Abstract
Abstract
Objective
Investigating the correlation between body mass index (BMI) and cognitive decline among elderly people in the Chinese community.
Design
A non-random sampling method was employed to conduct a cross-sectional, mixed methods survey among elderly individuals in the Chinese community.
Setting
This research was conducted across the country, focusing on 20 distinct communities (2 rural and 18 urban) situated in the eastern, central and western parts of China.
Participants
The China Longitudinal Aging Study (cohort 1) and Shanghai Brain Aging study (cohort 2) were the sources of the present data. Cohort 1 consisted of 2947 individuals aged 60 and above, who were subjected to a comprehensive screening procedure encompassing a physical examination, medical background and initial evaluations of cognitive abilities through an in-person interview. Cohort 2 comprised an extra 226 older adults, including 54 patients with mild cognitive impairment (MCI) and 174 normal adults, and unlike cohort 1, all of them underwent T1 phase MRI scans.
Outcome measures
The cognitive abilities, BMI and structural magnetic resonance properties of elderly individuals in the Chinese community.
Results
In cohort 1, we discovered that having a BMI below 18.5 kg/m2 posed a significant risk for MCI (p=0.005, OR=2.000, 95% CI: 1.228 to 3.255), regardless of age, gender and other significant variables. Despite this, there was no correlation between dementia and various BMIs. In cohort 2, we found that both left and right hippocampal volumes were significantly smaller in patients with MCI than in normal older adults, and there was a clear mediating effect between the right hippocampus, BMI and cognitive impairment (r=2.182, p=0.030).
Conclusions
BMI below 18.5 kg/m2 is associated with an increased likelihood of mild cognitive decline, which may be related to the effect of BMI on the volume of the right hippocampus.
Keywords: Aged, Dementia, Body Mass Index
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The research conclusions are more convincing because our data comes from a nationally representative sample.
The considerable number of participants in our study is a major advantage.
In contrast to prior research, we incorporated MRI into the second group, which is a remarkable advancement of our study.
Despite the cross-sectional nature of the current study, it is not possible to establish a causal relationship between low body mass index and mild cognitive impairment.
The current study is significantly hindered by the lack of intervention experiments.
Introduction
It is thought that dementia and Alzheimer’s disease (AD) are caused by a combination of genetic predisposition and environmental factors.1 The prevention and control of dementia mainly focus on the other factors that can be modified, as genes, age and sex are irreversible.2 An increasing number of people are exploring the potential of straightforward and achievable methods of obesity to anticipate the onset of dementia.3 4 Cao et al studied 502 528 people from the UK Biobank and tracked them for 8.1 years, concluding that those with a lower weight had a 1.91-fold increased chance of developing dementia within 8.1 years (HR=1.91, 95% CI: 1.24 to 2.97) compared with those with a healthy weight.5 Over a span of 2 years, Nam3 et al meticulously tracked 167 876 individuals diagnosed with type 2 diabetes, aged 40 years or above, and discovered a noteworthy U-shaped correlation between the alteration in body weight percentage and the likelihood of developing all-cause dementia (p<0.001). Additionally, weight loss exceeding 10% exhibited a 1.26-fold increase in the risk of developing AD within 2 years, when compared with the control group (HR 1.26 (95% CI 1.01 to 1.59)). The research conducted by Qizilbash et al revealed that individuals with a body mass index (BMI) below 20 kg/m2 during their mid-life exhibited a 34% increased risk to dementia.6 Other studies have suggested that a low BMI or reduced BMI is a risk factor for future cognitive impairment.4 7 8 Nevertheless, a meticulous examination and meta-analysis of potential studies also revealed that the risk of developing dementia, AD and vascular dementia during mid-life was notably heightened, when BMI exceeded 29, 30 and 32 kg/m2. Consequently, the results of the research are not uniform and the discrepancies may be linked to the disparities in ethnicity and the techniques used for investigation.
It is essential to carry out comparable investigations in the elderly Chinese population, given that the aforementioned studies were mainly conducted in Western countries and the prevalence of dementia and obesity parameters varied by race. Yuan et al’s research revealed that elderly men with a greater BMI were two times as prone to developing mild cognitive impairment (MCI).9 In Zhang et al’s study, it was found that there are different associations between BMI and cognitive impairment among rural/urban groups.10 Huang et al’s research revealed that elderly people who were obese in the abdomen and had low levels of physical activity had poorer cognitive abilities.11 Domestic studies have yielded divergent results, and no dependable explanation has been suggested as to why varying BMI has an impact on cognitive status.
MRI is an effective means of examining the anatomy and performance of the brain. The Braak stage of upstream tangles deposition and downstream neuropsychological deficits in AD coincide precisely with changes in brain structure.12 The atrophy of volume in the whole brain, entorhinal cortex, hippocampus and temporal lobe is considered to be a biomarker of AD because of this.13 In our previous research, we additionally showcased that the disparity between the hippocampus and amygdala is a distinctive indication of subjective cognitive dysfunction.14 In addition, some studies have suggested that there may be an intrinsic relationship between BMI and hippocampal volume. For example, one study from the UK Biobank showed that individuals with a high BMI had significantly smaller hippocampus volume than normal controls.15 Another study also points out that higher BMI was associated with lower hippocampal and amygdala connectivity to regions of prefrontal cortex implicated in cognitive control and emotion regulation.16 Therefore, we propose that there is an intrinsic association between BMI and cognitive brain area and that BMI is likely to affect cognitive function by affecting the structure and function of hippocampal and amygdala.
In order to validate our research hypothesis, we conducted an extensive nationwide cohort study and a comparatively limited MRI cohort sample (including magnetic resonance data) to examine the impact of varying BMI on cognitive function and potential mechanisms, with a primary focus on the hippocampus and amygdala.
Materials and methods
Patients and public involvement
Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research.
Participants
Cohort 1 was included in the China Longitudinal Aging Study (CLAS), which has been extensively documented in prior studies.17 18 In 2011, the CLAS study was initiated across the nation, focusing on 20 distinct communities (2 rural and 18 urban) in eastern, central and western China.19 20 As per the 2010 National census, a database was populated with individual aged 60 and above who held permanent residency. In order to be eligible for the study, participants had to meet the following criteria: being 60 years or older, being a permanent resident of the community, having no evidence of serious physical illness or mental illness, such as schizophrenia and major depression, and agreeing to participate. The following conditions were not included in the study: (1) acute stress state, such as acute medical disorders; (2) serious mental illness or serious physical illness; and (3) the participants or guardians declined to take part. All participants would be subjected to a comprehensive evaluation that encompassed physical and neurological assessments, medical records and cognitive appraisals. In addition, clinical physicians conducted face to face interviews with the elderly to determine if they were suffering from dementia or not. Subsequently, we chose 2947 people with diagnosis and full weight information at random for our research (out of the 2947 participants, 596 had MCI, 188 had dementia and 2168 had normal cognitive function).
A total of 226, elderly people including 54 patients with MCI and 172 normal controls were included in cohort 2 (the Shanghai Brain Aging study). In contrast to the initial group, every participant in the subsequent group underwent initial T1 phase MRI scans. The investigation items and screening procedures remain identical to those employed in cohort 1 (the evaluators are also in agreement). We mainly focused on the relationship between BMI and the hippocampus, and amygdala based on our and others’ previous work. The study flow chart is shown in online supplemental material.
Measurement of BMI
The calculation of BMI involves dividing weight (kg) by height2 (m2). The scale was used by all participants, and they were calibrated prior to measurement. The classification of BMI was based on the revised Asia-Pacific BMI standard, established by the WHO,21 which categorised it into underweight (BMI<18.5 kg/m2), normal weight (18.5 kg/m2≤BMI<24.0 kg/m2), overweight (24.0 kg/m2≤BMI<28.0 kg/m2) and obesity (BMI≥28.0 kg/m2).
T1 phase structure MRI
The Magnetom Verio V.3.0T scanner (Siemens, Munich, Germany) was used to capture the brain structure images. The MPRAGE sequence parameters obtained through T1-weighted three-dimensional magnetisation were as follows: repeat time (TR)=2300 ms, echo time (TE)=2.98 ms, matrix size=240×256; flip angle of 9°, slice thickness=1.2 mm, field of view=240×256 mm. The evaluation of volume data was conducted using automated procedures, as outlined by Wolz et al.22 Each subject’s hippocampus, amygdala and brain size index were analysed for volume and asymmetry using FreeSurfer.
Neuropsychological tests and diagnostic criteria
The entire group had been thoroughly assessed through a clinical assessment, physical inspection and psychological assessments. The subjects’ overall cognitive function was evaluated using the Beijing edition of the Montreal Cognitive Assessment (MoCA)23 and Mini-Mental State Examination (MMSE).24 The revised Petersen’s diagnostic algorithm25 and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition20 were used to determine the diagnosis of MCI and dementia, respectively.
Covariates
A standardised questionnaire was used to gather general demographic data (such as age, gender, education), daily living details (including smoking history, alcohol consumption) and disease information (such as hypertension and diabetes). The variables that varied among dementia, MCI and normal groups were taken into account as covariates.
Bias
To prevent any diagnostic errors or biases in the data survey, we ensure that all the evaluators receive consistent training before commencing the project, and they will be assigned tasks only after completing the relevant assessment examination successfully.
Study size
Given the anticipated inclusion of around 20 factors in cohort 1, it is imperative to compute the sample size as 20 multiplied by the overall number of factors, encompassing a minimum of 400 patients or participants with outcomes. Taking into account the reported occurrence of outcomes in literature, which stands at approximately 20% (400/20%=2000), it is approximated that a total of 2000 patients ought to be encompassed. Subsequently, a predictive model was constructed using a random selection process involving 70% of the patients (n=1400), while an additional 30% (n=600) were chosen to authenticate cohort 1. Linear regression analysis requires a sample size that is at least two times as large as that of the independent variable. At present, there are 16 independent variables, thus necessitating a minimum sample size of 32. Cohort 2 incorporated 226 variables to enhance the explanation of the findings.
Quantitative variables
In this research, gender, age and educational attainment will be taken into account as the independent variables. For a detailed breakdown of the analysis, please refer to the Statistical analysis section.
Statistical analysis
Mean±SD was used to express continuous variables, while frequencies (%) were used to express categorical variables. We used a Kolmogorov-Smirnov test to determine if the data followed a normal distribution. Subsequently, we employed an independent sample t-test and Kruskal-Wallis H to contrast the normal and non-normal data between the various cognitive groups, respectively. We also employed χ2 tests to contrast the classification variables. Subsequently, hierarchical multiple regression models were employed to delve deeper into the correlation between BMI and various cognitive states (with diagnosis as the dependent variable): model a represents a fundamental model devoid of any control over variables; model b accounted for age, male and education; model c further accounted for smoking, hypertension and diabetes using model b. Subsequently, correlation analysis was used to examine the relationship among BMI, brain structure and cognitive scores. The relationship among age, BMI and hippocampus and amygdala volume was explored through the utilisation of linear regression analysis, employing a mediating model. All analyses were conducted using two-tailed tests with a significance level of p<0.05. The OR value is frequently employed as an index in epidemiological studies for case–control purposes. When the OR value is 1, it signifies that the factor does not influence the occurrence of the disease; conversely, if the OR value surpasses 1, it signifies that the factor is a risk factor; and if the OR value falls below 1, it indicates that the factor acts as a protective factor. SPSS V.22.0 was employed to examine the data.
Results
Characteristic of subjects with different cognitive states (cohort 1)
The comparison of MCI, dementia and normal ageing groups is illustrated in table 1. The three groups exhibited significant differences in age, males, smoker, diabetes, hypertension, BMI, MMSE and MoCA (p<0.05), whereas there was no significant disparity (p>0.05) in education and drinker among the three groups. More specifically, the age of the normal elderly, the elderly with MCI and the elderly with dementia increased sequentially. Men and smokers had the highest proportion in normal old age, the second in MCI and the lowest in dementia. The proportion of hypertension, diabetes and underweight in normal aged people was the lowest, the second in MCI and the highest in dementia. Simultaneously, MMSE and MOCA scores declined in the sequence of typical ageing, MCI and dementia.
Table 1. Comparison of general demographic data of the three groups.
| Variables | MCI (n=596) | Dementia (n=188) | Normal (n=2163) | F or X2 | P value |
| Age, years | 73.78±8.232 | 78.71±7.524 | 70.05±7.495 | 141.786 | <0.001* |
| Education, years | 6.70±3.217 | 6.54±2.994 | 6.85±3.512 | 0.966 | 0.381 |
| Male, n (%) | 226 (37.9) | 67 (35.6) | 1045 (48.3) | 28.083 | <0.001* |
| Smoker, n (%) | 143 (24.0) | 41 (21.8) | 633 (29.3) | 9.986 | 0.007* |
| Drinker, n (%) | 117 (19.6) | 29 (15.4) | 458 (21.2) | 3.851 | 0.146 |
| Diabetes, n (%) | 106 (17.8) | 49 (26.1) | 337 (15.6) | 14.307 | <0.001* |
| Hypertension, n (%) | 288 (48.3) | 110 (58.5) | 1003 (46.4) | 10.405 | 0.006* |
| BMI | |||||
| <18.5 kg/m2 | 49 (8.2) | 23 (12.2) | 95 (4.4) | 40.465 | <0.001* |
| ≥18.5, <24.0 kg/m2 | 314 (52.7) | 95 (50.5) | 1071 (49.5) | ||
| ≥24.0, <28.0 kg/m2 | 175 (29.4) | 47 (25.0) | 782 (32.2) | ||
| ≥28.0 kg/m2 | 58 (9.7) | 23 (12.2) | 215 (9.9) | ||
| MMSE | 22.43±5.636 | 13.96±7.433 | 26.85±3.445 | 892.522 | <0.001* |
| MoCA | 16.75±6.160 | 9.10±6.274 | 22.86±5.135 | 728.191 | <0.001* |
means pP<0.05; BMI means ; MMSE means Mini-mental State Examination; MoCA means Montreal Cognitive Assessment.
BMIbody mass indexMCImild cognitive impairmentMMSEMini-Mental State ExaminationMoCAMontreal Cognitive Assessment
Results of hierarchical multiple regression models (cohort 1)
The correlation between BMI and MCI is demonstrated in table 2. The OR value was used in this part to determine whether the survey variable was a protective or a risk factor. Our findings in model (a), without accounting for any variables, indicated a significant association between a BMI below 18.5 kg/m2 and MCI (p<0.001, OR=2.361, 95% CI: 1.514 to 3.681), implying that as BMI decreased, the likelihood of MCI also increased. This association remained statistically significant even after accounting for age, males and education in model (b) (p=0.006, OR=1.983, 95% CI: 1.222 to 3.1218). Despite making additional adjustments for smoker, hypertension and diabetes in model (c), the outcomes remained unchanged (p=0.005, OR=2.000, 95% CI: 1.228 to 3.255). The area under the receiver operating characteristic (ROC) curve (AUC) is a worldwide metric that evaluates the capacity to differentiate between the existence or non-existence of particular conditions. AUC of 0.5 implies that the test does not discriminate. AUC of 1.0 suggests that the test is completely discerning, which is no better than a probability.26 Using the ROC curve, we engaged in a comprehensive discussion regarding the potential of BMI to forecast MCI, resulting in a final area under the curve of 0.46 (p=0.006, 95% CI: 0.437 to 0.490), thereby indicating that BMI had negligible influence on the prediction of MCI. The results are depicted in figure 1.
Table 2. Multivariate logistics regression analysis results, with MCI/dementia as the dependent variables.
| Variables | B | SE | Wald | df | P value | OR | 95% CI |
| MCI | |||||||
| Model† | |||||||
| BMI<18.5 kg/m2 | 0.859 | 0.227 | 14.366 | 1 | <0.001* | 2.361 | 1.514 to 3.681 |
| Model‡ | |||||||
| BMI<18.5 kg/m2 | 0.685 | 0.247 | 7.684 | 1 | 0.006* | 1.983 | 1.222 to 3.218 |
| Age | 0.042 | 0.006 | 41.322 | 1 | <0.001* | 1.043 | 1.029 to 1.056 |
| Male | −3.065 | 0.294 | 108.939 | 1 | <0.001* | 0.047 | 0.026 to 0.083 |
| Education | −0.407 | 0.043 | 89.247 | 1 | <0.001* | 0.666 | 0.612 to 0.725 |
| Model§ | |||||||
| BMI<18.5 kg/m2 | 0.693 | 0.249 | 7.771 | 1 | 0.005* | 2.000 | 1.228 to 3.255 |
| Age | 0.041 | 0.007 | 38.856 | 1 | <0.001* | 1.042 | 1.028 to 1.055 |
| Male | −3.029 | 0.295 | 105.354 | 1 | <0.001* | 0.048 | 0.027 to 0.086 |
| Education | −0.414 | 0.043 | 90.657 | 1 | <0.001* | 0.661 | 0.607 to 0.720 |
| Smoker | −0.161 | 0.137 | 1.375 | 1 | 0.241 | 0.851 | 0.650 to 1.114 |
| hypertension | −0.017 | 0.102 | 0.029 | 1 | 0.866 | 0.983 | 0.804 to 1.201 |
| Diabetes | 0.122 | 0.135 | 0.815 | 1 | 0.367 | 1.130 | 0.867 to 1.474 |
| Dementia | |||||||
| Model† | |||||||
| BMI<18.5 kg/m2 | 1.142 | 0.330 | 11.937 | 1 | 0.001* | 3.132 | 1.641 to 5.980 |
| Model‡ | |||||||
| BMI<18.5 kg/m2 | 0.467 | 0.378 | 1.526 | 1 | 0.217 | 1.596 | 0.760 to 3.351 |
| Age | 0.112 | 0.011 | 103.746 | 1 | <0.001* | 1.119 | 1.095 to 1.144 |
| Male | −3.754 | 0.511 | 53.926 | 1 | 0.001* | 0.023 | 0.009 to 0.064 |
| Education | −0.503 | 0.076 | 43.320 | 1 | <0.001* | 0.605 | 0.521 to 0.702 |
| Model§ | |||||||
| BMI<18.5 kg/m2 | 0.621 | 0.383 | 2.635 | 1 | 0.105 | 1.861 | 0.879 to 3.938 |
| Age | 0.112 | 0.011 | 99.472 | 1 | <0.001* | 1.119 | 1.094 to 1.144 |
| Male | −3.741 | 0.515 | 52.741 | 1 | <0.001* | 0.024 | 0.009 to 0.065 |
| Education | −0.516 | 0.077 | 44.340 | 1 | <0.001* | 0.597 | 0.513 to 0.695 |
| Smoker | −0.154 | 0.227 | 0.457 | 1 | 0.499 | 0.858 | 0.550 to 1.338 |
| hypertension | 0.318 | 0.171 | 3.440 | 1 | 0.064 | 1.374 | 0.982 to 1.922 |
| Diabetes | 0.652 | 0.197 | 10.918 | 1 | 0.001* | 1.920 | 1.304 to 2.827 |
means a basic model, no adjustmentP<0.05.
Means pBasic model, no adjustment.
aAdjusted for age, and male.
aAdjusted for age, male, education, smoker, diabetes, and hypertension.
BMIbody mass indexMCImild cognitive impairment
Figure 1. The sensitivity and specificity of body mass index in predicting mild cognitive impairment.
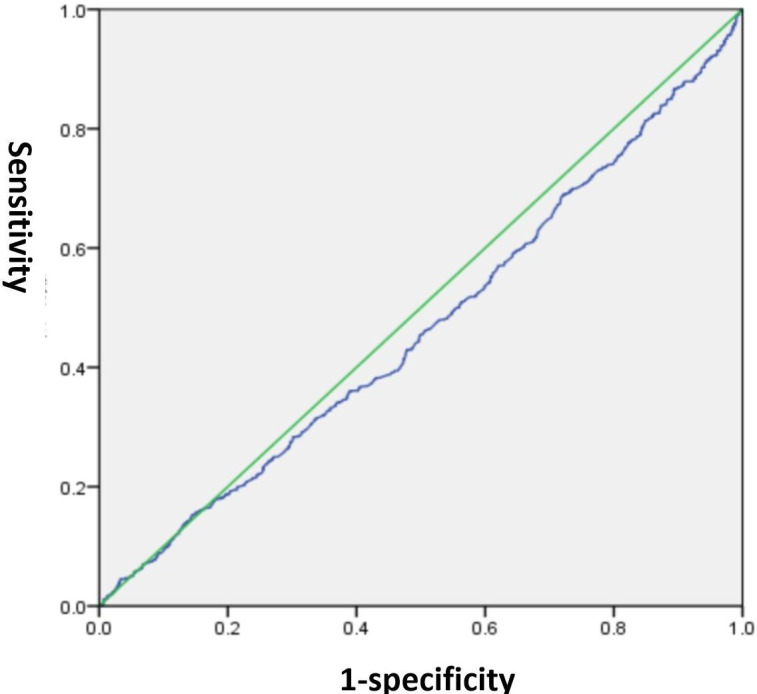
The correlation between BMI and dementia is also demonstrated in table 2. Our findings in model a, without accounting for any variables, indicated a significant association between a BMI below 18.5 kg/m2 and dementia (p<0.001, OR=3.132, 95% CI: 1.641 to 5.980), implying that as BMI decreased, the likelihood of developing dementia also decreased. Once age, gender, education and other variables were taken into account, BMI was no longer linked to dementia (p>0.05), suggesting that BMI is not a major factor in dementia.
Results of correlation analysis (BMI and hippocampus and amygdala volume) (cohort 2)
Cohort 2 included a total of 226 older adults, including 54 patients with MCI and 172 normal controls, matched for age, sex and education. We found that BMI, hippocampal volume and amygdala volume were significantly lower in the MCI group than in the normal control group (table 3). Next, the linear regression analysis-mediated model was used to explore the association between cognitive brain area, BMI and cognitive impairment, with hippocampus or amygdala volume as the dependent variable and BMI and cognitive diagnosis as the independent variable. Ultimately, we found that right hippocampal volume was associated with BMI and cognitive diagnosis, suggesting that BMI was likely to influence cognitive function by affecting right hippocampal volume. The results are depicted in figure 2.
Table 3. Comparison of general demographic data, BMI and brain structure between MCI and normal elderly people (cohort 2).
| Variables | MCI (n=54) | Normal (n=172) | X2 or t-test | P value |
| Age, years | 68.91±8.68 | 67.78±6.92 | 0.968 | 0.334 |
| Education, years | 10.93±3.94 | 11.55±3.18 | −1.191 | 0.235 |
| Males, n (%) | 27 (50.0) | 69 (40.1) | 1.643 | 0.211 |
| BMI, kg/m2 | 22.08±2.93 | 23.51±3.56 | −2.635 | 0.009* |
| Left hippocampus, cm3 | 3.04±0.63 | 3.65±0.39 | −8.472 | <0.001* |
| Right hippocampus, cm3 | 3.17±0.55 | 3.86±0.39 | −10.290 | <0.001* |
| Left amygdala, cm3 | 1.06±0.27 | 1.43±0.20 | −11.099 | <0.001* |
| Left amygdala, cm3 | 1.28±0.27 | 1.60±0.21 | −9.156 | <0.001* |
P<0.05.
BMIbody mass indexMCImild cognitive impairment
Figure 2. The mediating relationship among body mass index (BMI), right hippocampus and mild cognitive impairment (MCI). *mean p<0.05.
Discussions
The purpose of this study was to investigate whether different BMI affects cognitive status and, if so, explore possible mechanisms. In two large cohort studies, we found that (1) a BMI of less than 18.5 kg/m2 was a risk factor for MCI (but not dementia), independent of sex, age and other factors and (2) BMI is likely to influence cognitive function by affecting right hippocampal volume.
MCI is commonly thought to be an early stage of dementia with a higher risk of dementia transition. However, MCI is highly heterogeneous and not all MCI patients necessarily transition to dementia.27 Previous studies have shown that lowering initial BMI and losing weight during follow-up increase the risk of developing dementia in MCI patients.28 29 In addition, Jingzhu et al found an inverse relationship between BMI and MCI in older adults.30 Therefore, our research conclusions are consistent.
In our current study, we found that a BMI of less than 18.5 kg/m2 was also a risk factor for dementia, regardless of other variables. However, these associations disappeared when age, sex and other variables were included, suggesting BMI is not a central factor in dementia. The link between BMI and dementia is complex and studies are inconsistent. For example, Zhi et al found that BMI is an independent predictor of dementia and that BMI is associated with inverse monotonic and dose–response associations.5 Qizilbash et al found that being underweight in middle age increased risk of dementia within 20 years.6 In Albanese et al’s study, they found that being obese in middle age increases the risk of dementia.31
Our study differs from others for several reasons. First of all, our study is only a cross-sectional study and lacks long-term observation and follow-up, which may lead to some bias in the results. Second, in our study, we found that dementia patients are older, less educated and less likely to be man. Given that all three factors have a greater impact on dementia, it is not hard to understand why the study did not conclude that BMI has an impact on dementia. Finally, differences in enrolment population can also have an impact on the results. For example, we recruited all elderly people aged 60 years and above, while Cao et al’s study recruited individuals aged 37 years and above, moreover, they were all British population.5 Racial and genetic differences can also lead to quite the opposite conclusion. Therefore, the association between BMI and dementia needs to be further confirmed in larger follow-up cohort studies.
To investigate why a BMI of less than 18.5kg/m2 increases the risk of MCI, we added structural MRI to a second cohort. In this cohort, we included two groups of people, an MCI group and a normal control group, matched for sex, age and education. Similar to the first cohort, we found that the BMI of the MCI group was significantly lower than that of the normal control group, and the volume of the hippocampus and amygdala was also smaller than that of the normal control group. Using a mediating model of linear regression analysis, we found a clear association between right hippocampal volume, BMI and cognitive diagnosis, suggesting that BMI was likely to affect cognitive function by affecting right hippocampal volume.
The hippocampus is a brain structure involved in the higher regulation of eating behaviour. Thus, a dysfunctional hippocampus may help maintain a vicious cycle that predisposes people to obesity.32 In addition to our current study, previous studies have also explored the link between BMI and hippocampal structure and function. For example, in the study of Li G et al, they found that obesity was strongly associated with abnormal hippocampal/amygdala brain function. They also note that resting state activity in the hippocampus/amygdala may influence how these areas respond to food cues.33 In the study of Rucker and Ikuta, they found that there was a negative association between BMI and the pituitary gland functional connectivity with the hippocampus, putamen, orbitofrontal cortex and temporal lobe.34 In addition, in the study by Parcet et al, they also found that the higher the BMI, the smaller the hippocampal volume.32 So our findings are consistent.
We must concede that our research is not without its drawbacks. Initially, the two cohort in the current study were conducted in a cross-sectional manner, and they were unable to demonstrate a causal relationship between BMI and MCI. In the second cohort, although we found a correlation between the right hippocampus and BMI, there were no intervention experiments to test whether intervention in the right hippocampus could modulate BMI and cognitive function.
Conclusions
A BMI below 18.5 kg/m2 is linked to a heightened likelihood of experiencing MCI, potentially influenced by the impact of BMI on the volume of right hippocampal.
supplementary material
Footnotes
Funding: This study was supported by grants from the China Ministry of Science and Technology (STI2030-Major Projects-2022ZD0213100), Shanghai public health projects (GWVI-11.2-XD24), the Fei xiang Program of Shanghai Mental Health Center (2020-FX-03), the National Natural Science Foundation of China (82101564), Shanghai Clinical Research Center for Mental Health (19MC1911100) and the clinical research centre project of Shanghai Mental Health Center (CRC2017ZD02). This project was also funded by the Shanghai Elderly Brain Health Cohort Institute, Chinese Academy of Sciences (XDA12040101).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-076622).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: The research ethics committee of the affiliated mental health centre of Shanghai Jiao Tong University School of Medicine approved this study, and written informed consent was obtained from all participants before the study. The study was conducted in accordance with the Code of Ethics of the World Medical Association (Helsinki Declaration of 1964, as revised in 2018).
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Materials and methods section for further details.
Contributor Information
Wei Li, Email: 822203867@qq.com.
Lin Sun, Email: xiaosuan2004@126.com.
Ling Yue, Email: bellinthemoon@hotmail.com.
Shifu Xiao, Email: xiaoshifu@msn.com.
Data availability statement
Data are available on reasonable request.
References
- 1.Xu W, Tan L, Wang HF, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–66. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 3.Nam GE, Park YG, Han K, et al. BMI, Weight Change, and Dementia Risk in Patients With New-Onset Type 2 Diabetes: A Nationwide Cohort Study. Diabetes care. 2019;42:1217–24. doi: 10.2337/dc18-1667. [DOI] [PubMed] [Google Scholar]
- 4.Sobów T, Fendler W, Magierski R. Body mass index and mild cognitive impairment-to-dementia progression in 24 months: a prospective study. Eur J Clin Nutr. 2014;68:1216–9. doi: 10.1038/ejcn.2014.167. [DOI] [PubMed] [Google Scholar]
- 5.Cao Z, Xu C, Yang H, et al. Associations of BMI and Serum Urate with Developing Dementia: A Prospective Cohort Study. J Clin Endocrinol Metab. 2020;105:dgaa638. doi: 10.1210/clinem/dgaa638. [DOI] [PubMed] [Google Scholar]
- 6.Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–6. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 7.Astell-Burt T, Navakatikyan MA, Feng X. Behavioural change, weight loss and risk of dementia: A longitudinal study. Prev Med. 2021;145:S0091-7435(20)30417-5. doi: 10.1016/j.ypmed.2020.106386. [DOI] [PubMed] [Google Scholar]
- 8.Chen YC, Chen TF, Yip PK, et al. Body mass index (BMI) at an early age and the risk of dementia. Arch Gerontol Geriatr. 2010;50:S48–52. doi: 10.1016/S0167-4943(10)70013-3. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Li J, Zhang N, et al. Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. BMC Psychiatry. 2021;21:54. doi: 10.1186/s12888-021-03059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JJ, Li L, Liu D, et al. Urban-Rural Disparities in the Association Between Body Mass Index and Cognitive Impairment in Older Adults: A Cross-Sectional Study in Central China. J Alzheimers Dis . 2021;83:1741–52. doi: 10.3233/JAD-210295. [DOI] [PubMed] [Google Scholar]
- 11.Huang ZZ, Zhang YC, Zheng Y, et al. Associations of obesity and physical activity with cognition in people aged 50 and above in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:273–9. doi: 10.3760/cma.j.issn.0254-6450.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Vemuri P, Whitwell JL, Kantarci K, et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008;42:559–67. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Femminella GD, Thayanandan T, Calsolaro V, et al. Imaging and Molecular Mechanisms of Alzheimer’s Disease: A Review. Int J Mol Sci. 2018;19:3702. doi: 10.3390/ijms19123702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue L, Wang T, Wang J, et al. Asymmetry of Hippocampus and Amygdala Defect in Subjective Cognitive Decline Among the Community Dwelling Chinese. Front Psychiatry. 2018;9:226. doi: 10.3389/fpsyt.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambikairajah A, Tabatabaei-Jafari H, Walsh E, et al. Longitudinal Changes in Fat Mass and the Hippocampus. Obesity (Silver Spring) 2020;28:1263–9. doi: 10.1002/oby.22819. [DOI] [PubMed] [Google Scholar]
- 16.Donofry SD, Lesnovskaya A, Drake JA, et al. Obesity, Psychological Distress, and Resting State Connectivity of the Hippocampus and Amygdala Among Women With Early-Stage Breast Cancer. Front Hum Neurosci. 2022;16:848028. doi: 10.3389/fnhum.2022.848028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao S, Li J, Tang M, et al. Methodology of China’s national study on the evaluation, early recognition, and treatment of psychological problems in the elderly: the China Longitudinal Aging Study (CLAS) Shanghai Arch Psychiatry. 2013;25:91–8. doi: 10.3969/j.issn.1002-0829.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Yue L, Xiao S. Prospective Associations of Tea Consumption With Risk of Cognitive Decline in the Elderly: A 1-Year Follow-Up Study in China. Front Nutr. 2022;9:752833. doi: 10.3389/fnut.2022.752833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Wang Y, Yuan Y, et al. Gender differences in the protective effects of green tea against amnestic mild cognitive impairment in the elderly Han population. Neuropsychiatr Dis Treat. 2018;14:1795–801. doi: 10.2147/NDT.S165618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Wang T, Xiao S. Type 2 diabetes mellitus might be a risk factor for mild cognitive impairment progressing to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2016;12:2489–95. doi: 10.2147/NDT.S111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B-F, Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci . 2002;15:83–96. [PubMed] [Google Scholar]
- 22.Wolz R, Schwarz AJ, Yu P, et al. Robustness of automated hippocampal volumetry across magnetic resonance field strengths and repeat images. Alzheimers Dement. 2014;10:430. doi: 10.1016/j.jalz.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Ciesielska N, Sokołowski R, Mazur E, et al. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50:1039–52. doi: 10.12740/PP/45368. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Jia J, Yang Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J Alzheimers Dis . 2016;53:487–96. doi: 10.3233/JAD-160119. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoo ZH, Candlish J, Teare D. What is an ROC curve? Emerg Med J . 2017;34:357–9. doi: 10.1136/emermed-2017-206735. [DOI] [PubMed] [Google Scholar]
- 27.Sanford AM. Mild Cognitive Impairment. Clin Geriatr Med. 2017;33:325–37. doi: 10.1016/j.cger.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Moody JN, Valerio KE, Hasselbach AN, et al. Body Mass Index and Polygenic Risk for Alzheimer’s Disease Predict Conversion to Alzheimer’s Disease. J Gerontol A Biol Sci Med Sci. 2021;76:1415–22. doi: 10.1093/gerona/glab117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cronk BB, Johnson DK, Burns JM, et al. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010;24:126–30. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu J, Liu Q, Du Y, et al. Age- and Sex-Specific Prevalence and Modifiable Risk Factors of Mild Cognitive Impairment Among Older Adults in China: A Population-Based Observational Study. Front Aging Neurosci. 2020;12:578742. doi: 10.3389/fnagi.2020.578742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–78. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parcet MA, Adrián-Ventura J, Costumero V, et al. Individual Differences in Hippocampal Volume as a Function of BMI and Reward Sensitivity. Front Behav Neurosci. 2020;14:53. doi: 10.3389/fnbeh.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Hu Y, Zhang W, et al. Resting activity of the hippocampus and amygdala in obese individuals predicts their response to food cues. Addict Biol. 2021;26:e12974. doi: 10.1111/adb.12974. [DOI] [PubMed] [Google Scholar]
- 34.Rucker P, Ikuta T. Pituitary Gland Functional Connectivity and BMI. Front Neurosci. 2019;13:120. doi: 10.3389/fnins.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]



