Abstract
Abstract
Objectives
To evaluate the improvements in the mean Short Form-36 (SF-36) score (95% CI) from predischarge to postdischarge among prospective participants of a Swiss Outpatient Parenteral Antimicrobial Therapy (OPAT) programme using Patient Reported Outcomes.
Design
Prospective cohort study.
Setting
A public tertiary care hospital in Switzerland.
Participants
Patients enrolled in the University Hospital Zurich’s OPAT programme between October 2020 and September 2022. They were interviewed predischarge (interview 1) and 7–14 days postdischarge (interview 2) using a shortened, four-domain version of the validated SF-36) questionnaire, complemented by four additional questions gauging patient satisfaction.
Co-primary outcomes
The primary outcomes were the scores in four domains of the SF-36 questionnaire.
Results
33 patients participated in the study. Univariate analysis revealed substantial improvement in three of the four SF-36 domains. Specifically, participants reported improvements in the mean SF-36 score (95% CI) from interviews 1 to 2 for ‘emotional role’ (24.2 (5.0–43.5)), ‘social functioning’ (22.0 (95%CI 10.8 to 33.2)) and ‘emotional well-being’ (11.9 (95%CI 5.6 to 18.2)). Furthermore, 97% of patients would recommend OPAT to others.
Conclusion
Patients experienced significant improvements in Health-Related Quality of Life (HRQoL) while enrolled in OPAT and the programme yields high patient satisfaction. Hospitals considering new OPAT programmes should include both patient satisfaction and HRQoL impact in their argument repertoire for the introduction of OPAT.
Keywords: Patient Reported Outcome Measures, Quality of Life, INFECTIOUS DISEASES
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We obtained data from a broad range of Outpatient Parenteral Antimicrobial Therapy (OPAT) patients regarding comorbidities, infections, OPAT settings and administration devices.
By comparing the characteristics of patients of all OPAT patients (Zurich outpatient parenteral antimicrobial therapy cohort) with the OPAT patients included in this study (Zurich outpatient parenteral antimicrobial therapy cohort; illuminating HRQoLife cohort) we were able to show that the two groups did not differ significantly.
Our trained interviewers were not directly involved in patient care, ensuring unbiased answers from participants.
Noncomplex OPAT cases, such as neurosyphilis with a standard 14-day benzylpenicillin treatment course, were ineligible for this study, as these patients did not require inpatient care.
The small number of patients enrolled hindered us from analysing further subgroups that could provide insights into the impact of OPAT on their Health-Related Quality of Life.
Introduction
Antimicrobial therapies are often extensive, complex, and in case of the need for intravenous administration thus require prolonged hospitalisations. This is associated with high costs, increased infection risks and other adverse events.1 2 Outpatient Parenteral Antimicrobial Therapy (OPAT) has emerged as a pivotal approach in administering intravenous antibiotics outside traditional hospital settings.3,6 OPAT also reflects a comprehensive approach to patient care aligning with antimicrobial stewardship (AMS) principles.7,9 OPAT represents a paradigm shift in the delivery of antimicrobial therapy, allowing patients to receive necessary treatments from the comfort of their homes. This transition from inpatient to outpatient care is a key contributor to the positive experiences reported by patients undergoing OPAT.10 Several factors might contribute to the patient satisfaction associated with OPAT. Primarily, it highlights the freedom it provides patients without the constraints of a hospital stay. Patients maintain their normal daily routine, minimising disruptions to work, family life and social engagement. Moreover, the close outpatient care provided by the professional OPAT team7 are factors influencing patient satisfaction with OPAT. By administering treatment in a home environment, the likelihood of exposure to hospital-acquired infections is reduced,11 possibly fostering a sense of safety and well-being among patients.
To determine which approach best meets the patient’s needs, values and preferences, it is essential to incorporate the patient’s perspective. Patient Reported Outcome (PRO) is a type of clinical outcome assessment where the report comes directly from the patient. Patients respond to questions about their health condition without any alteration or interpretation.12 13 Instruments used to measure and record PRO are known as Patient Reported Outcome Measures (PROMs). Some PROMs instruments aim to describe or measure health in general (or generic) manner, allowing the same questions to be used for patients with diverse conditions. The concepts measured by these PROs should encompass broader aspects of Health-Related Quality of Life (HRQoL).12 HRQoL, according to CDC,14 refers to an individual’s or group’s perceived physical and mental health over time. HRQoL encompasses various dimensions, such as physical, mental, emotional and social functioning.12 15 Serving as a patient-centred measure, HRQoL complements objective disease indicators and has the potential to predict morbidity and mortality.16 It is recognised that patients with infections may experience impaired HRQoL,12 and such impairments can endure even after the infection has resolved. With this study, we aimed to assess the improvements in the mean SF-36 score (95% CI) from interview 1 (predischarge) to 2 (postdischarge) among prospective participants of a Swiss OPAT programme.
Methods
Setting
The OPAT programme at the USZ in Switzerland started in November 2018. Preceding OPAT assignment, consultation with an infectious disease (ID) physician is advised but not mandatory. Referrals to the OPAT team are made when it is clear during the inpatient stay that parenteral antibiotic therapy will extend beyond the required hospitalisation period or when parenteral antibiotic therapy is needed for targeted treatment, but hospitalisation is not necessary. Both the OPAT programme and the ID consultation service are an integral component of the hospital’s AMS programme.
The dedicated OPAT team comprises nurse practitioners, ID physicians, a consultant pharmacist and an economist.7 The programme is accessible to both hospital inpatients and outpatients, as well as patients referred by general practitioners and other hospitals. Close clinical supervision is mandatory on admission to our OPAT service. Patients are monitored with at least 1 weekly check either in our ID department or by their attending physician.
There are three settings for antimicrobial administration:
Hospital OPAT: patients receive antimicrobial therapy in an outpatient clinic at the hospital, with dedicated nursing staff overseeing the treatment.
Homecare OPAT: antimicrobial therapy by home care nurses or family doctors.
Self-administered OPAT: patients administer antimicrobial therapy themselves after receiving prior training from an OPAT nurse.
Parenteral antimicrobial medicines are preferably administered via a central venous line, such as a peripherally inserted central venous catheter or a port-a-cath. The choice of vascular access is contingent on the infusion system type and therapy duration. Antimicrobial treatment is delivered via intermittent or continuous infusion, facilitated by either elastomeric (Easypump II, B.Braun) or battery-driven infusion pumps (MiniRhythmic). Elastomeric pumps are employed for antibiotics with a time-dependent killing mechanism and can be commercially compounded for self-administered or homecare OPAT settings. Alternatively, nurses prepare pumps on the ward for hospital OPAT, connecting them directly to the patient’s vascular access. Battery-driven infusion pumps are exclusive to homecare-OPAT.
Study design and participants
ZOPATlife (Zurich outpatient parenteral antimicrobial therapy cohort; illuminating HRQoLife) was a single centre, prospective, open interval cohort study conducted at the USZ, which focused on patients receiving parenteral antimicrobial therapy within the local OPAT programme. Patients were included over 23 months from October 2020 to September 2022. The eligibility criteria included patients aged >18 years old, referred to the OPAT programme after a hospital stay of at least 2 days, with a minimum of 7 days of therapy remaining. Additionally, participants needed to comprehend and respond to the assessment questions in German or English, provide written informed consent and obtain general consent for the subsequent use of their personal health data for research purposes. Enrollment was limited to one occurrence per participant. The baseline was established at the time of the first interview and start of OPAT. The study received approval from the Independent Ethics Committee of Zurich (BASEC 2020–02438).
Data collection and definitions
In the ZOPAT prospective cohort study (BASEC-Nr: 2020–00866)7 sociodemographic and clinical data were collected. The data extracted from the health records included age, gender, body mass index (BMI), marital status, education level, employment status, infection type, antimicrobial substance, administration device, intravenous access, antibiotic therapy duration, complications and comorbidities. Complex Outpatient Antibiotic Therapy (cOPAT) offers long-term oral antibiotic management with frequent monitoring and reviews. The cOPAT team, part of the OPAT service, provides intravenous antibiotics for patients who do not need hospitalisation.
A condensed version of the validated Short Form-36 (SF-36)17 questionnaire in German or English, as shown in online supplemental table S1, was employed to assess patients’ HRQoL. The original SF-3617 comprises eight health domains, each ranging from 0 to 100, with higher values indicating better health. The validated German translation18 was used for the survey in German. The questionnaire was shortened, excluding non-essential domains for evaluating HRQoL changes due to the OPAT programme without changing the structure of the questionnaire domains. Additionally, time indications in questions were modified to cover the past 7 days instead of 30 days. This adaptation focused on four domains (1) mental health (‘emotional well-being’; five items) (2) role limitations due to physical problems (‘role physical’; four items) (3) role limitations due to emotional problems (‘role emotional’; three items) and (4) social functioning (two items). Consequently, Mental and Physical Component Summary Scale Scores could not be calculated.
A trained investigator conducted the interviews, strictly adhering to the SF-36 to ensure no misinterpretation. The first interview took place shortly before discharge (as an inpatient) and the second interview took place 7 to 14 days after discharge (as an outpatient). The second interview included additional questions about evaluating the local OPAT programme. The inpatient interviews were conducted in person, and the outpatient interviews were conducted either in person or by phone. Both interviews needed to be completed for inclusion in the study (no missing data). We made three attempts to contact the patients. Study size was determined by the number of patients included after this foreseen period of time (23 months).
We created a Microsoft Access relational database to collect baseline information such as gender, age, height and weight, comorbidities, Charlson Comorbidity Index (CCI),19 pathogens, antimicrobial substances, mode of administration, duration of therapy and type of vascular access. We evaluated patient outcomes, including adverse drug events, line-related events, readmission and clinical cure at the conclusion of OPAT and 30 days afterwards. The same database was used for the collection of the answers from the interviews. To detect any selection bias, populations ZOPAT and ZOPATlife were compared.
The SF-36 scores reached by the ZOPATlife patients were also compared with the SF-36 scores observed in the Swiss population.20
Statistics
We employed descriptive statistics for demographic and clinical data. Continuous variables were expressed as medians and IQRs. Categorical variables were presented as numbers and percentages. The Fisher’s exact test was used for comparing categorical variables, while the Wilcoxon rank-sum test was used for continuous variables. We calculated the average scores with 95% CIs for each dimension of the SF-36 score and calculated differences between interview 1 and interview 2 by Wilcoxon rank-sum tests. P values <0.05 were considered to indicate statistically significant results. Statistical analyses were conducted using Stata/SE 17.0 (StataCorp, College Station, Texas, USA).
Patient and public involvement
At the design stage of the study, one patient was involved to get feedback about comprehensibility of the patient information accompanying the informed consent.
Reporting guidelines
We used the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist when writing our report.21
Results
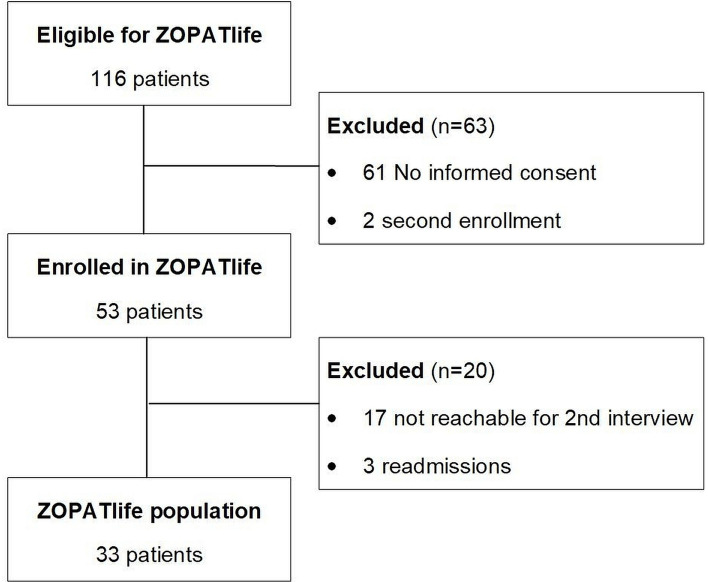
During the 23-month study period, 223 patients were enrolled in the local OPAT programme (ZOPAT cohort). Therefore, 116 participants were eligible for the HRQoL assessment. Of these, 53 patients (45.7%; 53/116) provided informed consent to participate, while 61 (52.6%) did not. Additionally, two patients (1.7%) were already included in ZOPATlife during their initial OPAT episode, meeting an exclusion criterion. Of the enrolled patients, 33 patients completed both the first as well as the second interview. 20 patients completed only the first interview, and their datasets were subsequently excluded from the analysis. The reasons for not completing both interviews included patients not being reachable for the second interview within the predefined time from 7 to 14 days after discharge (n = 17) or patients being readmitted to the hospital (n = 3) (figure 1).
Figure 1. Flow chart of patient recruitment. Zurich OPAT cohort HRQoL, ZOPATlife. HRQoL, Health-Related Quality of Life; OPAT, Outpatient Parenteral Antimicrobial Therapy; ZOPATlife, Zurich outpatient parenteral antimicrobial therapy cohort; illuminating HRQoLife.
Baseline characteristics
Table 1 summarises the characteristics of the patients who were enrolled in ZOPATlife (n=33) and those enrolled in ZOPAT but not in ZOPATlife (n=190). The majority of the ZOPATlife patients were male (n=26; 79%), with a median age of 56 years (IQR 43–71). Endovascular and osteoarticular infections constitute the primary types of infections. Most patients received treatment either in the hospital-OPAT (14/33; 46%) or in the homecare-OPAT setting (12/33; 36%). More than half of the ZOPATlife patients were treated with antimicrobial substances administered via an elastomeric pump (18/33; 55%).
Table 1. Patient characteristics and their treatment.
| Characteristics | Patients with two interviewsn = 33 | Patients with OPATn = 190 | P value |
| Gender | |||
| Female sex, n (%) | 7 (21) | 68 (21) | 0.11 |
| Male sex, n (%) | 26 (79) | 122 (64) | |
| Age, median years (IQR) | 56 (43–71) | 57 (18–92) | 0.63 |
| BMI, median kg/m2 (IQR) | 23 (22–25) | 25 (16–53) | 0.006 |
| Charlson comorbidity index, median (IQR) | 3 (1–4) | 3 (0–13) | 0.48 |
| OPAT setting | |||
| Homecare-OPAT, n (%) | 12 (36) | 92 (48) | 0.16 |
| Hospital-OPAT, n (%) | 14 (42) | 78 (41) | |
| Self-administered, n (%) | 7 (21) | 20 (11) | |
| Administration device | |||
| Battery-operated pump, n (%) | 1 (3) | 11 (5.8) | 0.06 |
| Elastomeric pump, n (%) | 18 (55) | 61 (32) | |
| none, n (%) | 14 (42) | 118 (62) | |
| Indication for OPAT (stratified by ICD-10 codes) | 0.22 | ||
| Foreign body-associated infections,* n (%) | 8 (24) | 29 (15) | |
| Other infections and parasitic diseases, n (%) | 5 (15) | 36 (19) | |
| Urinary tract infections, n (%) | 4 (12) | 54 (28) | |
| Hepatobiliary infections, n (%) | 4 (12) | 9 (5) | |
| Infective endocarditis, n (%) | 4 (12) | 9 (5) | |
| Osteoarticular infections, n (%) | 3 (9) | 16 (8) | |
| Central nervous system infections, n (%) | 3 (9) | 21 (11) | |
| Respiratory tract infections, n (%) | 1 (3) | 7 (4) | |
| Ear, nose and throat infections, n (%) | 1 (3) | 4 (2) | |
| Intraabdominal infections, n (%) | 0 (0) | 5 (3) | |
| OPAT duration, median days (IQR) | 15 (6–27) | 9 (6–19) | 0.25 |
| Adverse events, line-related, n (%) | 2 (6) | 7 (3.7) | 0.53 |
| Adverse events, drug-related, n (%) | 3 (9) | 30 (16) | 0.82 |
Includes prosthetic joint infections, vascular graft infections, breast implant infections.
BMIbody mass indexICDInternational Classification of DiseasesOPATOutpatient parenteral antimicrobial therapy
Half of the patients were married (17/33; 52%), while the remaining half were single, divorced or widowed (16/33; 48%). 61% of the patients were still employed (21/33), 33% had already retired (11/33) and 6% were currently without employment (2/33). The groups ‘ZOPATlife’ and ZOPAT, ‘not ZOPATlife’ did not differ in demographic characteristics, such as age (p=0.635), gender (p=0.114) or CCI (p=0.441) but in median BMI (p=0.006). Furthermore, there was no discernable difference between the two groups in terms of the OPAT setting, OPAT duration or number of adverse events. However, a distinction was observed in the choice of administration device. Patients enrolled in ZOPATlife were more frequently treated using an elastomeric pump to deliver the antimicrobial substance than were those not enrolled in ZOPATlife (18/33 (55%) versus 60/190 (32%), p = 0.038; table 1). No differences were identified when comparing populations that underwent only one interview to those that had two interviews, except in ‘role emotional’ baseline (see online supplemental tables S2 and S3). Figure 1 shows why some individuals did not complete both paired interviews (n=20): three were because of readmission of which one was due to clinical deterioration, and another 17 were not reachable.
HRQoL assessment
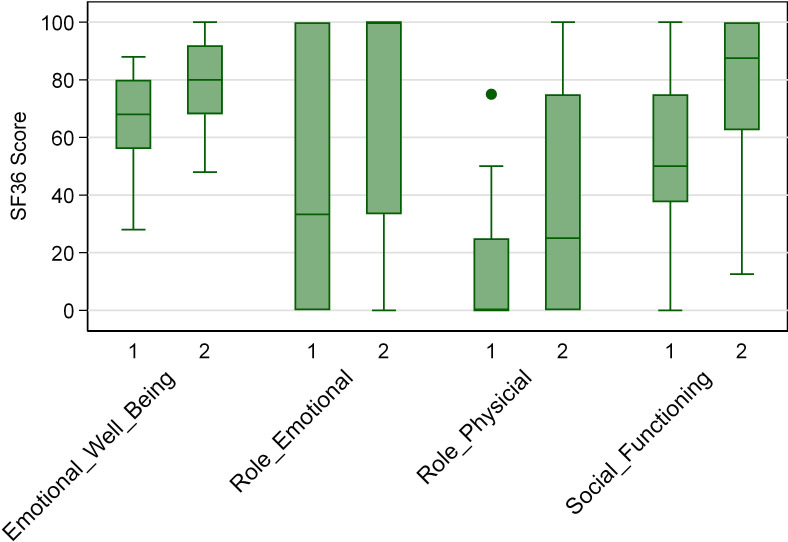
Table 2 reports the mean scores for the four domains measured by the shortened SF-36 questionnaire of inpatients before discharge and outpatients 7–14 days after discharge (n = 33 for each group). Swiss population averages are included for comparison.20 The SF-36 scores for all domains were higher for outpatients compared with the baseline for inpatients. However, the SF-36 scores for both inpatients and outpatients did not reach the values observed in the Swiss population. Figure 2 presents the median values and IQRs of the SF-36 scores obtained during the first interview as inpatients and the second interview as outpatients.
Table 2. Outcomes of interviews as mean scores (95% CI) for each domain compared with the Swiss population.
| Domain | Inpatient (n= 33)mean (95% CI) | Outpatient (n= 33)mean (95% CI) | ΔMean (95% CI) | P value | Swiss populationmean (95% CI) |
| Emotional well-being | 66.5 (60.6 to 72.3) | 78.4 (72.9 to 83.9) | 11.9 (5.6 to 18.2) | 0.0034 | 87.64 (86.50 to 88.78) |
| Role emotional | 47.5 (31.3 to 63.7) | 71.7 (56.9 to 86.5) | 24.2 (5.0 to 43.5) | 0.0376 | 86.41 (85.22 to 87.60) |
| Role physical | 19.2 (10.6 to 27.8) | 36.4 (22.5 to 50.2) | 17.2 (4.7 to 29.7) | 0.1232 | 75.02 (74.07 to 75.98) |
| Social functioning | 56.8 (46.1 to 67.5) | 78.8 (71.0 to 86.6) | 22.0 (10.8 to 33.2) | 0.0028 | 85.84 (84.66 to 87.03) |
Figure 2. Box and whisker plots for the SF-36 scores for the four domains ‘emotional well being’, ‘role emotional’, ‘role physical’ and ‘social functioning’ at interviews 1 (as inpatients) and 2 (as outpatients). SF-36, Short Form-36.
Analyses revealed a substantial improvement in three out of the four investigated SF-36 domains. Specifically, participants reported significant improvements in emotional well-being (p = 0.0034), their role emotional (p = 0.0376) and social functioning (p = 0.0028).
The box represents the IQR, which is the range between the first quartile (Q1) and the third quartile (Q3) of the data. The length of the box indicates the spread of the central 50% of the data. The line inside the box represents the median. The whiskers include values up to 1.5 times the IQR.
Patient evaluation of the program
97% of the patients stated that important questions were comprehensively answered by the OPAT team and anxieties or fears about their condition or treatment were discussed (32/33). 82% of patients felt adequately involved in the decision-making process regarding their therapy (27/33), while in 76% of cases, patients indicated that they were thoroughly informed about the purpose of the drug therapy (25/33). Additionally, 58% of the patients reported being educated about possible side effects of the medicines (19/33) and about red flags or danger signals of the treatment course in 79% of cases (26/33) by someone from the OPAT team. Overall, the majority of patients felt secure during OPAT treatment (94%; 31/33) and would recommend OPAT to other patients in a similar situation (97%; 32/33).
Discussion
Hospital stays for parenteral antimicrobial treatment pose a considerable burden on care and costs for hospitals and impact the HRQoL of patients.10 22 OPAT provides a safe and efficient alternative,3 7 allowing patients to maintain their usual daily roles.23 24 This single-centre study is the first, to our knowledge, to compare the HRQoL of inpatients before discharge and shortly after initiating OPAT. The findings of this study demonstrated significant improvements in HRQoL for patients, as evidenced by comparing SF-36 scores shortly before discharge to those during OPAT. In particular, there is an improvement in ‘social functioning’ allowing individuals to participate in family activities and be present for friends and neighbours during home-based therapy. However, it seems that this impact is not only tied to physical proximity to loved ones but also encompasses emotional preparedness. The significantly enhanced scores for ‘role emotional’ indicate that patients felt emotionally stable enough to carry out their roles as spouses, parents, friends or employees.
Although these analyses were mostly descriptive, the inference of an improvement in emotional well-being becomes apparent. While the scores for emotional well-being were already the highest among the four domains shortly before discharge, they demonstrated a further significant improvement after discharge. This finding suggests that the combination of disease recovery and home treatment has a significant impact on HRQoL. This seemed to be independent of the potentially higher workload from the individual’s perspective during OPAT: patients have to actively organise medicines and supplies and home care together with the OPAT nurse, as well as managing daily activities such as cooking or personal care. All of which can be compensated for by additional support. For patients with a second person in the household, this support could play a compensatory role. Looking at the patients enrolled, the support seemed to be available as half of the patients were married, indicating that they were not living alone. Still, living alone might be a hindrance for an OPAT as was also mentioned by Twiddy and colleagues.25
The only domain that showed a trend without significant improvement was ‘physical functioning’. This finding aligns with findings from the study of Goodfellow and colleagues,26 which indicated that the component summaries reflecting physical strength did not significantly change during OPAT. This is not surprising, considering, that patients are often comorbid (CCI of 3, IQR 1–4) and require antimicrobial treatment for life-threatening and recurrent infections, possibly associated with additional symptoms. This may explain why we cannot estimate the percentage of patients returning to work after discharge, as was done by Wee and colleagues.27 In addition, patients are frequently on sick leave shortly after hospital discharge in Switzerland. Moreover, the period between the first and the second interviews (7–14 days after discharge) was too short to evaluate this aspect, and a third of the patients were already retired (11/33; 33%).
Nonetheless, patients felt highly supported by the OPAT team and questions regarding their fears about their condition or treatment were answered comprehensively. Mirroring this, OPAT patients did feel that their own will and purpose for their therapy influenced their decision-making together with the treatment team. In contrast, some patients did state that they were not sure about the possible side effects of the drugs or danger signals that should alert them to contact the OPAT team. It is unclear, whether these patients were not educated at all or whether it happened in a manner that was not comprehensive enough for the patient or not sustainable over the period of 2 weeks. In any case, measures will be taken to improve the education of the patient. However, most of the patients felt secure during their OPAT treatment. Overall, patient satisfaction was very high, with 97% of OPAT patients expressing a willingness to recommend the service to other patients in a similar situation. This aligns with findings from Saillen and colleagues,28 where 97% of patients expressed a preference for this type of care, and would have recommended the local OPAT programme to others. This finding is also consistent with the results of Quintens and colleagues.8 28
We also tried to minimise a possible selection bias by comparing the ZOPAT cohort to the ZOPATlife cohort. The groups showed no significant differences in demographic characteristics but patients included in ZOPATlife more frequently received their therapy through an elastomeric pump. This likely stems from the fact that these therapies are often initiated for patients undergoing extended antimicrobial treatment, increasing their likelihood of enrolling in ZOPATlife. Our study has several limitations. The OPAT team of the USZ primarily handles cOPAT, resulting in a case mix characterised by high comorbidity and complex situations, involving recurrent infections and/or highly resistant pathogens. The small patient number hindered us from analysing further subgroups that could provide insights into the impact of OPAT on their HRQoL. In contrast, non-complex OPAT cases, such as neurosyphilis with a standard 14-day benzylpenicillin treatment course, were ineligible for this study, as these patients did not require inpatient care. The data suggest a potential association between outpatient treatment and better HRQoL outcomes, though this finding should be interpreted cautiously. As patients convalesce, their quality of life may naturally improve, which could occur with inpatient care as well. While it is plausible that OPAT has contributed to better scores, we could not demonstrate this in a controlled manner. We could not establish a control group of potential OPAT candidates without outpatient therapy. Furthermore, it is important to note that the original SF-36 was not designed to capture HRQoL for the last 7 days, as was the case in this study. It has been validated for mapping HRQoL only over the past month. Nevertheless, it seems unlikely that the modified timeframe influenced the usefulness of the questionnaire since the questions of the used domains pointed to feelings and well-being that might change from day to day and do not need a longer time period to evolve as physical fitness for instance. We could not perform the second interview in 38% of patients, which may lead to relevant selection bias. However, when comparing patients with one or two interviews, we did not detect significant differences besides the emotional role.
It is important to note that we conducted the first interview shortly before discharge. At this point, the only reason for the patient’s inpatient therapy was the need for parenteral antimicrobial therapy. This indicates that the patient’s health condition might not have changed significantly in the following 7 to 14 days, which would not account for the major improvements in HRQoL domain scores. Regarding the patient’s evaluation of the programme, it should be noted that the interviews were not anonymous, which could influence the answers given by the patient. However, a trained person who was not directly involved in the patient’s treatment conducted the interview in order to counteract this bias. Finally, it should be mentioned that patient enrollment occurred during the COVID-19 pandemic. During these times, patient visits on the wards were highly restricted, potentially exaggerating the impact of discharge on HRQoL domains such as social functioning.
One future objective in studying OPAT programmes should involve the identification of risk factors for complications, such as readmission during OPAT due to adverse events or clinical deterioration. In this context, exploring whether the enhancement of HRQoL influences treatment outcomes, such as clinical cure, and understanding how patients can be best supported to successfully complete their therapy at home, as also suggested by Keller and colleagues,29 would be advantageous.
Conclusion
Patients experienced significant improvements in HRQoL domains from just before discharge to the period in OPAT. It remains unclear whether this was due to the OPAT programme itself or to the circumstances of improved health in general. Either way, the shift to outpatient care improves communication between healthcare providers and patients. Regular follow-up visits and close monitoring of patients in their home environment create a more personalised and attentive care experience. This increased interaction and support helps patients better understand their treatment plan and fosters a sense of partnership, resulting in high patient satisfaction as shown in this study. As hospitals consider the establishment or expansion of OPAT programmes, recognising the impact on patient satisfaction becomes crucial. Incorporating patient satisfaction metrics into the evaluation of OPAT programmes can provide valuable insights into the success and effectiveness of these initiatives. Moreover, patient and public involvement should be used in the planning of a future study for assessing outcomes meaningful for the patient. By prioritising patient-centred care and continuously improving the OPAT experience, healthcare institutions can enhance not only the clinical outcomes but also the overall satisfaction and well-being of individuals undergoing outpatient antimicrobial therapy.
supplementary material
Acknowledgements
The authors thank the participants of ZOPATlife and the clinical nurses, Sabine Willach and Albina Boesch-Tajic for their excellent work. We also thank Judith Bergada-Pijuan for support in statistical questions and Claudine Reiber for the initial effort to start this study.
Footnotes
Funding: This work was supported by the Clinical Research Priority Program (CRPP) of the University of Zurich for the CRPP Precision medicine for bacterial infections (to AZ/BH). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084727).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: The study was conducted in accordance with the Declaration of Helsinki and approved by the Independent Ethics Committee of Zurich approved the quality assurance study (BASEC 2020-024386; 2020). Informed consent was obtained from all subjects involved in the study.
Data availability free text: The data are not publicly available to keep confidentiality and to comply with ethical considerations. Anonymised data are available upon request from the first author.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Andrea R Burch, Email: andrea.burch@usz.ch.
Bruno Ledergerber, Email: ledergb@gmail.com.
Martin Ringer, Email: martin.ringer@usz.ch.
Annelies S Zinkernagel, Email: annelies.zinkernagel@usz.ch.
Nadia Eberhard, Email: nadia.eberhard@kssg.ch.
Marisa B Kaelin, Email: marisa.kaelin@usz.ch.
Barbara Hasse, Email: barbara.hasse@usz.ch.
Data availability statement
Data are available upon reasonable request.
References
- 1.Tamma PD, Avdic E, Li DX, et al. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern Med. 2017;177:1308–15. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briquet C, Cornu O, Servais V, et al. Clinical characteristics and outcomes of patients receiving outpatient parenteral antibiotic therapy in a Belgian setting: a single-center pilot study. Acta Clin Belg. 2020;75:275–83. doi: 10.1080/17843286.2019.1608396. [DOI] [PubMed] [Google Scholar]
- 3.Erba A, Beuret M, Daly ML, et al. OPAT in Switzerland: single-center experience of a model to treat complicated infections. Infection. 2020;48:231–40. doi: 10.1007/s15010-019-01381-8. [DOI] [PubMed] [Google Scholar]
- 4.Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapya. Clin Infect Dis. 2019;68:1–4. doi: 10.1093/cid/ciy867. [DOI] [PubMed] [Google Scholar]
- 5.Voumard R, Gardiol C, André P, et al. Efficacy and safety of continuous infusions with elastomeric pumps for outpatient parenteral antimicrobial therapy (OPAT): an observational study. J Antimicrob Chemother. 2018;73:2540–5. doi: 10.1093/jac/dky224. [DOI] [PubMed] [Google Scholar]
- 6.Hatcher J, Costelloe C, Cele R, et al. Factors associated with successful completion of outpatient parenteral antibiotic therapy (OPAT): A 10-year review from a large West London service. Int J Antimicrob Agents. 2019;54:207–14. doi: 10.1016/j.ijantimicag.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Burch AR, Ledergerber B, Ringer M, et al. Improving antimicrobial treatment in terms of antimicrobial stewardship and health costs by an OPAT service. Infection. 2024;52:1367–76. doi: 10.1007/s15010-024-02194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintens C, Steffens E, Jacobs K, et al. Efficacy and safety of a Belgian tertiary care outpatient parenteral antimicrobial therapy (OPAT) program. Infection. 2020;48:357–66. doi: 10.1007/s15010-020-01398-4. [DOI] [PubMed] [Google Scholar]
- 9.Seaton RA, Gilchrist M. Making a case for outpatient parenteral antimicrobial therapy (OPAT) J Antimicrob Chemother. 2024;79:1723–4. doi: 10.1093/jac/dkae183. [DOI] [PubMed] [Google Scholar]
- 10.Alzahrani N. The effect of hospitalization on patients’ emotional and psychological well-being among adult patients: An integrative review. Appl Nurs Res. 2021;61:S0897-1897(21)00095-1. doi: 10.1016/j.apnr.2021.151488. [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Robertson C, Kennedy S, et al. Personalized infection prevention and control: identifying patients at risk of healthcare-associated infection. J Hosp Infect. 2021;114:32–42. doi: 10.1016/j.jhin.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Powers JH, III, Howard K, Saretsky T, et al. Patient-Reported Outcome Assessments as Endpoints in Studies in Infectious Diseases. Clin Infect Dis. 2016;63:S52–6. doi: 10.1093/cid/ciw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration . Focus area: patient-reported outcomes and other clinical outcome assessments silver. Spring MD: FDA; https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-patient-reported-outcomes-and-other-clinical-outcome-assessments Available. [Google Scholar]
- 14.US Government . Centers for Disease Control and Prevention (CDC) Washington: US Government; 2020. https://www.cdc.gov Available. [Google Scholar]
- 15.Megari K. Quality of Life in Chronic Disease Patients. Health Psychol Res. 2013;1:e27. doi: 10.4081/hpr.2013.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevarez-Flores AG, Chappell KJ, Morgan VA, et al. Health-Related Quality of Life Scores and Values as Predictors of Mortality: A Scoping Review. J Gen Intern Med. 2023;38:3389–405. doi: 10.1007/s11606-023-08380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JJK, Keller SD. SF-36(R) physical and mental health summary scales: a user’s manual. Boston: The Health Institute; 1994. [Google Scholar]
- 18.Morfeld M, Kirchberger I, Bullinger M. SF-36 Fragebogen Zum Gesundheitszustand: Deutsche Version Des Short Form-36 Health Survey, 2nd edn. Hogrefe-Verlag; 2011. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Roser K, Mader L, Baenziger J, et al. Health-related quality of life in Switzerland: normative data for the SF-36v2 questionnaire. Qual Life Res. 2019;28:1963–77. doi: 10.1007/s11136-019-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque de Almeida F, Al MJ, Koymans R, et al. Impact of hospitalisation on health-related quality of life in patients with chronic heart failure. Health Qual Life Outcomes. 2020;18:262. doi: 10.1186/s12955-020-01508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Board N, Brennan N, Caplan GA. A randomised controlled trial of the costs of hospital as compared with hospital in the home for acute medical patients. Aust N Z J Public Health. 2000;24:305–11. doi: 10.1111/j.1467-842X.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 24.Corwin P, Toop L, McGeoch G, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ . 2005;330:129. doi: 10.1136/bmj.38309.447975.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twiddy M, Czoski Murray CJ, Mason SJ, et al. A qualitative study of patients’ feedback about Outpatient Parenteral Antimicrobial Therapy (OPAT) services in Northern England: implications for service improvement. BMJ Open. 2018;8:e019099. doi: 10.1136/bmjopen-2017-019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodfellow AF, Wai AO, Frighetto L, et al. Quality-of-life assessment in an outpatient parenteral antibiotic program. Ann Pharmacother. 2002;36:1851–5. doi: 10.1345/aph.1C153. [DOI] [PubMed] [Google Scholar]
- 27.Wee LE, Sundarajoo M, Quah WF, et al. Health-related quality of life and its association with outcomes of outpatient parenteral antibiotic therapy. Eur J Clin Microbiol Infect Dis. 2020;39:765–72. doi: 10.1007/s10096-019-03787-6. [DOI] [PubMed] [Google Scholar]
- 28.Saillen L, Arensdorff L, Moulin E, et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis. 2017;36:1387–92. doi: 10.1007/s10096-017-2944-5. [DOI] [PubMed] [Google Scholar]
- 29.Keller SC, Williams D, Levering M, et al. Health-Related Quality of Life in Outpatient Parenteral Antimicrobial Therapy. Open Forum Infect Dis. 2018;5:ofy143. doi: 10.1093/ofid/ofy143. [DOI] [PMC free article] [PubMed] [Google Scholar]