Abstract
Abstract
Introduction
The autoimmune encephalitides (AE) are a heterogeneous group of neurological disorders with significant morbidity and healthcare costs. Despite advancements in understanding their pathophysiology, uncertainties persist regarding long-term prognosis and optimal management. This study aims to address these gaps, focusing on immunotherapeutic strategies, neoplastic associations and functional outcomes.
Methods and analysis
The Retrospective Linkage Study of Autoimmune Encephalitis project will use data linkage techniques to establish a retrospective 10-year population cohort of Australian patients with AE. Two cohorts will be analysed, the Reference Cohort (clinically confirmed AE cases obtained from hospital medical records, n=145) and the Operationally Defined Cohort (AE cases identified through administrative coding data, n≈5000). Univariate statistical methods will identify candidate coding elements for use in the operational case definition and multivariate models and evaluation methods used to identify and internally validate the optimal coding algorithms. The two study cohorts will be analysed separately due to the high likelihood of overlap. Primary outcomes include relapse rate, prevalence and control of epilepsy, cognitive disability, poor educational attainment, delayed tumour diagnosis and mortality. Statistical analyses, including random mixed-effects regression models, will assess treatment effects, covariates and outcomes.
Ethics and dissemination
This project has been approved by the leading investigators’ institutional Human Research Ethics Committee (HREC), the St Vincent’s Hospital Melbourne HREC, as well as the Australian Institute of Health and Welfare HREC and relevant jurisdictional HRECs where required. The dissemination of findings through peer-reviewed publications and patient advocacy channels will maximise the impact of this research.
Keywords: Neurology, EPIDEMIOLOGY, IMMUNOLOGY
STRENGTHS AND LIMITATIONS OF THE STUDY.
The Retrospective Linkage Study of Autoimmune Encephalitis project will leverage Australia’s robust data linkage infrastructure to create the first comprehensive population-level cohort of patients with autoimmune encephalitides (AE).
The accuracy of identifying these AE cases in administrative datasets will be enhanced by the utilisation of a Reference Cohort (consisting of clinically confirmed AE cases) to develop and validate an operational case definition.
Consumer consultation and the inclusion of diverse standardised datasets (including educational attainment, childhood development, cancer registries and disability datasets) ensure a patient-centred focus on functional outcomes and the influence of therapy on patients’ lives.
Relative limitations include temporal resolution challenges limiting the analysis of treatment timing during the acute phase of AE and a reporting gap for medications administered in the inpatient setting which will be addressed using data extracted from medical records for the Reference Cohort.
Introduction
The autoimmune encephalitides (AE) are a diverse group of neurological disorders featuring a largely antibody-mediated process targeting specific cell surface antigens (often receptors, proteins or channels).1 This autoimmune attack results in several clinically recognisable acute neurological syndromes (figure 1). Symptoms can be severe, including status epilepticus, dysautonomia and coma, and is associated with significant healthcare costs.2 3 The last two decades have seen significant progress in understanding the pathophysiology of these conditions; however, the long-term prognosis is less well described, and optimal management strategies are still debated.
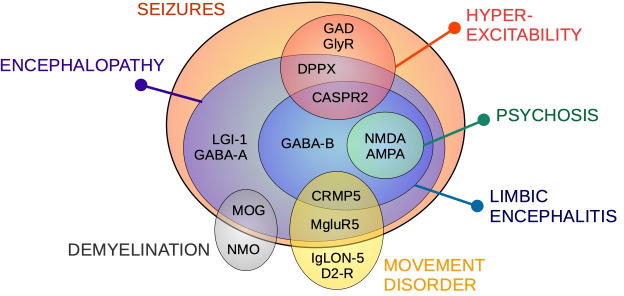
Figure 1. Autoimmune encephalitis subtypes. Clinical presentations associated with autoimmune encephalitis antibodies. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CASPR2, contactin-associated protein-like 2 receptor; CRMP5, collapsin response mediator protein 5; D2-R, dopamine receptor 2; DPPX, dipeptidyl-peptidase-like protein-6; GABA, gamma aminobutyric-acid receptor; GAD, glutamic acid decarboxylase; GlyR, glycine receptor; LGI-1, leucine-rich glioma-inactivated 1; MgluR5, metabotropic glutamate receptor 5; MOG, myelin oligodendrocyte glycoprotein; NMDA, N-methyl-D-aspartate; NMO, neuromyelitis optica.
Immunotherapy appears to provide benefits in the acute phase of illness, and a tiered approach to treatment intensity has been widely adopted, following expert recommendations informed by observational studies.4 5 However, accumulating evidence indicates that long-term residual impairments are common in patients with AE, even after intensive treatment. Around two-thirds experience residual cognitive impairment,6 7 around half require long-term psychiatric medication,8 and 18% require long-term treatment for movement disorders.8 Maintenance therapy is important for preventing the recurrence of symptoms and maintaining disease stability over the long term. However, the lack of adequate evidence has led to uncertainties in guiding patient selection and duration of therapy in this maintenance phase, leading to heterogeneous management practices.9 Further, there are currently no recommendations to guide non-immunotherapeutic management such as acute symptomatic seizure management, post-encephalitic epilepsy surgery and rehabilitation.
The association between AE and underlying neoplasms is an important consideration in the management of these disorders. It is recognised that 20–30% of patients with AE have an underlying tumour at the time of AE onset. This is perhaps best described for ovarian teratoma in patients with AE associated with antibodies to the N-methyl-D-aspartate (NMDA) receptor,10 11 although various tumours have been associated with other forms of AE.1 12 13 Removal or treatment of the underlying tumour has been associated with improved prognosis in patients with AE.14 Unfortunately, the full spectrum of tumour associations in AE are not comprehensively characterised, and screening guidelines are currently untargeted.5 15 Furthermore, patients without identifiable tumours at the time of AE onset and who respond unfavourably or incompletely to immunosuppression are often rescreened for malignancy intermittently, and the yield of this rescreening is currently unknown.
Obtaining quality evidence to address these knowledge gaps has proven challenging, with clinical heterogeneity, low disease incidence and case dispersal across a range of treating specialities. This makes it difficult to establish large cohorts with the requisite clinical and outcome information to address these outstanding research questions. Data linkage analysis can overcome such hurdles to create large cohorts of patients with uncommon conditions, including the provision of data regarding important medical history and health and related outcomes. Data linkage units, acting under strict privacy governance policies, identify patients with particular diagnoses from administrative health databases and combine this information with that from separate data-rich but siloed health administrative and welfare datasets to create a large and informative ‘joined-up’ dataset with the power to detect important treatment effects and disease associations. Australia has considerable data linkage infrastructure and expertise following several decades of government investment and as such is ideally positioned to undertake a population level study of AE.
The RESILIENCE (Retrospective Linkage Study of Autoimmune Encephalitis) project will use data linkage techniques to create a national cohort of patients with AE. Our team will produce a retrospective cohort of all Australian patients with a presumed diagnosis of autoimmune encephalitis over a 10-year period. Our team will use this cohort to estimate disease prevalence and incidence and provide high-quality evidence on optimal immunotherapeutic and epilepsy management strategies, describe detailed neoplastic associations and describe functional outcomes including disability and educational attainment.
Research questions
The primary aim of the RESILIENCE study is to describe the prognosis of AE in Australia and prognostic factors associated with disease outcomes. The primary outcomes of interest are relapse rate, prevalence and control of epilepsy, cognitive disability, poor educational attainment, delayed tumour diagnosis and mortality.
Methods and analysis
Study cohorts
This study will examine two cohorts of patients with AE. The first is a multicentre retrospective cohort of patients with clinically confirmed definite or probable autoimmune encephalitis,16 referred to as the Reference Cohort. The second cohort will be created using an epidemiological operational definition of AE using administrative coding data, referred to as the Operationally Defined Cohort.
Reference Cohort
The Reference Cohort consists of 145 individuals meeting Graus et al consensus diagnostic criteria for AE.16 This cohort was retrospectively recruited from 12 adult Australian tertiary neurology centres (see online supplemental appendix 1). Patients were included if they met consensus diagnostic criteria for definite or probable AE after a review of the medical record, and symptom onset occurred between January 2008 and December 2019. Definite AE includes patients with encephalitis-associated antibodies, a suggestive clinical presentation (subacute short-term memory loss, altered mental status or psychiatric symptoms) and at least one line of evidence for brain inflammation (new focal neurology, new seizures, cerebrospinal fluid pleocytosis or MRI features suggestive of encephalitis). Probable AE includes antibody-negative cases with a suggestive clinical syndrome and multiple lines of evidence for brain inflammation with reasonable exclusion of other causes. Graus et al also define a category of ‘possible AE’, describing patients with a suggestive clinical syndrome, at least one line of evidence for brain inflammation and reasonable exclusion of other causes. Possible AE is intended as a prompt to investigate further for AE with antibodies and additional tests for brain inflammation, rather than a definitive diagnosis, and thus patients meeting criteria for possible AE were not included in the Reference Cohort. An accompanying Mimicker Cohort consists of approximately 200 patients suspected to have AE but ultimately given an alternative diagnosis, determined through medical records review. Data have been collected for the Reference Cohort for granular data elements not available in accessible linked administrative datasets, including presenting clinical features and inpatient immunotherapy.
Operational case definition
Using the Reference and Mimicker Cohorts, we will develop and validate an operational case definition of AE, this being an algorithm to accurately identify patients with AE using coding elements available from linked administrative datasets. The algorithm will be a list of criteria, each stating a data element or combination of elements that must be present (or absent if so stipulated) for an individual to be included in the cohort, thus functioning as a set of inclusion and exclusion criteria.
Following a presentation to a hospital in Australia, all diagnoses and inpatient procedures are coded using national standard coding systems and submitted to jurisdictional datasets for statistical reporting (see table 1). The use of national standard coding systems for diagnoses and inpatient procedures is a common practice in many healthcare systems worldwide to ensure consistency and interoperability. In Australia, the key coding systems used for this purpose are the International Statistical Classification of Diseases17 and the Australian Classification of Health Interventions,18 used for coding diagnoses and procedures in hospitals and other healthcare settings. Once diagnoses and procedures are coded using these standard systems, the data are submitted to jurisdictional datasets. Each Australian state and territory maintains its own health data repository or database. These datasets serve as valuable resources for statistical reporting, epidemiological studies, healthcare planning and policy development.
Table 1. Source datasets.
| Dataset | Dataset description | Key variables | Custodian | Data linkage unit | Availability | Quality |
| Screening cohort | Patients tested for AE antibodies 2008–2019. | Date, specimen type and test result. | Laboratory | N/A | N/A | High |
| Reference cohort | Patients meeting consensus diagnostic criteria for definite or probable AE. | Clinical presentation (seizures, psychosis, memory impairment), immunotherapy. | Hospital | N/A | N/A | High |
| Hospital | Separations from all public and most private hospitals. | Dates, diagnostic and procedure codes, intensive care and assisted ventilation hours. | State or territory | Jurisdictional | Variable | Generally good48 49 |
| Emergency | Presentations to emergency departments at public hospitals. | Dates of presentations and diagnostic codes. | State or territory | Jurisdictional | Variable | Generally fair50 51 |
| MCD | Medicare is Australia’s universal health insurance provider. The dataset stores personal details for registered individuals. | Year of birth, sex, geographical location. | Commonwealth | AIHW | 2008–2021 | High |
| PBS | Claims for prescription medicines with a government benefit. | Anticonvulsants, immunotherapy, cancer therapy, comorbidities. | Commonwealth | AIHW | 2003–2021 | High |
| MBS | Health service claims that qualify for a government benefit. | Cancer treatment, neurological investigations. | Commonwealth | AIHW | 1984–2021 | High |
| DS NMDS | Disability services utilisation and client assessments. | Functional performance, carer data, employment, income. | Commonwealth | AIHW | 2003–2019 | Variable52 53 |
| ACD | Registry of all new cases of cancer diagnosed in Australia. | Date and type of cancer diagnosis. | State or territory | AIHW | 1982–2021 | High54 |
| AEDC | Reports early childhood development. | Scores in all domains (physical, social, emotional, language/cognitive, communication). | Commonwealth | AIFS | 2009, 2012, 2015, 2018 | High55 |
| NAPLAN | Reports academic performance. | Scores in all domains (reading, writing, spelling, grammar and numeracy). | State or territory | AIFS | 2003–2021 | High56 |
| NDI | Coded data of all deaths in Australia. | Date and cause of death. | State or territory | AIHW | 2008–2021 | Good57 |
ACDAustralian Cancer DatabaseAEautoimmune encephalitidesAEDCAustralian Early Development CensusAIHWAustralian Institute of Health and WelfareDS NMDSDisability Services National Minimum Data SetMBSMedicare Benefits ScheduleMCDMedicare Consumer DirectoryNAPLANNational Assessment Program – Literacy and NumeracyNDINational Death IndexPBSPharmaceutical Benefits Scheme
These codes and other routinely collected administrative data will be obtained for the Reference and Mimicker Cohorts. Data elements with a high sensitivity and specificity for AE will be identified for potential inclusion in the operational case definition, and combinations of these elements will be explored using multivariable logistic regression and measures of best fit (described in detail in the Data analysis section below). The best-performing algorithm will function as an operational definition of AE, able to select patients with AE from administrative datasets using routinely collected coding elements.
Operationally Defined Cohort
The Operationally Defined Cohort will then be created by applying the operational case definition to a screening population, consisting of all individuals tested for AE antibodies in Australia from January 2008 (when antibody testing first became commercially available in Australia) to December 2019. We assume that all patients diagnosed with AE have been tested for AE antibodies at some time and hence should improve the sensitivity of the operational case definition by applying it to people who have been tested. Commercial testing for AE antibodies in Australia is primarily performed in only four laboratories (PathWest, Queensland Pathology, the Institute of Clinical Pathology And Medical Research at New South Wales Health Pathology, and the Sydney Children’s Hospital Network); all four are collaborating in this study, thereby providing comprehensive coverage of the screening population. The size of the Operationally Defined Cohort is estimated to be approximately 5000 individuals.
This screening population will include both adult and paediatric individuals. Patients tested for the following antibodies using cell-based assays will be included: anti-NMDA receptor; anti-leucine-rich glioma-inactivated 1 (LGI-1); anti-contactin-associated protein-like 2 receptor (CASPR2); anti-gamma aminobutyric-acid receptor (GABA); anti–α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA); anti-dipeptidyl-peptidase like protein-6 (DPPX); anti-glutamic acid decarboxylase (GAD); anti-myelin oligodendrocyte glycoprotein (MOG); and anti-aquaporin-4 (AQP4). MOG (resulting in MOG-associated disease) and anti-AQP4 (associated with neuromyelitis optica) are emerging as important causes of AE with demyelinating features19 20 and high-titre GAD as a cause of immunotherapy-responsive autoimmune epilepsy.21 Less specific antibody testing (voltage-gated potassium channel (VGKC) radio-immunoassay, thyroid antibodies) will not be included due to the excessive risk of false positives.
Primary outcomes
Primary outcomes to be examined are relapse rate, frequency of the development of epilepsy and its severity, cognitive disability, educational attainment, timing of tumour diagnosis and death. Operational definitions used to estimate primary and secondary outcomes and covariates will be derived from published algorithms where available or developed with input from study collaborators and validated using the Reference Cohort where possible. Operational definitions have been provided in online supplemental appendix 2. These operational definitions may be modified if development and validation using the Reference Cohort demonstrate better performance of an alternative operational definition or there is interim publication of other validated definitions. This will be justified and discussed when presenting the results in publication.
Relapse rate
Relapse rate has been reported in several studies; however, the reported rate is variable, ranging from 15% to 68%.22,24 Some of this variability relates to the heterogeneous nature of AE but also highlights the difficulties of reporting episodic outcomes in small cohorts with short follow-ups. Risk factors associated with relapses are similarly variable, with findings inconsistent across studies.24,27 High-quality data will allow a more thorough examination of intermediate and long-term relapse rates. We will determine the 1-, 2- and 5-year relapse rates for AE and its subtypes and examine prognostic factors associated with relapse rate.
Epilepsy risk and seizure control
Seizure incidence and response to immunotherapy during the acute phase of AE have been well studied; however, the risk of post-encephalitic epilepsy in the longer term is less clear, with rates reported from 10% to 40%28,30 at 2 years after disease onset. This study will determine the risk of post-encephalitic epilepsy in patients with AE and its subtypes.
Cognitive disability
Cognitive impairment persists for many years after the acute phase of illness in most patients with AE. At least 50% demonstrate clinically significant impairment on formal neuropsychological testing,31 32 even higher in some AE subtypes.6 33 34 Impairment is predominantly in executive function and memory.6 Studies to date have examined small numbers of patients, and residual cognitive impairment and its functional impact have yet to be fully characterised. We will estimate the 2- and 5-year prevalence of cognitive disability in this cohort using Australia’s national disability services dataset, with cognitive disability defined as the need for assistance in the domain of ‘learning, applying knowledge and general tasks and demands’.
Educational attainment
Children constitute around half of all patients with AE. Small studies have shown that 2 years following a diagnosis of AE, 92% still require allied health assistance, 40% require special needs assistance or attend a special needs school, and almost half have residual attention or executive deficits on formal testing.35 However, the impact on educational attainment and childhood development is largely unknown. In this study, we will describe the academic performance of children with AE using results of standardised examinations performed as part of a national scheme for comparison and benchmarking of schools (National Assessment Program – Literacy and Numeracy (NAPLAN)). The NAPLAN reports the academic performance of school-aged children in five academic domains (reading, writing, spelling, grammar and numeracy). The performance of children with AE in these domains will be compared with national benchmarks and the proportion under the benchmark compared with the national average. We will also examine individual students’ performance over time to determine whether the onset of AE is associated with falls in performance, how well this recovers with time and factors associated with improved educational outcomes, including treatment strategy.
Delayed tumour diagnosis
Some types of AE have a strong association with neoplasms, with molecular mimicry in tumour tissue thought to be a potential trigger for the development of these pathogenic auto-antibodies.10 The cancer type and strength of cancer association vary according to the specific AE syndrome.36 37 For example, approximately one-third of patients with anti-NMDA-receptor encephalitis have an associated ovarian teratoma,10 11 14 and half of patients with anti-GABAb encephalitis have small-cell lung cancer.38 The neoplastic associations in less common AE sub-types are not completely understood, with either numerous or no cancer associations reported.36 37
Further, regarding patients where a tumour is not identified at the time of AE diagnosis, there is concern that some of these patients may harbour an occult tumour not identified on initial screening, particularly those who respond poorly to immunotherapy. Indeed, there are case reports of patients with refractory anti-NMDA-receptor encephalitis who have undergone empirical bilateral oophorectomy for imaging-negative ovarian teratoma. A few such cases describe microscopic ovarian teratomas subsequently identified on pathology;39 however, expert authors have stated that in their experience, the yield of empirical oophorectomy in imaging-negative cases is low.12 Given that anti-NMDA-receptor encephalitis primarily affects females of childbearing potential who are unable to consent for themselves at the time oophorectomy is being considered, there is a dire need for systematic data regarding the rates of occult teratomas in these patients. Similarly, in other types of AE, no longitudinal data are available describing the subsequent diagnosis of tumours in patients who are tumour-negative at the time of AE diagnosis to guide surveillance recommendations. We will describe the incidence and types of tumours diagnosed more than 90 days after the onset of AE, using diagnoses and dates from the National Cancer Registry and hospital discharge diagnosis codes.
Death
Mortality in most cases of acute AE is 5–15% although is as high as 40–60% in some of the less common sub-types.40 Death is usually due to severe disease, especially status epilepticus or dysautonomia resulting in respiratory failure, or associated malignancy. Unfortunately, mortality has been infrequently and inconsistently measured across various studies, and reliable prognostic factors to assist in clinical decision-making have proven elusive.41 We will calculate crude and cause-specific 2-year and 5-year mortality rates from the date of AE onset.
Secondary outcomes
In addition to the primary aims and outcomes described above, we will undertake a number of secondary analyses. We will describe the epidemiology of AE in Australia including its prevalence, annual incidence and demographic features. Further aspects of disease prognosis will be described including movement disorders, mood disorders and psychotic illness, which will be estimated using prescriptions data. In patients who develop AE-associated epilepsy, we will examine seizure control and factors associated with seizure control, including epilepsy surgery. In patients accessing disability services, we will describe the domains of disability reported to the disability dataset. We will describe the disability support services used by these patients, their carer arrangements, income sources and labour force status. In the paediatric subgroup, we will also examine childhood development, as reported to the Australian Early Development Census.
Covariates
Covariates to be included in the study of prognostic factors include age at onset of AE, gender, socioeconomic status (estimated by mapping postcode of residence to the Socioeconomic Index for Area score)42, AE severity, comorbid disease (estimated using a validated prescription coding algorithm, RxRisk-V) and type and timing of immunotherapies.
Source datasets
Medicare is Australia’s public health system, which provides free or low-cost access to most healthcare services including all public hospital and emergency services, services provided by general practitioners and medical specialists, medicines, investigations and selected allied health services. Medicare covers all Australian citizens and permanent residents and international citizens from countries with reciprocal rights. Medicare accounts for approximately 70% of Australia’s annual healthcare expenditure,43 44 thereby capturing high-quality data relating to the majority of Australia’s healthcare contact and treatment.
The study cohorts will be linked to a range of health and welfare datasets to determine study covariates and outcomes (table 1). Hospital admissions and emergency presentation data will be obtained from jurisdictional datasets. Prescription information will be obtained using data from the Pharmaceutical Benefits Scheme (PBS), an Australian federal government programme that subsidises the cost of certain prescription medications. Data regarding neurological investigations and procedures, general practitioner and specialist visits, and cancer treatment will be obtained from the Medicare Benefits Scheme, Australia’s publicly funded universal healthcare system that subsidises the cost of a range of medical services and treatments. Impairment and disability services data will be obtained from the Disability Services National Minimum Data Set, a dataset that collects data about disability support services in Australia provided under the National Disability Agreements (NDA) and their clients. Cancer diagnosis data will be obtained from the Australian Cancer Database (ACD), a national registry of all new diagnoses of cancer, which is a notifiable disease in all Australian states and territories. Mortality will be estimated using data from the National Death Index. Educational attainment will be estimated using results of NAPLAN testing, an annual standardised assessment programme administered to students in years 3, 5, 7 and nine in all Australian schools to monitor and assess students’ progress in essential literacy and numeracy skills over time. Childhood development data will be obtained from the Australian Early Development Census, a compulsory assessment performed by teachers every 3 years that scores developmental performance in multiple domains.
Hospital admissions, emergency presentations and prescription medications will be obtained from 2003 onwards to provide a minimum 5-year look-back period from the date of antibody testing to determine the date of disease onset. Cancer and Medicare billing data will be obtained from the inception of these datasets (1982 and 1984, respectively) to maximise the look-back period for cancer diagnosis and comorbidities. Data will be obtained until December 2021 to provide a minimum 2-year follow-up period following antibody testing.
Data preparation
This project will engage the services of multiple government-accredited integrating authorities, including the Australian Institute of Health and Welfare (AIHW) which will act as a coordinating linkage centre and link Commonwealth datasets, the Australian Institute of Family Studies (AIFS) which will link education datasets, and jurisdictional data linkage units in each Australian state/territory which will link jurisdictional hospital and emergency datasets.
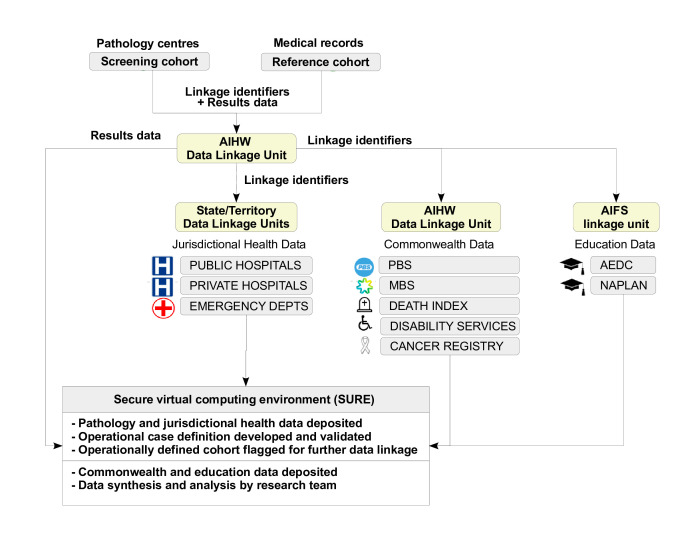
The stages of the project are summarised in figure 2. Linkage identifiers for the Reference and Mimicker Cohorts and the screening population will be provided to the AIHW, who will securely on-provide them to jurisdictional data linkage units for linkage to hospital and emergency datasets. De-identified linked data will be provided to researchers within the Secure Unified Research Environment (SURE), a secure virtual computing environment designed for analysis of de-identified unit record level data. Researchers will use this data to ascertain the operational case definition and create the Operationally Defined Cohort. The Operationally Defined Cohort and Reference Cohort will then be linked by the AIHW to Commonwealth datasets and by AIFS to education datasets, and de-identified linked data provided to researchers in SURE. Data analysis will be conducted within the SURE environment.
Figure 2. Project data flow. Flow of identifiers and research data among participating institutions and data linkage centres. AEDC, Australian Early Development Census; AIHW, Australian Institute of Health and Welfare; MBS, Medicare Benefits Schedule; NAPLAN, National Assessment Program – Literacy and Numeracy; PBS, Pharmaceutical Benefits Scheme.
Data analysis
Development and validation of autoimmune encephalitides (AE) operational definitions
Development of the operational definition will be performed as previously described,45 using statistical methods to determine and define the best potential coding algorithms for identifying cases of AE. This will be performed using the Reference Cohort (n=145 cases of AE, n≈200 mimickers). Initial univariate analysis using χ2/Fisher’s exact test (p<0.25) will be used to identify candidate variables. Univariate analysis will be restricted to 2×2 tables with a frequency >10 in each cell. Multivariate logistic regression models will then be constructed to test how various combinations of the candidate variables fit the data. The Hosmer–Lemeshow test will be used for model evaluation to define the best potential coding algorithms for identifying cases.
The diagnostic characteristics of each candidate variable and coding algorithm (sensitivity, specificity, positive predictive value and negative predictive value) will be defined and receiver operating characteristic curves created.
The best model will be internally validated to ensure robustness via bootstrap resampling.46 The mean of the coefficients and standard errors of the random samples will be examined for coherence and compared with the final model to look for evidence of overfitting.
Estimation of covariates and outcomes
Primary outcomes will be determined using the Operationally Defined Cohort (n≈5000). Some prognostic factors and secondary outcomes will be determined using the Reference Cohort, for covariates not accessible through linked datasets such as inpatient first-line immunotherapy treatment (n=145). The two study cohorts will be analysed separately due to the high likelihood of overlap. Operational definitions for outcomes and covariates will be based on published, validated definitions where available or new operational definitions developed with input from study collaborators where validated definitions are unavailable. Operational definitions have been provided in online supplemental appendix 2. These operational definitions may be modified if development and validation using the Reference Cohort demonstrate better performance of an alternative operational definition or there is interim publication of other validated definitions. This will be justified and discussed when presenting the results in publication.
Statistical approaches
Linked patient data will be stored, cleaned and analysed using Stata, R and Python in the SURE provided by the Sax Institute, Australia. The level of significance for primary research hypotheses will be defined at p<0.05.
For each outcome, initial statistical analysis will be conducted on samples with complete data. Proportion and assumptions missing values for each variable in the complete dataset will be described, and we will then employ various imputation methods to address missing data using Multiple Imputation with Denoising Autoencoders in Python (MIADASpy) and R for Missing Completely at Random and Missing at Random variables while conducting sensitivity analyses for Missing Not at Random data to evaluate the impact of various assumptions on our results. Continuous and categorical variables will be presented as mean±SD and number (percentage), respectively. Normality of continuous variables will be assessed, and variables that violate assumptions of normality will be transformed if appropriate. Differences in continuous variables will be assessed using the independent sample t-test or its equivalent non-parametric Mann-Whitney U test. Differences between categorical variables will be assessed using the χ2 test or Fisher’s exact test, where appropriate.
Random mixed effects regression models will be used to evaluate treatment effects on outcomes, accounting for covariates and allowing for the repeated measures design. The logit link will be used for binary outcomes and the ordinal link for ordered categorical outcomes. The correlation matrix structure will be chosen depending on the underlying data structure. Cox proportional hazards will be used for time-to-event analysis.
Methodological considerations
Patient and public involvement
Consumer consultation (International Autoimmune Encephalitis Society (IAES), Portland, USA) revealed disappointment in the lack of research focus on functional outcomes. Our engagement with the IAES has strengthened the need for our research aims towards investigating functional outcomes described in disability and educational datasets and the influence of therapy on these outcomes.
Limitations
There are several methodological considerations specific to this study that warrant consideration. First, medications administered to patients while admitted to Australian hospitals are not routinely reported to any administrative datasets. This will be addressed using the Reference Cohort, with information on inpatient prescriptions of immunosuppressive agents for AE obtained directly from medical records. Other medications administered during hospital admission for disease symptoms or comorbidities will not be captured. This will be taken into consideration during the interpretation of study findings.
Most of the datasets included in this study are good to high quality, with low rates of missing values and high content accuracy (see table 1). Most have data available for the entire period of interest; however, the DS NMDS is less complete. Until 2019 Australia’s disability services were governed by the National Disability Agreement (NDA), in which state and territory governments were responsible for specialist disability services, these jurisdictions collected data on the disability support services provided under NDA and provided this to the DS NMDS. The data therefore vary by jurisdiction and year regarding the services provided under the NDA, the accuracy of the statistical linkage key, and missing reports and non-response items from service providers. Further, the NDA was replaced in 2019 with a unified national scheme, the National Disability Insurance Scheme (NDIS), under which disability services and support are coordinated and funded by a federal government agency, and data regarding service users are reported directly to the Commonwealth NDIS dataset by disability services providers. The DS NMDS is therefore only available until 2019. These data will be interrogated using data quality assessment methods, examining the degree and pattern of data missingness to assess for potential sources of bias and considering these factors carefully in the interpretation of results.
Cancer data will be obtained from the ACD. Cancer is a notifiable disease in all Australian states and territories, with legislation requiring various institutions to report new diagnoses of cancer to jurisdictional registries. This includes malignant or invasive neoplasms and excludes basal and squamous cell carcinomas of the skin and most benign neoplasms, including several known to be associated with AE, including non-malignant ovarian teratomas and thymomas.1 12 13 Neoplasms excluded from cancer registries will be captured using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification diagnosis codes attached to hospital admissions, with non-malignant neoplasms classified under category D (D00-D48) codes, including benign neoplasms of the ovary (D27) and thymus (D15.0).
Timing of treatment is recognised as an important factor determining response to immunotherapy in patients with AE, with treatment delays associated with poorer outcomes.47 Examination of treatment timing using data linkage techniques is limited by unmeasurable time windows between symptom onset and any medical encounters that generate administrative data, and the coarse temporal resolution with which data is reported to some administrative datasets. Regarding timing of treatment, this study will primarily examine the impact of maintenance therapy on outcomes, with duration of therapy in the sub-acute and chronic phases of illness able to be studied within the resolution of administrative datasets used in this project. Timing of therapy in the acute phase will be explored, although it is likely to be limited by the above factors.
Ethics and dissemination
This project, including data linkage and waiver of consent, has been approved by the leading investigators’ institutional HREC, the St Vincent’s Hospital (Melbourne) HREC (Reference number LRR LRR137.21), as well as the AIHW HREC (EO2021/2/1249), and jurisdictional HRECs as required in New South Wales (NSW Population and Health Services Research Ethics Committee, 2021/ETH01322), Western Australia (Department of Health WA HREC, PRN RGS4297), Tasmania (University of Tasmania HREC, Project ID 25036) and Australian Capital Territory (Calvary Public Hospital Bruce HREC, Project ID 42–2021). Results will be published in peer-reviewed journals and peer-reviewed findings disseminated to patients and advocates via the IAES.
supplementary material
Footnotes
Funding: This work is supported by funding from UCB Global, Belgium. The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084664).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Collaborators: Australian Adult Comprehensive Epilepsy Centres Consortium: Mark Cook, John Dunne, Lisa Gillinder, Udaya Seneviratne, Emma Whitham, Nicholas Lawn, Elaine Pang, Mastura Monif, Saxon Douglass, Ernest Butler, Wendyl D’Souza.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Amy Jean Halliday, Email: amy.halliday@svha.org.au.
Katrina Lambert, Email: k.lambert@latrobe.edu.au.
Christine Bundell, Email: chris.bundell@health.wa.gov.au.
Andrew McLean-Tooke, Email: Andrew.Mclean-Tooke@health.wa.gov.au.
David Gillis, Email: David.Gillis@health.qld.gov.au.
Kerri M Prain, Email: kerri.prain@health.qld.gov.au.
Greg Bryson, Email: Greg.Bryson@health.qld.gov.au.
Lisa Gillinder, Email: l_gillinder@hotmail.com.
David Brown, Email: david.brown1@sydney.edu.au.
Sudarshini Ramanathan, Email: sudarshini.ramanathan@sydney.edu.au.
Russell Dale, Email: russell.dale@health.nsw.gov.au.
Fabienne Brilot, Email: Fabienne.brilot@sydney.edu.au.
Nerissa Jordan, Email: Nerissa.Jordan@health.wa.gov.au.
Nicholas Lawn, Email: nicholas.lawn@health.wa.gov.au.
Alan Lai, Email: alan.lai@unimelb.edu.au.
James Boyd, Email: James.Boyd@latrobe.edu.au.
Australian Adult Comprehensive Epilepsy Centres Consortium, Email: notapplicable@notapplicable.com.
Ximena Camacho, Email: ximena.camacho@unimelb.edu.au.
Wendyl Jude D’Souza, Email: wendyl.dsouza@svha.org.au.
Australian Adult Comprehensive Epilepsy Centres Consortium:
Mark Cook, John Dunne, Lisa Gillinder, Udaya Seneviratne, Emma Whitham, Nicholas Lawn, Elaine Pang, Mastura Monif, Saxon Douglass, Ernest Butler, and Wendyl D’Souza
References
- 1.Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med. 2018;378:840–51. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 2.Li A, Gong X, Guo K, et al. Direct economic burden of patients with autoimmune encephalitis in western China. Neurol Neuroimmunol Neuroinflamm. 2020;7:e891. doi: 10.1212/NXI.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J, Sotoca J, Gandhi S, et al. Autoimmune encephalitis: A costly condition. Neurology (ECronicon) 2019;92:e964–72. doi: 10.1212/WNL.0000000000006990. [DOI] [PubMed] [Google Scholar]
- 4.Nosadini M, Mohammad SS, Ramanathan S, et al. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15:1391–419. doi: 10.1586/14737175.2015.1115720. [DOI] [PubMed] [Google Scholar]
- 5.Abboud H, Probasco JC, Irani S, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry . 2021;92:757–68. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine J, Kopp UA, Klag J, et al. Long-Term Cognitive Outcome in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Ann Neurol. 2021;90:949–61. doi: 10.1002/ana.26241. [DOI] [PubMed] [Google Scholar]
- 7.Binks SNM, Veldsman M, Easton A, et al. Residual Fatigue and Cognitive Deficits in Patients After Leucine-Rich Glioma-Inactivated 1 Antibody Encephalitis. JAMA Neurol. 2021;78:617–9. doi: 10.1001/jamaneurol.2021.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abboud H, Briggs F, Buerki R, et al. Residual symptoms and long-term outcomes after all-cause autoimmune encephalitis in adults. J Neurol Sci. 2022;434:120124. doi: 10.1016/j.jns.2021.120124. [DOI] [PubMed] [Google Scholar]
- 9.Macher S, Zimprich F, De Simoni D, et al. Management of Autoimmune Encephalitis: An Observational Monocentric Study of 38 Patients. Front Immunol. 2018;9:2708. doi: 10.3389/fimmu.2018.02708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bost C, Chanson E, Picard G, et al. Malignant tumors in autoimmune encephalitis with anti-NMDA receptor antibodies. J Neurol. 2018;265:2190–200. doi: 10.1007/s00415-018-8970-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Liu X, Jiang X-Y, et al. Late-onset anti-N-methyl-d-aspartate receptor encephalitis in China. Epilepsy Behav. 2018;84:22–8. doi: 10.1016/j.yebeh.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Uy CE, Binks S, Irani SR. Autoimmune encephalitis: clinical spectrum and management. Pract Neurol. 2021;21:412–23. doi: 10.1136/practneurol-2020-002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husari KS, Dubey D. Autoimmune Epilepsy. Neurotherapeutics. 2019;16:685–702. doi: 10.1007/s13311-019-00750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18:19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Casemix & Classification Centre U of Wollongong, National Centre for Classification in Health (Australia) The international statistical classification of diseases and related health problems: 10th revision: Australian modification (ICD-10-AM) 2013 [PubMed]
- 18.National Centre for Classification in Health, World Health Organization . Australian classification of health interventions ACHI; 2019. International statistical classification of diseases and related health problems. [Google Scholar]
- 19.Armangue T, Olivé-Cirera G, Martínez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19:234–46. doi: 10.1016/S1474-4422(19)30488-0. [DOI] [PubMed] [Google Scholar]
- 20.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75:411–28. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daif A, Lukas RV, Issa NP, et al. Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav. 2018;80:331–6. doi: 10.1016/j.yebeh.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Qiao S, Wu H-K, Liu L-L, et al. Characteristics and Prognosis of Autoimmune Encephalitis in the East of China: A Multi-Center Study. Front Neurol. 2021;12:642078. doi: 10.3389/fneur.2021.642078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan W, Yang H, Wang Q. Neuronal Surface Antibody-Medicated Autoimmune Encephalitis (Limbic Encephalitis) in China: A Multiple-Center, Retrospective Study. Front Immunol. 2021;12:621599. doi: 10.3389/fimmu.2021.621599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Xie Q, Liu L, et al. Factors influencing prognosis and relapse in patients with anti-N-methyl-D-aspartate receptor encephalitis. Mult Scler Relat Disord. 2023;74:104697. doi: 10.1016/j.msard.2023.104697. [DOI] [PubMed] [Google Scholar]
- 25.Guo K, Liu X, Lin J, et al. Clinical characteristics, long-term functional outcomes and relapse of anti-LGI1/Caspr2 encephalitis: a prospective cohort study in Western China. Ther Adv Neurol Disord. 2022;15:17562864211073203. doi: 10.1177/17562864211073203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Zhang O, Gu L, et al. Analysis of risk factors for a poor functional prognosis and relapse in patients with autoimmune encephalitis. J Neuroimmunol. 2022;369:577899. doi: 10.1016/j.jneuroim.2022.577899. [DOI] [PubMed] [Google Scholar]
- 28.Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30:345–53. doi: 10.1097/WCO.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesselingh R, Broadley J, Buzzard K, et al. Prevalence, risk factors, and prognosis of drug-resistant epilepsy in autoimmune encephalitis. Epilepsy Behav. 2022;132:108729. doi: 10.1016/j.yebeh.2022.108729. [DOI] [PubMed] [Google Scholar]
- 30.Chen S-S, Zhang Y-F, Di Q, et al. Predictors and prognoses of epilepsy after anti-neuronal antibody-positive autoimmune encephalitis. Seizure. 2021;92:189–94. doi: 10.1016/j.seizure.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Hébert J, Day GS, Steriade C, et al. Long-Term Cognitive Outcomes in Patients with Autoimmune Encephalitis. Can J Neurol Sci. 2018;45:540–4. doi: 10.1017/cjn.2018.33. [DOI] [PubMed] [Google Scholar]
- 32.Matricardi S, Farello G, Savasta S, et al. Understanding Childhood Neuroimmune Diseases of the Central Nervous System. Front Pediatr. 2019;7:511. doi: 10.3389/fped.2019.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galioto R, Aboseif A, Krishnan K, et al. Cognitive outcomes in anti-LGI-1 encephalitis. J Int Neuropsychol Soc. 2023;29:541–50. doi: 10.1017/S1355617722000509. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Li C, Li A, et al. Long-term cognitive and neuropsychiatric outcomes of anti-GABABR encephalitis patients: A prospective study. J Neuroimmunol. 2021;351:577471. doi: 10.1016/j.jneuroim.2020.577471. [DOI] [PubMed] [Google Scholar]
- 35.Flet-Berliac L, Tchitchek N, Lépine A, et al. Long-term outcome of paediatric anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2023;65:691–700. doi: 10.1111/dmcn.15429. [DOI] [PubMed] [Google Scholar]
- 36.Newman MP, Blum S, Wong RCW, et al. Autoimmune encephalitis. Intern Med J. 2016;46:148–57. doi: 10.1111/imj.12974. [DOI] [PubMed] [Google Scholar]
- 37.Sechi E, Flanagan EP. Diagnosis and Management of Autoimmune Dementia. Curr Treat Options Neurol. 2019;21:11. doi: 10.1007/s11940-019-0550-9. [DOI] [PubMed] [Google Scholar]
- 38.Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology (ECronicon) 2013;81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson D, Nathoo N, Henry M, et al. Oophorectomy in NMDA receptor encephalitis and negative pelvic imaging. Pract Neurol. 2021;21:57–60. doi: 10.1136/practneurol-2020-002676. [DOI] [PubMed] [Google Scholar]
- 40.Trewin BP, Freeman I, Ramanathan S, et al. Immunotherapy in autoimmune encephalitis. Curr Opin Neurol. 2022;35:399–414. doi: 10.1097/WCO.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 41.Broadley J, Seneviratne U, Beech P, et al. Prognosticating autoimmune encephalitis: A systematic review. J Autoimmun. 2019;96:24–34. doi: 10.1016/j.jaut.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Trewin D. SEIFA 2016 Technical Paper (5). 2016 p. 39. Report No.: ABS Catalogue No. 2033.0.55.001
- 43.Harris B, Craike M, Dunkin R, et al. Melbourne: Victoria University; 2019. Is medicare fair? I. the distribution of medicare benefits across the states and territories. (Mitchell institute policy issues paper). Report no.: 2–2019. [Google Scholar]
- 44.Canberra: Australian Institute of Health and Welfare; 2023. Health expenditure Australia 2021-22. (Health expenditure Australia) [Google Scholar]
- 45.Tan M, Wilson I, Braganza V, et al. Development and validation of an epidemiologic case definition of epilepsy for use with routinely collected Australian health data. Epilepsy Behav. 2015;51:65–72. doi: 10.1016/j.yebeh.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Steyerberg EW, Harrell FE, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 47.Gastaldi M, Mariotto S, Giannoccaro MP, et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol. 2019 doi: 10.1111/ene.14139. [DOI] [PubMed] [Google Scholar]
- 48.SA NT DataLink Integrated South Australian activity collection data quality statemen. 2010. https://www.phrn.org.au/media/81302/dqs_isaac_may2010_v100.pdf Available.
- 49.Government of Western Australia Department of Health Data quality statement: hospital morbidity data collection. 2020. https://www.datalinkage-wa.org.au/wp-content/uploads/2020/09/Data-Quality-Statement_HMDC_Aug2020_V1.0.pdf Available.
- 50.SA NT DataLink Emergency department data collection data quality statement. 2010. https://www.phrn.org.au/media/81300/dqs_eddc_may2010_v100.pdf Available.
- 51.Government of Western Australia Department of Health Data quality statement: emergency department data collection. 2020. https://www.datalinkage-wa.org.au/wp-content/uploads/2020/09/Data-Quality-Statement_EDDC_Aug2020_V1.0.pdf Available.
- 52.Australian Institute of Health and Welfare Disability services national minimum data set (DS NMDS) - data quality statement. Report no.: 728139. 2020 https://meteor.aihw.gov.au/content/index.phtml/itemId/728139 Available.
- 53.Disability services national minimum data set 2018-19; quality statement. 2021. https://meteor.aihw.gov.au/content/index.phtml/itemId/728139 Available.
- 54.Australian cancer database 2011 data quality statement. 2021. https://meteor.aihw.gov.au/content/index.phtml/itemId/586979 Available.
- 55.The social research centre. Australian early development census data user guide. 2014
- 56.Jason P, Bentley NN. Sydney, New South Wales, Australia: Menzies Centre for Health Policy, School of Public Health, University of Sydney; 2017. The quality of record linkage between population-based birth and children’s early child development and school test result (NAPLAN) records in New South Wales, Australia.https://ses.library.usyd.edu.au/bitstream/handle/2123/17823/Bentley2017_Data%20Linkage%20Technical%20Report.pdf?sequence=4&isAllowed=y Available. [Google Scholar]
- 57.National Death Index (NDI) Data quality statement. 2021. https://meteor.aihw.gov.au/content/index.phtml/itemId/480010 Available.