0.011); 89.2% (95% CI 84.2 to 93.0) and 85.0% (95% CI 79.5 to 89.5) in the male population (p=0.035); 92.6% (95% CI 87.8 to 95.9) and 83.0% (95% CI 76.8 to 88.1) in the female population (p<0.001); 94.8% (95% CI 90.3 to 97.6) and 92.4% (95% CI 87.4 to 95.9) in the younger population (p=0.219); and 87.8% (95% CI 82.8 to 91.7) and 77.7% (95% CI 71.8 to 83.0) in the older population (p<0.001).
The PPVs for the POC and laboratory assays were, respectively, 14.3% (95% CI 11.1 to 18.2) and 11.3% (95% CI 9.1 to 14.0) in the overall population; 17.9% (95% CI 12.9 to 24.2) and 13.5% (95% CI 10.2 to 17.7) in the male population; 12.5% (95% CI 7.9 to 19.1) and 5.9% (95% CI 4.4 to 7.9) in the female population; 25.0 (95% CI 15.0 to 38.6) and 18.8% (95% CI 12.0 to 28.0) in the younger population; and 12.5% (95% CI 9.2 to 16.8) and 7.3% (95% CI 5.8 to 9.1) in the older population.
Discussion
To the best of our knowledge, this study is the first to compare diagnostic performance between a POC hs-cTnI assay and a laboratory hs-cTnI assay performed using blood samples (collected at two time points) from the overall, male, female, younger and older populations of Chinese patients with chest pain. Both assays exhibited equivalent sensitivity values and NPVs at both time points, indicating similar efficacy in ruling out MI in the specified populations. However, the POC assay outperformed the laboratory assay in terms of specificity and PPV at both time points, suggesting the superior ability of the POC assay to rule in MI in the study populations. Similarly, Gunsolus et al15 reported that the sensitivity and specificity values, NPVs and PPVs of the POC hs-cTnI assay were comparable to those of two laboratory hs-cTnI assays performed using initial blood samples and those collected 2 hours later in the overall, male and female populations of patients from the USA.
The between-assay differences in specificity were nonsignificant in the younger population and were the most significant in the older population. In the younger population, the specificity values for the POC and laboratory hs-cTnI assays were >94% and >92%, respectively, at both time points; in the older population, these values were >87% and <80% at both time points.
The sensitivity values and NPVs for the assays remained consistent at both time points across the overall, male, female, younger and older populations. Approximately 90% of all patients had chest pain for >3 hours by the time the initial blood collection was completed, suggesting that the majority had sufficiently elevated cTnI levels at both time points. Gunsolus et al15 found that the sensitivity values and NPVs for the assays were lower for the initial blood samples than for the subsequent samples in their overall, male and female populations of American patients. However, the researchers presented no information regarding the onset of chest pain, which hindered direct comparison with our findings.
This study has several strengths and limitations. Subgroup analysis by age was performed using sex-specific 99th percentile URLs as the cut-off values for MI diagnosis, thereby mitigating the influence of sex-related confounders. However, the exclusion of patients with chronic kidney disease might have limited the generalisability of our findings to this group. Furthermore, the prevalence of MI was relatively low in the study cohort, resulting in wide CI values for sensitivity and affecting the sensitivity estimation of the assays at the two time points. Approximately 30% of all patients were discharged or admitted without a second hs-cTnI assay in the ED; this reduced the sample size, further influencing the sensitivity estimation of the assays performed using the subsequent blood samples. A prospective approach is widely acknowledged to offer significant advantages in assessing the diagnostic performance of a test compared with a retrospective approach. The advantages include but are not limited to obtaining a sample that is better defined in terms of clinical characteristics and standardising the methods for performing and interpreting a test and gold standard procedure. Nonetheless, a potential limitation resides in the fluctuating prevalence of the target condition, especially for rare and life-threatening conditions.25 This prospective study revealed an unexpectedly low prevalence of MI in the study cohort, leading to broad CI values for sensitivity and affecting the sensitivity estimation at the two time points. Stratification is one of the strategies proposed to achieve a precise sensitivity estimation in subsequent investigations. In this strategy, Chinese patients with chest pain may be stratified based on the prevalence of MI in various subpopulations in terms of history of or risk factors for coronary artery disease, and they are optimally sampled from the strata to accrue those with MI efficiently.25 Notwithstanding the impact of low MI prevalence on sensitivity estimation, the current sample size was sufficiently large to yield an accurate specificity estimation. The sample size was 585 in the present study, while the minimum sample size for the specificity estimation securing the statistical power should be 51. This is estimated using the aforementioned formula and values, except that the prevalence of MI is revised to 2.4%.21
Our findings corroborate and enhance those of Gunsolus et al,15 which exhibited similar sensitivity values and NPVs between the POC hs-cTnI assay and two laboratory hs-cTnI assays at two time periods across the overall, male and female populations of patients with chest pain. We additionally discovered that the POC hs-cTnI assay surpassed the laboratory hs-cTnI assay for specificity values and PPVs at both time points, especially in older patients with chest pain. Our findings, along with those of Gunsolus et al,15 support the use of the POC hs-cTnI assay to expedite clinical decision-making and shorten LOS, which accounts for a major proportion of the total healthcare cost in chest pain management.3 10 Early diagnosis of MI and timely initiation of treatments may reduce myocardial damage and associated mortality.10 12 The POC analyser’s portability and minimal sample requirement—only a drop of whole blood—facilitate its use by healthcare professionals in non-laboratory settings such as ambulances, clinics and small hospitals.14 15 In addition, results of the POC assay can be transmitted from out-of-hospital settings to hospitals in advance, thus expediting diagnosis and treatment and ultimately improving clinical outcomes.
The present study lays the foundation for future studies exploring the effects of using the POC hs-cTnI assay on LOS, treatment duration, clinical outcomes and healthcare utilisation. Future studies should explore its effects on cTn level–based risk score for predicting the risk of cardiac events. In addition, studies should assess the effects of implementing the POC assay on treatment timing and clinical outcomes in out-of-hospital settings. Sex-specific 99th percentile URLs for the assay have been determined using blood samples from apparently healthy individuals belonging to diverse racial and age groups; the results indicate that the diagnostic performance of the POC assay varies across populations with varying cTnI levels.14 16 17 Future studies should determine whether using sex–race–age-specific 99th percentile URLs improves the diagnostic performance of the POC assay.
Our findings suggest that compared with a laboratory hs-cTnI assay, the POC hs-cTnI assay exhibits similar rule-out efficacy and superior rule-in ability, particularly in older Chinese patients with chest pain. Implementing the POC assay may shorten LOS, expedite treatment, enhance clinical outcomes and reduce healthcare costs. Evaluating the effects of its real-world implementation and the use of sex–race–age-specific 99th percentile URLs in improving its diagnostic performance should be prioritised in the future.
Acknowledgements
We would like to thank the patients who participated in this study.
Footnotes
Funding: This work was supported by Hong Kong Metropolitan University Grant (RIF/2022/2.2, RD/2022/2.10).
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Research Ethics Committee (Kowloon Central / Kowloon East) (reference number: KC/KE-22-0208/ER-4). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability free text: Data are available upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Jonathan Ka Ming Ho, Email: kamho@hkmu.edu.hk.
Janet Yuen Ha Wong, Email: jyhwong@hkmu.edu.hk.
Gary Tse, Email: gatse@hkmu.edu.hk.
Andy Chun Yin Chong, Email: andychong@twc.edu.hk.
Calvin Chi Wai Chau, Email: chaucw2@ha.org.hk.
Chi Yip Wong, Email: bwcyz06@ha.org.hk.
Johnson Wai Keung Tse, Email: twk336@ha.org.hk.
Jeremy Yan Hon Tam, Email: tamyanhon@gmail.com.
Simon Ching Lam, Email: simonlam@twc.edu.hk.
Data availability statement
Data are available upon reasonable request.
References
- 1.Khan MA, Hashim MJ, Mustafa H, et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 3.Forberg JL, Henriksen LS, Edenbrandt L, et al. Direct hospital costs of chest pain patients attending the emergency department: a retrospective study. BMC Emerg Med. 2006;6:6. doi: 10.1186/1471-227X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Case BC, Yerasi C, Wang Y, et al. Admissions Rate and Timing of Revascularization in the United States in Patients With Non-ST-Elevation Myocardial Infarction. Am J Cardiol. 2020;134:24–31. doi: 10.1016/j.amjcard.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Choi KH, Lee JM, et al. Prognostic Implications of Door-to-Balloon Time and Onset-to-Door Time on Mortality in Patients With ST -Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Heart Assoc. 2019;8:e012188. doi: 10.1161/JAHA.119.012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed S, Jafri L, Raheem A, et al. Performance Evaluation of Cardiac Troponin I Assay: A Comparison Between the Point-of-care Testing Radiometer AQT90 FLEX and the Central Laboratory Siemens Advia Centaur Analyzer. Cureus. 2019;11:e4231. doi: 10.7759/cureus.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–51. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 8.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate Use Criteria for Coronary Revascularization in Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:570–91. doi: 10.1016/j.jacc.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Ko HF, Lee HY, Ho HF. A 2-Hour Accelerated Chest Pain Protocol to Assess Patients with Chest Pain Symptoms in an Accident and Emergency Department in Hong Kong. Hong Kong J Emerg Med. 2014;21:261–9. doi: 10.1177/102490791402100105. [DOI] [Google Scholar]
- 10.Venge P, van Lippen L, Blaschke S, et al. Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay. Clin Chim Acta. 2017;469:119–25. doi: 10.1016/j.cca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Loten C, Attia J, Hullick C, et al. Point of care troponin decreases time in the emergency department for patients with possible acute coronary syndrome: a randomised controlled trial. Emerg Med J. 2010;27:194–8. doi: 10.1136/emj.2008.069427. [DOI] [PubMed] [Google Scholar]
- 12.Wilke P, Masuch A, Fahron O, et al. Diagnostic performance of point-of-care and central laboratory cardiac troponin assays in an emergency department. PLoS One. 2017;12:e0188706. doi: 10.1371/journal.pone.0188706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammad OH, Naushad VA, Purayil NK, et al. Diagnostic Performance of Point-of-Care Troponin I and Laboratory Troponin T in Patients Presenting to the ED with Chest Pain: A Comparative Study. Open Access Emerg Med. 2020;12:247–54. doi: 10.2147/OAEM.S259726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apple FS, Schulz K, Schmidt CW, et al. Determination of sex-specific 99th percentile upper reference limits for a point of care high sensitivity cardiac troponin I assay. Clin Chem Lab Med. 2021;59:1574–8. doi: 10.1515/cclm-2021-0262. [DOI] [PubMed] [Google Scholar]
- 15.Gunsolus IL, Schulz K, Sandoval Y, et al. Diagnostic performance of a rapid, novel, whole blood, point of care high-sensitivity cardiac troponin I assay for myocardial infarction. Clin Biochem. 2022;105–106:70–4. doi: 10.1016/j.clinbiochem.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Kalaria TR, Harris N, Sensi H, et al. High-sensitivity cardiac troponin I: is ethnicity relevant? J Clin Pathol. 2021;74:709–11. doi: 10.1136/jclinpath-2020-206951. [DOI] [PubMed] [Google Scholar]
- 17.Kong N, Chua RFM, Besser SA, et al. A retrospective analysis of high sensitivity cardiac troponin-T ranges in non-myocardial infarction emergency department visits. BMC Cardiovasc Disord. 2021;21:283. doi: 10.1186/s12872-021-02089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Jeinsen B, Brandebussemeyer S, Gruen D, et al. Evaluation of the effect of older age on the diagnostic specificity of high-sensitivity troponin I and high-sensitivity troponin T in patients with suspected acute myocardial infarction. Eur Heart J. 2020;41 doi: 10.1093/ehjci/ehaa946.1692. [DOI] [Google Scholar]
- 19.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long B, Belcher CN, Koyfman A, et al. Interpreting troponin in renal disease: A narrative review for emergency clinicians. Am J Emerg Med. 2020;38:990–7. doi: 10.1016/j.ajem.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193–204. doi: 10.1016/j.jbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Laboratories A. ARCHITECT: STAT High Sensitive Troponin-I. Package Insert. Abbott Park, IL: Abbott Laboratories; 2018. [Google Scholar]
- 23.Slagman A, von Recum J, Möckel M, et al. Diagnostic performance of a high-sensitive troponin T assay and a troponin T point of care assay in the clinical routine of an Emergency Department: A clinical cohort study. Int J Cardiol. 2017;230:454–60. doi: 10.1016/j.ijcard.2016.12.085. [DOI] [PubMed] [Google Scholar]
- 24.Hawass NE. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997;70:360–6. doi: 10.1259/bjr.70.832.9166071. [DOI] [PubMed] [Google Scholar]
- 25.Obuchowski NA, Zhou XH. Prospective studies of diagnostic test accuracy when disease prevalence is low. Biostatistics. 2002;3:477–92. doi: 10.1093/biostatistics/3.4.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.