Abstract
Introduction
Optimally measuring improvements in chronic breathlessness in clinical practice and research continues to evolve. The aim of this study was to consider the performance of uni-dimensional measures in chronic breathlessness limiting exertion.
Methods
We report five measures of breathlessness (intensity: worst, best and average in the previous 24 hours; breathlessness now; and an affective component unpleasantness now) and two clinical thresholds over baseline on their 0–100 mm visual analogue scale (8.9 mm absolute improvement; and 15% relative improvement) collected in a multi-site, randomised, double-blind, parallel-arm, placebo-controlled trial of regular, low-dose, sustained-release morphine for people with chronic breathlessness with optimally treated underlying causes.
Results
Participants (n=284) were mostly elderly men with severe, chronic breathlessness. Worst breathlessness in the previous 24 hours showed improvement in people with more severe breathlessness and chronic obstructive pulmonary disease. By contrast, breathlessness now and average breathlessness in the previous 24 hours generated similar patterns of response, as did unpleasantness now and breathlessness now. Best breathlessness added little value. The two clinical thresholds showed differing patterns of significance.
Discussion
Consistent with other recent work, worst breathlessness may be an important uni-dimensional outcome in evaluating chronic breathlessness clinically and in research. This study does not support a differential between unpleasantness now and breathlessness now, previously observed in laboratory-generated, acute-on-chronic breathlessness. Timeframe for recall (now or the last 24 hours) and the threshold for a clinical meaningful improvement (absolute (8.9 mm) or relative (15%)) affect assessment performance.
Keywords: Perception of Asthma/Breathlessness, COPD Exacerbations, Drug reactions
WHAT IS ALREADY KNOWN ON THIS TOPIC
There are many measures of breathlessness available to clinicians and researchers and the timeframes of recall that they cover. Understanding the performance of each tool is necessary to refine the choices made for each of these settings.
WHAT THIS STUDY ADDS
This study provides prospectively collected data of five uni-dimensional tools collected simultaneously and two different thresholds to compare and contrast their performance.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
For clinicians, the use of an absolute change in patient-reported rating of worst breathlessness in the previous 24 hours is a reasonable tool when evaluating an intervention. Average breathlessness in the previous 24 hours may also add some value in assessing people with long-term breathlessness.
Introduction
Chronic breathlessness at rest or on minimal exertion is prevalent in people with advanced disease and is associated with poorer perceived health,1 disability,2 reduced quality of life,3 increased levels of anxiety and depression,4 increased unplanned healthcare contact5 6 and increased mortality.3 7 Despite its widespread consequences, chronic breathlessness is often invisible because people progressively adapt to the symptom by avoiding activities that trigger or worsen chronic breathlessness, especially in late-stage disease.8,10 Chronic breathlessness needs to be proactively diagnosed, assessed and managed in people at risk of developing the symptom.
How best to measure chronic breathlessness continues, its impacts on patients and their caregivers, and any responses to symptomatic therapies continue to evolve.11 Many uni- and multi-dimensional measures of breathlessness have been proposed, each with their own advantages and disadvantages.12 The setting in which the tool will be used will also influence the choice: a clinical tool in the outpatient or community health setting is likely to need to be brief and used as a symptom screening tool where, if positive, a more detailed history will be required. In research, a more comprehensive (and hence longer) overview of chronic breathlessness and its impacts may be required from every participant in a study. Of the uni-dimensional tools, a recent mixed-methods study of 22 participants in a randomised controlled trial of mirtazapine for the symptomatic reduction of chronic breathlessness suggests that worst breathlessness in the previous 24 hours may most closely reflect the qualitative changes reported by participants.13
Identifying how best to measure chronic breathlessness would help to optimise the evaluation of interventions that sought to reduce the symptom in clinical trials. In clinical practice, a screening question aimed at the aspect of chronic breathlessness most responsive to interventions may help to increase emphasis on the recognition, assessment and management of this symptom.8 14 By providing a tool that can be used easily by all clinicians (allied health practitioners, nurses and medical practitioners), the outcomes for people with chronic breathlessness may be systematically improved.
The aim of this pre-planned exploratory analysis of a large, multi-site, double-blind, placebo-controlled, parallel-arm, randomised controlled trial (where the primary outcome was breathlessness now) of regular, low-dose, sustained release morphine for the symptomatic reduction of chronic breathlessness15 was to report five measures of breathlessness (intensity: worst, best and average breathlessness in the previous 24 hours) and an affective component (unpleasantness now) alongside the primary outcome of the study: breathlessness now.
Methods
Setting
This was a multi-site study in 14 Australian respiratory and palliative services across four states. Participants were drawn from inpatient, outpatient and community settings.
Participants
Participants had clinician-rated modified Medical Research Council (mMRC) breathlessness scale rating (measuring level of breathlessness limiting exertion) ≥2 at screening despite physician-confirmed optimal treatment of underlying cause(s).15 (Of note, participants’ self-rating showed a wider spread of mMRC assessments at baseline (table 1).
Table 1. Baseline characteristics of participants and completion rates in a multi-site, placebo-controlled, parallel-arm study of 20 mg morphine daily for chronic breathlessness by study arm (n=284).
| Intention-to-treat—whole population | |||||
| Morphine n=145 | Placebo n=139 | ||||
| Clinico-demographic data | |||||
| Age (years) | Mean (SD) | 74.0 (9.6) | 74.5 (9.1) | ||
| Range | 44.8, 94.1 | 44.3, 89.4 | |||
| Sex n (%) | Female | 52 (35.9%) | 52 (37.4%) | ||
| Functional status (Australia-modified Karnofsky Performance Score: 0–100) | Mean (SD) | 61 (12) | 62 (10) | ||
| Range | 30, 90 | 40, 80 | |||
| Body mass index (kg/m2) | Mean (SD) | 25.2 (7.6) | 25.9 (7.0) | ||
| Range | 13.0, 66.1 | 12.3, 47.8 | |||
| Charlson Co-morbidity Index (CCI) | Mean (SD) | 3.3 (2.5) | 3.2 (2.5) | ||
| Range | 0, 12 | 1, 13 | |||
| Baseline chronic breathlessness assessments | |||||
| Primary clinician-assessed cause for chronic breathlessness n (%) | COPD | 82 (56.6%) | 82 (59.0%) | ||
| Mixed/Other | 37 (25.5%) | 35 (25.2%) | |||
| Cancer | 26 (17.9%) | 22 (15.8%) | |||
| Self-rated modified Medical Research Council (mMRC) score at baseline (0–4)* n (%) | 1 | 18 (14.1%) | 12 (10.3%) | ||
| 2 | 22 (17.2%) | 25 (21.6%) | |||
| 3 | 33 (25.8%) | 33 (28.4%) | |||
| 4 | 55 (43.0%) | 46 (39.7%) | |||
| Baseline mean (SD) breathlessness scores0–100 mm visual analogue scale (VAS) | Intensity/severity† | In the previous 24 hours | Worst | 58.5 (23.8) | 60.7 (24.9) |
| Best | 28.3 (21.3) | 30.1 (20.5) | |||
| Average | 41.2 (18.5) | 43.8 (20.6) | |||
| Now | Now | 40.9 (22.0) | 42.9 (23.1) | ||
| Affective‡ | Unpleasantness | 37.5 (22.0) | 37.4 (23.7) | ||
| Oxygen use n (%) | Yes | 87 (60.0%) | 75 (54.0%) | ||
| Completion of all 7 days of therapy in the allocated arm n (%) | 112 (77.2%) | 121 (87.1%) | |||
This is in contrast to clinician-rated mMRC at screening.
Anchored at ‘none’ and ‘worst’ possible.
Anchored at ‘none’ and ‘the most unpleasant that I have ever felt.’.
COPDchronic obstructive pulmonary disease
Intervention
Participants were randomised at baseline to either 20 mg of once-daily sustained release morphine or identical placebo for 1 week in this parallel arm study. This allowed for approximately 5 days of therapy after the participant achieved steady state of the sustained release preparation. Additionally, both groups could take up to six doses per day of 2.5 mg, of ‘as needed’, open-label immediate-release morphine which was a requirement of the primary Human Research Ethics Committee overseeing the trial.
Outcomes
The study’s primary outcome compared baseline breathlessness now on a 0–100 mm visual analogue scale (VAS) with the mean scores from days 5–7 on an intention-to-treat basis. Secondary VAS outcomes included worst, best and average breathlessness in the previous 24 hours (intensity) and unpleasantness of breathlessness now (an affective component) each averaged over days 5–7 and compared with baseline.
Two outcome thresholds were compared: an absolute reduction of 8.9 mm based on earlier studies of chronic breathlessness that defined a minimal clinically important decrease in chronic breathlessness16 17 and a relative reduction of 15% over baseline (arbitrarily derived from similar studies in pain).18
Analyses
Analyses were dichotomised for mMRC breathlessness scale self-reported assessment at baseline between mMRC 1,2 and mMRC 3,4—the latter group reflecting the trial population originally envisaged in this study.15 (A key eligibility criterion was expanded two-thirds the way through the study where clinician-rated screening mMRC scores were increased from mMRC 3,4 to mMRC 2–4.) The second dichotomisation was for chronic obstructive pulmonary disease (COPD) compared with other underlying causes as COPD accounted for approximately one-half of the study population and was considered a reasonable clinical distinction to make, given that there are apparent differences in response to opioids by underlying diagnoses.19
Multiple imputation was used for missing data using Markov Chain Monte Carlo multiple imputation with 50 samples redrawn for all reported primary and secondary outcomes in this exploratory study.
The population is described. Analysis of covariance assessed the changes from baseline to the mean of days 5–7 for the continuous visual analogues scale (VAS) variables. Logistic regression was used to analyse changes in the four secondary breathlessness measures captured using a 0–100 mm VAS each using the two thresholds of response (8.9 mm and ≥15% (the basis of the original power calculation for the study). Factors included in the model were strata, subgroup (COPD—yes/no and mMRC—1,2 or 3,4), and treatment group; and subgroup-by-treatment testing an interaction term. Stratification factors were: centre for the COPD and mMRC/COPD subgroup analyses; centre and baseline cause of breathlessness for the mMRC subgroup analysis. Analyses were performed with SAS V.9.4 (SAS, Carey, NC, USA). Given that this was an exploration of secondary outcomes, no adjustment was made for multiple testing.
Consent, ethics, trial registration and reporting
The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12609000806268) before any recruitment commenced. The study was conducted in accordance with the principles of Good Clinical Practice.20 This study’s reporting reflects the relevant Consolidated Standards of Reporting Trials statement.21
Results
284 trial participants consented to the study reflecting a typical population of people with chronic breathlessness: mean±SD age was 74±9 years; 63% were male; 59% had a self-rated mMRC ≥3 and 58% used supplementary oxygen; mean body mass index was 26±7 kg/m2 and mean Australia-modified Karnofsky Performance Status was 62±11.15 Baseline scores by arm to which people were randomised (table 1) and by diagnosis (table 2) were similar.
Table 2. Baseline characteristics of participants and completion rates in a multi-site, placebo-controlled, parallel arm study of 20 mg of sustained release morphine daily by dominant underlying cause of chronic breathlessness (n=284).
| Dominant cause of chronic breathlessness | |||||
| COPD n=164 | Other n=120 | ||||
| Chronic breathlessness assessments | |||||
| Baseline mean (SD) breathlessness scores0–100 mm visual analogue scale (VAS) | Intensity/severity* | In the previous 24 hours | Worst | 60.3 (23.2) | 58.5 (24.8) |
| Best | 28.7 (20.8) | 29.7 (21.2) | |||
| Average | 42.1 (19.3) | 42.8 (20.0) | |||
| Now | Now | 41.4 (22.4) | 42.5 (22.9) | ||
| Affective† | Unpleasantness | 36.4 (23.1) | 39.0 (22.4) | ||
| Completion of all7 daysof therapy n (%) | 135 (82.3%) | 98 (81.7%) | |||
Anchored at ‘none’ and ‘worst’ possible.
Anchored at ‘none’ and ‘the most unpleasant that I have ever felt.’.
COPDchronic obstructive pulmonary disease
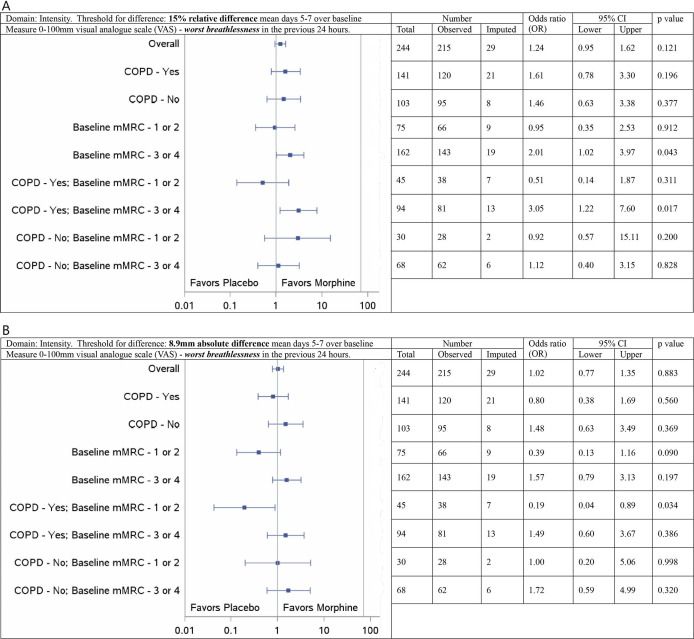
Based on a 15% improvement in worst breathlessness in the previous 24 hours, there was a significant signal favouring morphine in the subgroup of people with mMRC ≥3 with COPD as the main cause of breathlessness (OR of a response compared with placebo 3.05, 95% CI 1.22, 7.60; figure 1A). Based on the minimal clinically important improvement (an absolute 8.9 mm), the same subgroup did not have a significant result (OR 1.49, 95% CI 0.60, 3.67; figure 1B). Based on a 15% improvement, there was no signal for people with mMRC ≤2 and COPD (OR 0.51, 95% CI 0.14, 1.87; figure 1A) noting that the point estimate favoured placebo; the direction of signal was similar when response was based on an absolute difference of 8.9 mm (OR 0.19, 95% CI 0.04, 0.89; figure 1B).
Figure 1. Subgroup analysis from a parallel arm, randomised, placebo-controlled clinical trial with multiple imputations for missing values: forest plot of treatment reporting ORs for visual analogue scores of mean worst breathlessness response at days 5–7 compared with baseline by subgroup on an intention to treat basis. (A) 15% improvement over baseline. (B) 8.9mm improvement over baseline. n=284. COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council breathless scale.
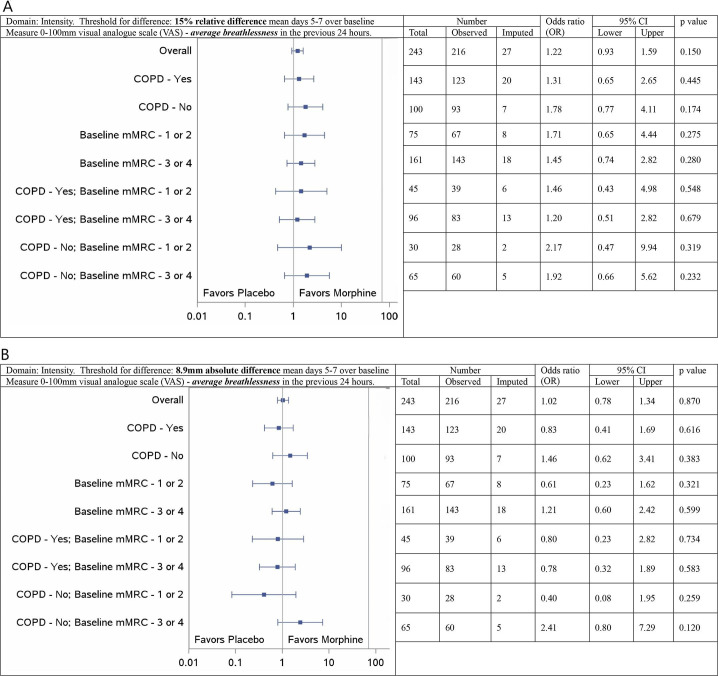
For average breathlessness in the previous 24 hours, there were no signals for differences between morphine and placebo with either clinical threshold (figure 2A,B), or for best breathlessness in the previous 24 hours (online supplemental web appendix 1). Breathlessness has already been reported but is included in this reporting format for completeness (online supplemental web appendix 2).
Figure 2. Subgroup analysis from a parallel arm, randomised, placebo-controlled clinical trial with multiple imputations for missing values: forest plot of treatment reporting ORs for visual analogue scores of mean average breathlessness response at days 5–7 compared with baseline by subgroup on an intention to treat basis. (A) 15% improvement over baseline. (B) 8.9mm improvement over baseline. n=284. COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council breathless scale.
At a threshold of 15% relative improvement, unpleasantness now in people with more severe breathlessness (mMRC 3,4) appeared to benefit from morphine (OR 2.09; 95% CI 1.05, 4.14), a signal that was further amplified in those with more severe breathlessness and a diagnosis other than COPD (OR 3.41; 95% CI 1.13, 10.23). Using an absolute threshold of 8.9 mm improvement over baseline, these signals disappeared (more severe chronic breathlessness (mMRC 3,4; OR 1.18; 95% CI 0.59, 2.38; more severe chronic breathlessness (mMRC 3,4) and a diagnosis other than COPD (OR 1.58; 95% CI 0.53, 4.69; online supplemental web appendix 3).
Discussion
The findings of this exploratory analysis show that the significance of effect differs depending on the uni-dimensional measure and the definition of clinical response. Although this is expected intuitively, quantifying these differences helps to optimise future study design and refine clinical care. The participants in this study are broadly representative of people with chronic breathlessness limiting exertion when compared with population surveys that are independent of health service utilisation.4 22 Despite activity-related differences in breathlessness by sex in laboratory-induced acute breathlessness in young healthy volunteers,23 this study did not see such differences in the setting of chronic breathlessness.
Scores for breathlessness now and unpleasantness of breathlessness now in chronic breathlessness from this data set were almost indistinguishable.24 This is in contrast to studies which reported differences between the two measures. A fundamental difference may be that the current study reports on people in day-to-day life while the paper by Leupoldt was in people with chronic breathlessness who had acute-on-chronic breathlessness generated in the laboratory.25
There were also similarities in the point estimates and distributions of breathlessness now and average breathlessness in the previous 24 hours. This may reflect the phenomenon of the ‘peak-end rule’ where the perception rated now relates most to the worst (peak) or most recent (end) measurement.26 Given the similarities in the outcomes, the ‘end’ appears to be more dominant than the ‘peak’ in this dataset. Best breathlessness was also measured but provided no useful signal in the study.
Any measurement of breathlessness intensity or the affective component of unpleasantness now requires standardisation of exertion or the period of rest before it is measured. Their utility without such standardisation is limited. Perception of breathlessness in this setting would differ with the level of exertion that precedes the measurement and likely with other factors including weather (temperature, humidity) or a person’s emotional state (anxiety, depression).
This study also highlights the differences in outcomes reported depending on whether absolute or relative changes over baseline are used. Between commencing recruitment and completing the study, the paper outlining the case for absolute differences (rather than relative differences) was published.17 Additionally, since this study was conceived, minimal clinically important improvements for each of the five measures have been reported.16 At a broader level, the challenge of new science becoming available between commencing a randomised controlled trial and its completion, especially when it relates to the primary outcome, requires further refinement. Randomised trials in frail populations are difficult to conduct and therefore take long periods of time to recruit to the calculated sample size, even in the setting of multi-site engagement. A potential solution is to consider whether outcome measures and analysis plans should be formally reviewed by the data safety monitoring committee of the study before the dataset is finally locked for analysis if such advances in the science of the topic being studied have been achieved.
A more recent randomised controlled trial in people with chronic breathlessness (mMRC ≥3) and COPD did not show any advantage of sustained release morphine (doses 8–32 mg) over placebo to reduce worst breathlessness.16 Another recent randomised controlled trial showed that sustained release morphine (20–30 mg/day) may improve health status in people with COPD and worst breathlessness in a similar cohort.17 It is unclear what contributes to these differences in outcomes. Although all three studies achieved steady state pharmacokinetically well before the measurement of the primary outcome, the pharmacodynamic effects of morphine on the symptomatic reduction beyond 1 week are yet to be defined and may help to explain some of the benefits reported in the study with the longer follow-up period.27
Implications for clinical practice
In clinical practice, this study suggests that how we ask about breathlessness is critically important to whether we will recognise the presence of the symptom and therefore how we further assess and manage this. Further work is needed, but questions about breathlessness may need to be specific to particular patient phenotypes. At best, the measures reported in this paper are rudimentary screening tools where, any positive response, requires more in-depth assessment. Questions such as which activities can now only be done with difficulty, reduced or ceased may better alert clinicians to the presence and impacts of chronic breathlessness.28 The threshold at which a patient suggests that s/he has had an appreciable response to a therapy continues to need additional research from other large, prospectively collected databases as this may also be dependent on the underlying cause(s), duration of breathlessness and its severity.
Implications for research
It is timely to consider whether greater consensus can be reached in how best to succinctly measure the presence, severity and impact of breathlessness in clinical trials. Given that there is a trade-off between the length of measurement tools and the quality and completeness of data, it is important to agree on the tool(s) that can provide the most information while being least burdensome with an understanding that clinical care and research may require different tools.
Strengths
This is a large dataset which was collected prospectively in the setting of a controlled clinical trial. The population of older people is largely representative of the people who report chronic breathlessness in population studies.4 In population studies, the sex of people reporting chronic breathlessness is equally split between males and females4 despite differences in the perceptions of breathlessness when measured in the laboratory,23 but those who report COPD (the major underlying aetiology of breathlessness in this study and in population studies) are more likely to be male. This study therefore reflects the population of people with chronic breathlessness seen in clinical consultations. Quality of the data was high.
Limitations
This study reports multiple exploratory analyses of secondary outcomes demonstrating variation, some of which will be random. Any interpretation of these results should be done with caution.
Conclusion
Worst breathlessness in the previous 24hours showed a signal for people with more severe breathlessness and COPD with a relative improvement over baseline as did unpleasantness now in people with more severe breathlessness but a diagnosis other than COPD. These signals disappeared with an absolute threshold for response. Exploring the meaning of such changes and optimising outcomes defined as important by patients and carers is key to improving our understanding of the symptomatic reduction of chronic breathlessness.
supplementary material
Footnotes
Funding: Funding was provided for the study by the Australian Government Department of Health through the National Palliative Care Programme. There is no grant/reference number attached to this funding.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants. The study was approved by the Southern Adelaide Clinical Research Ethics Committee (Dnr: EC00188) and site-specific approvals were provided by all sites before recruitment commenced at each site. All participants provided written informed consent before commencing the study.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Diana Ferreira, Email: diana.mbhf@gmail.com.
Magnus Ekström, Email: pmekstrom@gmail.com.
Sandra Louw, Email: Sandra.Louw@McCloudCG.com.
Philip McCloud, Email: philip.mccloud@McCloudCG.com.
Miriam Johnson, Email: Miriam.Johnson@hyms.ac.uk.
Katherine Clark, Email: katherine.clark@health.nsw.gov.au.
David Currow, Email: dcurrow@uow.edu.au.
Data availability statement
Data are available upon reasonable request.
References
- 1.Kochovska S, Chang S, Olsson M, et al. Associations in Perceived Health and Persistent Breathlessness: A Cross-Sectional Study. Palliat Med Rep . 2023;4:120–6. doi: 10.1089/pmr.2022.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochovska S, Ferreira D, Chang S, et al. Disability and long-term breathlessness: a cross-sectional, population study. BMJ Open Respir Res . 2024;11:e002029. doi: 10.1136/bmjresp-2023-002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currow DC, Chang S, Grande ED, et al. Quality of Life Changes With Duration of Chronic Breathlessness: A Random Sample of Community-Dwelling People. J Pain Symptom Manage. 2020;60:818–27. doi: 10.1016/j.jpainsymman.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Currow DC, Chang S, Reddel HK, et al. Breathlessness, Anxiety, Depression, and Function-The BAD-F Study: A Cross-Sectional and Population Prevalence Study in Adults. J Pain Symptom Manage. 2020;59:197–205. doi: 10.1016/j.jpainsymman.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Currow DC, Chang S, Ekström M, et al. Health service utilisation associated with chronic breathlessness: random population sample. ERJ Open Res. 2021;7:00415-2021. doi: 10.1183/23120541.00415-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karsanji U, Brunelli VN, Kochovska S, et al. Increasing medical research council breathlessness scores are associated with longer hospital stays. ERJ. 2023;62:PA5352. doi: 10.1183/13993003.congress-2023.PA5352. [DOI] [Google Scholar]
- 7.Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–40. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 8.Kochovska S, Chang S, Ferreira D, et al. Invisibility of breathlessness in clinical consultations: a cross-sectional, national online survey. Eur Respir J. 2022;60:2201603. doi: 10.1183/13993003.01603-2022. [DOI] [PubMed] [Google Scholar]
- 9.Kochovska S, Currow D, Chang S, et al. Persisting breathlessness and activities reduced or ceased: a population study in older men. BMJ Open Respir Res. 2022;9:e001168. doi: 10.1136/bmjresp-2021-001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson M, Björkelund AJ, Sandberg J, et al. Factors most strongly associated with breathlessness in a population aged 50-64 years. ERJ Open Res. 2024;10:00582-2023. doi: 10.1183/23120541.00582-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanania NA, O’Donnell DE. Activity-related dyspnea in chronic obstructive pulmonary disease: physical and psychological consequences, unmet needs, and future directions. Int J Chron Obstruct Pulmon Dis. 2019;14:1127–38. doi: 10.2147/COPD.S188141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bausewein C, Farquhar M, Booth S, et al. Measurement of breathlessness in advanced disease: a systematic review. Respir Med. 2007;101:399–410. doi: 10.1016/j.rmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Lovell N, Etkind SN, Bajwah S, et al. To What Extent Do the NRS and CRQ Capture Change in Patients’ Experience of Breathlessness in Advanced Disease? Findings From a Mixed-Methods Double-Blind Randomized Feasibility Trial. J Pain Symptom Manage. 2019;58:369–81. doi: 10.1016/j.jpainsymman.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi Z, Sandberg J, Shannon-Honson A, et al. Is chronic breathlessness less recognised and treated compared with chronic pain? A case-based randomised controlled trial. Eur Respir J . 2018;52:1800887. doi: 10.1183/13993003.00887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currow D, Louw S, McCloud P, et al. Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75:50–6. doi: 10.1136/thoraxjnl-2019-213681. [DOI] [PubMed] [Google Scholar]
- 16.Ekström M, Johnson MJ, Huang C, et al. Minimal clinically important differences in average, best, worst and current intensity and unpleasantness of chronic breathlessness. Eur Respir J . 2020;56:1902202. doi: 10.1183/13993003.02202-2019. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MJ, Bland JM, Oxberry SG, et al. Clinically important differences in the intensity of chronic refractory breathlessness. J Pain Symptom Manage. 2013;46:957–63. doi: 10.1016/j.jpainsymman.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MJ, Bland JM, Oxberry SG, et al. Measuring improvement in dyspnoea: should absolute or relative values be used? Eur Respir J . 2014;44:1700–3. doi: 10.1183/09031936.00108014. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira DH, Ekström M, Sajkov D, et al. Extended-Release Morphine for Chronic Breathlessness in Pulmonary Arterial Hypertension-A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J Pain Symptom Manage. 2018;56:483–92. doi: 10.1016/j.jpainsymman.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency . ICH Harmonised Tripartite Guideline E6: Note for Guidance on Good Clinical Practice (PMP/ICH/135/95) London: European Medicines Agency; 2002. [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulos LM, Ampon RD, Currow DC, et al. Prevalence and burden of breathlessness in Australian adults: The National Breathlessness Survey-a cross-sectional web-based population survey. Respirology. 2021;26:768–75. doi: 10.1111/resp.14070. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffer MR, Mendonca CT, Levangie MC, et al. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp Physiol. 2014;99:427–41. doi: 10.1113/expphysiol.2013.074880. [DOI] [PubMed] [Google Scholar]
- 24.Ekström M, Williams M, Johnson MJ, et al. Agreement Between Breathlessness Severity and Unpleasantness in People With Chronic Breathlessness: ALongitudinal Clinical Study. J Pain Symptom Manage. 2019;57:715–23. doi: 10.1016/j.jpainsymman.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Leupoldt A von, Bradley MM, Lang PJ, et al. Neural Processing of Respiratory Sensations when Breathing Becomes More Difficult and Unpleasant. Front Physiology. 2010;1 doi: 10.3389/fphys.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandberg J, Sundh J, Anderberg P, et al. Comparing recalled versus experienced symptoms of breathlessness ratings: An ecological assessment study using mobile phone technology. Respirology . 2022;27:874–81. doi: 10.1111/resp.14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currow DC, Quinn S, Greene A, et al. The longitudinal pattern of response when morphine is used to treat chronic refractory dyspnea. J Palliat Med. 2013;16:881–6. doi: 10.1089/jpm.2012.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochovska S, Chang S, Morgan DD, et al. Activities Forgone because of Chronic Breathlessness: A Cross-Sectional Population Prevalence Study. Palliat Med Rep . 2020;1:166–70. doi: 10.1089/pmr.2020.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.