Abstract
Introduction
Peripheral artery disease (PAD) is an atherosclerotic condition characterised by stenosis or occlusion of the arteries in the lower limbs. Patients with PAD commonly report intermittent claudication (leg pain/discomfort) during physical activities, which significantly limits the ability to walk and perform activities of daily living. Supervised exercise training is an effective therapy that can improve walking capacity in people with PAD. Emerging evidence also suggests that supervised exercise therapy following lower limb revascularisation can further enhance walking capacity when compared with revascularisation alone. However, access to dedicated exercise programmes for patients with PAD is limited in most countries, and there is a need to test the efficacy of alternative rehabilitation strategies and referral pathways. This randomised-controlled study aims to assess the efficacy of a cardiovascular rehabilitation (CR) programme versus usual care on walking capacity and quality of life in patients who have undergone lower limb revascularisation for PAD.
Methods and analysis
This will be a single-centre, prospective, parallel group, randomised-controlled trial. Sixty-six participants who have undergone a lower limb revascularisation procedure for PAD, in the previous 12 months, will be randomly allocated to a CR programme or a usual care (control) group. The CR programme will include two supervised exercise sessions per week for 6 weeks primarily consisting of intermittent treadmill walking at a moderate exercise intensity and home-based walking advice. During the 6-week programme, participants will also attend one education seminar (5.5 hours) which will cover topics such as diet, medications, exercise training and lifestyle modifications for the management of cardiovascular diseases. The control group will receive usual care and medical advice from their local doctor and vascular surgeon. The primary outcome will be 6-min walk distance. Secondary outcomes include pain-free walking distance during the six-minute walk test, maximal and pain-free walking time during a graded treadmill walking test, cardiorespiratory fitness, self-reported walking capacity, disease-specific quality of life, and self-reported and objectively measured physical activity levels. Exploratory outcomes include brachial artery flow-mediated dilation, arterial stiffness, ankle-brachial blood pressure index and biomarkers of cardiovascular disease risk. Outcomes will be assessed at baseline (week 1), following the CR/usual care period (week 8) and again at 6-month follow-up (week 34).
Ethics and dissemination
This study has received ethics approval from the Human Research Ethics Committees of Queensland Health Metro North Hospital and Health Service (94155) and the University of the Sunshine Coast (S231914). Findings from this study will be disseminated in peer-reviewed journals and through national and international conference presentations.
Trial registration number
ACTRN12623000190606.
Keywords: VASCULAR SURGERY, Exercise Test, Quality of Life, Cardiovascular Disease, Exercise
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The primary outcome of this study, 6-min walk distance, is an important clinical endpoint which correlates with mortality and morbidity rates in people with peripheral artery disease.
This study includes a large number of outcome measures aiming to assess the efficacy of cardiovascular rehabilitation (CR) on walking capacity, cardiorespiratory fitness, disease-specific quality of life, accelerometer-derived physical activity and cardiovascular function.
The same investigators who will deliver the CR programme will also be involved in the collection of outcome data; however, to reduce the risk of bias, all data analysis will be undertaken in blinded fashion using coded data.
Introduction
Peripheral artery disease (PAD) is an atherosclerotic condition characterised by stenosis or occlusion of the arteries of the lower limbs. Worldwide, PAD affects over 230 million adults, and its prevalence is expected to further increase over the coming years due to the ageing of the population.1 People with PAD are limited by intermittent claudication (leg pain/discomfort) which significantly impairs walking capacity, physical activity levels and quality of life.2,4 Reduced walking capacity and physical inactivity further contribute to the elevated risk of secondary cardiovascular events (stroke, myocardial infarction, cardiovascular death) and associated hospitalisation.5,8
The initial treatment for PAD includes medical management of symptoms and cardiovascular disease (CVD) risk factors with pharmacotherapies and lifestyle modification.9 In patients with advanced PAD, including limiting claudication or chronic limb-threatening ischemia, lower limb revascularisation procedures are indicated to restore blood flow and ‘save’ the affected limb.9 Lower limb revascularisation procedures are associated with improvements in limb blood flow,10 walking capacity11 and quality of life.12 13 However, despite improvements in limb blood flow, the improvements in walking capacity are generally only modest after lower limb revascularisation (~60% improvement) when compared with exercise therapy (~110%).14 Furthermore, the benefits of revascularisation for walking capacity and quality of life are short-lived, with prospective studies reporting deteriorations in walking capacity as early as 12 months after revascularisation.15,17 Reintervention rates are also high in people with PAD with a meta-analysis of 52 studies (n=6769 patients) reporting a reintervention rate of 18.2% (95%CI 14.5 to 22.6) at 12 months following endovascular revascularisation.18 This highlights an important limitation of lower limb revascularisation procedures for the long-term durability and improvement of walking capacity in patients with PAD.
Supervised exercise is an effective therapy that is widely recommended in several international guidelines for the management of patients with PAD.919,22 A large body of evidence suggests that supervised exercise programmes, incorporating aerobic and resistance exercises of the lower limbs, improve walking capacity,23,25 physical activity levels26 27 and quality of life28 29 in patients with PAD. A commonly used assessment of walking capacity for patients with PAD is the six-minute walk test (6MWT); and evidence shows gains in 6-min walk distance can range between 45 and 80 metres following supervised exercise programmes.2630,34 Beyond the recommendation that supervised exercise should be included as part of the initial treatment of PAD, there is emerging evidence that outcomes following lower limb revascularisation can also be enhanced when combined with exercise therapy.35,37 This aligns with a recent systematic review that reported significant improvements in maximum walking distance (mean difference range: 82–321 m) and pain-free walking distance (mean difference range: 38–408 m) favouring a combined therapy approach over supervised exercise training or revascularisation alone.38 Post-revascularisation exercise therapy has also been associated with reduction in the need for reintervention when compared with revascularisation39 or supervised exercise therapy alone (OR 0.19, 95% CI 0.09 to 0.40, p<0.0001).40
Despite this strong evidence supporting the benefits of supervised exercise therapy, access to dedicated exercise programmes is very limited for patients with PAD. Previous studies report that as few as 43–48% of vascular units in the USA and the UK have access to dedicated supervised exercise programmes for the referral of patients with PAD.41 42 Similarly, a survey of 378 vascular surgeons across 43 European countries reported that only 30% (n=115/378) of surgeons have access to supervised exercise programmes for the referral of patients with PAD.43 This highlights a need for alternative rehabilitation strategies and referral pathways to increase the access to supervised exercise therapy for patients with PAD.
Cardiovascular rehabilitation (CR) is a well-established multidisciplinary approach for the care and rehabilitation of patients with heart disease, particularly those recovering from myocardial infarction or cardiac surgery.44 CR programmes typically consist of supervised exercise training, dietary and lifestyle advice, psychological support and education on the management of CVD risk factors. Studies report that CR programmes are cost-effective for improving functional capacity, physical activity levels and quality of life and reducing the risk of secondary cardiovascular events in patients with cardiac diseases.45,48 While CR programmes are widely accessible in most countries, patients with PAD are historically seen as out of scope and are not usually referred for CR.44 49 To date, very few studies have investigated the effectiveness of routine CR for patients with PAD.50,55 Most of these studies have been limited to the investigation of patients with coronary artery disease referred for CR who also had PAD as a comorbidity.51,54 In Canada, of 23 215 patient referrals with coronary artery disease, 5.9% (n=1366 patients) were identified as having a comorbidity of PAD.51 The identified patients with PAD had significantly impaired cardiorespiratory fitness and a lower 10-year survival rate when compared with patients without PAD. Importantly, this study demonstrated that completion of CR led to significant reductions in mortality rate (adjusted HR 0.62, 95% CI 0.57 to 0.67) in patients with PAD, when compared with patients who did not attend CR.51
Recently, a small (n=20 participants), non-randomised pilot study of CR in patients who had undergone lower limb revascularisation for PAD reported that CR was safe and feasible and led to greater improvements in the 6-min walk distance (mean difference, 53 m; p=0.04) when compared with usual care.56 These findings highlight the potential for CR to be used as a standard referral pathway for patients with PAD who are recovering from a lower limb revascularisation procedure. To test this, we will conduct a randomised-controlled trial to assess the efficacy of a 6-week community-based CR programme versus usual care on walking capacity and quality of life in patients who have recently (< 12 months) undergone lower limb revascularisation for PAD.
Primary aim
To assess the efficacy of a 6-week community-based CR exercise programme versus usual care on 6-min walk distance in patients who have recently (< 12 months) undergone lower limb revascularisation for PAD.
Secondary aims
To assess the efficacy of a 6-week community-based CR exercise programme on: (1) pain-free walking distance during the 6MWT, (2) maximal walking time and pain-free walking time during a graded treadmill walking test, (3) cardiorespiratory fitness measured as peak oxygen uptake (VO2peak) during a graded treadmill walking test, (4) disease-specific quality of life and self-reported functional capacity and (5) self-reported and objectively measured physical activity levels.
Exploratory aims
To assess the efficacy of a 6-week community-based CR exercise programme on: (1) brachial artery flow-mediated dilation, (2) arterial stiffness (augmentation index (AIx), carotid-femoral artery pulse wave velocity (PWV)), (3) ankle-brachial blood pressure index and (4) circulating biomarkers of CVD risk.
Methods and analysis
Study design and overview
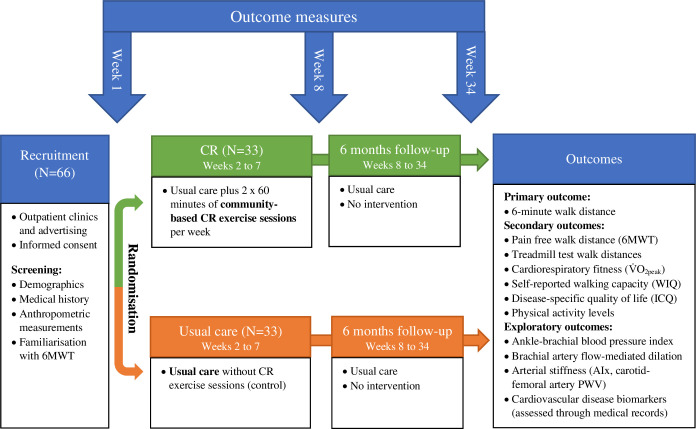
An overview of the study is shown in figure 1. This is a single-centre, prospective, parallel-group, randomised-controlled trial conducted at the University of the Sunshine Coast and the Sunshine Coast University Hospital (Australia). Patients with PAD who have recently (< 12 months) undergone a lower limb revascularisation procedure will be identified and randomly allocated to either usual care or usual care plus a 6-week community-based CR programme (n=33 per group; refer to power and sample size estimate). Participants allocated to the usual care group will receive usual care and medical advice from their local doctor and vascular surgeon. The community-based CR programme will comprise two supervised exercise sessions per week for 6 weeks, home-based exercise advice and an education seminar (5.5 hours). The CR programme will be delivered by the Cardiovascular Rehabilitation Service of the Sunshine Coast University Hospital. Primary, secondary and exploratory outcomes will be assessed at baseline (week 1), after the completion of the CR programme/usual care period (week 8) and again 6 months after the completion of the CR programme/usual care period (week 34). Maximal exercise assessments such as graded treadmill walking tests will be conducted at the Clinical Investigations Unit at the Sunshine Coast University Hospital to facilitate access to medical supervision. Other outcome measures will be conducted at the VasoActive Laboratory at the University of the Sunshine Coast. As per SPIRIT, a schedule of participant enrolment, intervention and assessments is presented in table 1.57 The study commenced in April 2024, and data collection is planned to be completed in January 2026.
Figure 1. Overview of the Saving Legs and Lives study. 6MWT, six-minute walk test; AIx, augmentation index; CR, cardiovascular rehabilitation; ICQ, intermittent claudication questionnaire; PWV, pulse wave velocity; VO2peak, peak oxygen uptake; WIQ, walking impairment questionnaire.
Table 1. Schedule of participant enrolment, intervention and assessment.
| Milestones | Activity | Screen | Baseline (preintervention) | Postintervention | Follow-up | ||
| Week | 0 | 1 | 8 | 34 | |||
| Visit (timepoint) | 1 | 2 | 3 | 4 | 5 | 6 | |
| Recruitment | Patient identification | X | |||||
| Prescreen checklist for eligibility | X | ||||||
| Enrolment and screening | Consent | X | |||||
| Confirm eligibility | X | ||||||
| Demographics and health history | X | ||||||
| Familiarisation with 6MWT | X | ||||||
| Randomisation | Stratification and randomisation | X | |||||
| Intervention | Usual care plus community-based CR programme (weeks 2 to 7) |

|
|||||
| Control | Usual care (weeks 2 to 7) |

|
|||||
| Primary outcome | 6MWT | X | X | X | |||
| Secondary outcomes | Treadmill walking test and cardiorespiratory fitness test with ECG* | X | X | ||||
| Quality of life (WIQ, ICQ) | X | X | X | ||||
| Physical activity levels (7 day accelerometer, physical activity survey) | X | X | X | ||||
| Exploratory outcomes | Ankle-to-brachial systolic blood pressure index | X | X | X | |||
| Brachial artery flow-mediated dilation assessment | X | X | X | ||||
| Arterial stiffness assessments (AIx, carotid-femoral artery PWV) | X | X | X | ||||
| Markers of CVD (total cholesterol, LDL, HDL triglycerides, HbA1c) | X | X | |||||
Note:All participants will continue to receive usual care and medical advice from their general practitioner (local doctor) and vascular surgeon, and they will be randomly allocation to a community-based cardiovascular rehabilitation programprogramme (intervention) or a usual care group (control) for 6-weeks weeks. Prior to randomisation, participants will be stratified to account for type of procedure (eg, open surgical vs endovascular procedure) and time since procedure (< 12 weeks vs >12 weeks). Outcome measures will be assessed at baseline (week 1), at the end of the intervention / usual care period (week 8) and again at 6-month follow-up (week 34).
As the treadmill walking test and the cardiorespiratory fitness test are secondary outcomes, participants will be given the option to opt out of performing those assessments. For the post-intervention assessments at weeks 8 and 34, the assessment window may be extended by up to 7 days days to accommodate unforeseen circumstances (eg, participant illness).
AIx, augmentation index; CR, cardiovascular rehabilitation; CVD, cardiovascular disease; HbA1c, haemoglobin A1cHDL, high-density lipoprotein; ICQ, intermittent claudication questionnaire; LDL, low-density lipoprotein; 6MWTsix-minute walk testPWV, pulse wave velocity; WIQ, Walking Impairment Questionnaire
Participants and eligibility criteria
Potential participants will be identified from the Sunshine Coast region through: (1) an existing database of participants who have previously provided consent to be contacted, (2) collaborating vascular surgery clinics including the Sunshine Coast University Hospital, and (3) community sources and advertising.
Participants will be eligible to participate in the study if they:
Are 18 years of age or older and have a formal diagnosis of PAD made by a vascular surgeon.
Have undergone a lower limb revascularisation procedure (endovascular procedure, open surgical procedure or hybrid procedure) in the previous 12 months.
Have clearance to participate from their treating vascular surgeon, including verification that they have adequately recovered from any lower limb revascularisation procedure.
Can understand and communicate in English sufficient to provide informed consent.
Participants will be excluded from participation if they meet any of the following criteria:
Unable to walk independently (eg, depend on assistance from a walking aid).
Previous lower limb amputation or current tissue necrosis (ulceration or gangrene) that limits the ability to undertake walking tests.
Deemed not eligible to participate in CR by the CR clinical staff as per standard contraindications for exercise.58 These contraindications include unstable angina, acute heart failure, recent cerebrovascular event, uncontrolled resting hypertension, symptomatic hypotension, uncontrolled diabetes, uncontrolled sinus tachycardia and uncontrolled/complex arrythmias.
Currently participating in a supervised exercise rehabilitation programme.
Terminal illness or other medical condition or planned treatment that may affect the ability to participate in or complete the trial.
Intervention
Eligible participants will be randomised in equal proportions (1:1) to one of the study groups.
Usual care (control group).
Usual care plus a 6-week community-based CR programme (intervention group).
Usual care
All participants will continue to receive usual care and medical advice from their local doctor and vascular surgeon throughout the study. Usual care for PAD may include management of CVD risk factors with lifestyle modifications (eg, smoking cessation, dietary modifications) and pharmacotherapies.9 While usual care for PAD will not be altered by this protocol, on consent to the study, each participant’s local doctor and vascular surgeon will be contacted and requested to provide their best possible medical care throughout the study. Furthermore, in order to assess the efficacy of the CR programme, each participant’s local doctor and vascular surgeon will be requested to refrain from giving specific advice regarding exercise until the completion of the study.
Usual care plus community-based CR programme
In addition to usual care, participants who are randomised to the community-based CR programme will be referred to the CR programme of the Sunshine Coast Hospital and Health Service. The CR programme will be delivered at a community fitness facility. The CR programme will be structured in accordance with current exercise recommendations for people with PAD.919,22 The CR programme will include twelve 60-min sessions of supervised exercises, delivered two times per week over a period of 6 weeks and one education seminar (5.5 hours with breaks). While the recommended duration of supervised exercise training for patients with PAD is 12 weeks,19 20 improvements in walking capacity are reported after 3–6 weeks.59,61 Furthermore, the recommended duration for CR ranges between 6 and 12 weeks.62 To ensure outcomes are applicable to a wide range of CR programmes, the minimum duration for CR was selected (ie, 6 weeks). During the 6-week CR programme, participants will also be provided with exercise guidelines and advice to complete at least three home-based walking sessions per week. Following the completion of the CR programme, participants will be provided with individualised exercise and physical activity advice with the goal to meet the recommended 150–300 min of moderate intensity physical activity levels per week.58
The programme exercise sessions will be supervised by CR staff (nurses, exercise physiologist) and research personnel. The research personnel will be responsible for the prescription and progression of the exercises. As outlined in online supplemental table 1, the supervised exercise sessions will primarily consist of bouts of intermittent treadmill walking that are interspersed by periods of upper body activity and lower limb resistance exercises. Each supervised exercise session will last for 60 min, including a warm-up and a cool-down (10 min each). The total duration of treadmill walking for each session will be 10 min (eg, 5×2 min bouts) at the beginning of the programme (ie, week 1) and will progress to 30 min (eg, 15×2 min bouts) by the end of the programme (ie, week 6). The total duration of upper body and lower limb resistance training for each session will begin at 30 min (eg, 15×2 min bouts) at the beginning of the programme and will decrease to 10 min (eg, 5×2 min bouts) by the end of the programme. Exercise intensity and severity of claudication pain will be monitored with the modified rate of perceived exertion Borg scale and the intermittent claudication pain scale, respectively.63 64 Participants will be instructed to exercise at moderate to near-maximal claudication pain thresholds (ie, 3/4 on claudication scale) or, if asymptomatic, exercise at a moderate exercise intensity (ie, 3/10 on Borg scale).19 65 The initial exercise intensity will be individually prescribed based on the exercise workload achieved during the baseline exercise tests (eg, workload achieved at stage prior to treadmill test cessation).
The home-based walking sessions will also align with the current PAD exercise recommendations.919,22 Participants will be provided with individualised weekly walking goals which will be set and reviewed by the study team. During the home-based walking sessions, participants will be instructed to complete intermittent bouts of walking separated by periods of rest. Participants will be instructed to complete their walking sessions outdoors (eg, local neighbourhood and parks). Similar to the supervised exercise sessions, the total duration of walking for each home-based session will begin at 10 min (eg, 5×2 min bouts) at the beginning of the programme (ie, week 1) and will progress to 30 min (eg, 15×2 min bouts) by the end of the programme (ie, week 6). The total period of rest for each home-based walking session will be 20 min at the beginning of the programme (eg, 10×2 min bouts) and will decrease until participants are able to walk continuously for 30 min. Exercise intensity and severity of claudication pain will be self-monitored using the modified rate of perceived exertion Borg scale and the intermittent claudication pain scale.63 64 Participants will be instructed to walk at moderate to near-maximal claudication pain thresholds (ie, 3/4 on claudication scale) or, if asymptomatic, walk at a moderate exercise intensity (ie, 3/10 on Borg scale).19 65 Participants will be provided with a diary to record their home-based walking sessions.
Participants in the CR programme will attend one education seminar (5.5 hours with breaks) during the 6-week CR programme. The education seminar will be delivered by health specialists (eg, nurse, dietitian, psychologist, exercise physiologist) and will cover topics such as diet, medications, exercise training, physical activity and lifestyle modifications for the management and prevention of CVDs. The seminar information will be based on the current Australian guidelines for the management of acute coronary syndromes.66,68
Adherence
Strategies are incorporated into the protocol to promote and monitor adherence to the study intervention. The importance of attending the weekly supervised exercise sessions and accumulating the recommended weekly amount of exercise and physical activity levels will be explained to the participants in the participant information and consent form (PICF) and on starting the CR programme. Participants will also be provided with individualised weekly goals for the supervised and the home-based exercise sessions which will be set and reviewed by the study team. Adherence to the supervised and home-based exercise sessions will be assessed by recording the number of exercise sessions that participants complete each week against the goal/target for that specific week. Participants will keep a daily diary to record their home-based exercise sessions that they complete during the 6-week CR programme. Attendance to the education seminar will be assessed using an attendance checklist. The assessment of protocol adherence for the purpose of statistical analysis is described in the statistical analysis section.
Screening and enrolment (visit 1)
Prior to screening assessments, participants will be required to provide their informed consent to participate in the study which will occur at the commencement of the initial study visit (visit 1). A trained study staff member authorised by the principal investigator will take the participant through the information sheet and obtain informed consent. All participants will be fully informed of the potential risks and benefits of the study. Participants will be screened for comorbidities and cardiovascular risk factors prior to inclusion in the study. During this visit, prescribed medications will be captured, and anthropometric measurements (eg, height, weight) and resting blood pressure will be conducted. Participants will also be familiarised with the 6MWT to minimise test variability.
Randomisation and blinding
Following baseline outcome measures (ie, visit 3), participants will be randomly allocated to either the usual care group (n=33) or the usual care plus community-based CR exercise group (n=33). To ensure allocation concealment, randomisation will be generated using a secure, independent web-based randomisation system (SealedEnvelope.com). Prior to randomisation, participants will be stratified to account for type of procedure (eg, open surgical vs endovascular procedure) and time since procedure (< 12 weeks vs >12 weeks). This will allow stratification of participants who have recently undergone a revascularisation procedure (< 12 weeks) from those who have undergone a revascularisation procedure more than 12 weeks ago and have fully resumed normal activities of daily living, recreation and work activities. Block randomisation, using random block sizes of two to four participants, will be used to ensure that group allocation at any point in time remains similar. Enrolment, allocation, follow-up and final analysis will be conducted and reported in accordance with the CONSORT statement for randomised clinical trials.69
The same investigators who will deliver the CR programme will also be involved in the collection of outcome data. Therefore, participants and data collectors will not be blinded to group allocation. While it is not feasible to blind participants and investigators to group allocation in an exercise intervention study, all data analysis will be undertaken in blinded fashion using coded data.
Outcome measures and procedures
As outlined in table 1, primary, secondary and exploratory outcomes will be assessed at baseline (week 1), after the completion of the 6-week CR programme/usual care period (week 8) and again 6 months after the completion of the CR programme/usual care period (week 34). During weeks 1 and 8, participants will carry out the assessments over two visits to ensure that participants are sufficiently recovered between walking tests. As the treadmill test is a secondary outcome measure, participants will be given the option to opt out of performing this test. The treadmill test requires participants to walk until maximal exertion. Although this is an important outcome measure, only 34 participants are required to establish an effect (refer to power and sample size estimate). Therefore, participants who are unwilling to exert themselves to maximal effort, or those who are unable to maintain the walking speed of the treadmill, will be given the option to opt out of this test. At 6-month follow-up (week 34), participants will make a single visit for the assessment of the 6-min walking test, quality of life, self-reported functional capacity, physical activity levels, vascular function and biomarkers of CVD risk. The 6-month follow-up visit aims to provide an indication of longer-term durability of the effect of CR following revascularisation. For the post-intervention and follow-up assessments at weeks 8 and 34, the assessment window may be extended by up to 7 days to accommodate unforeseen circumstances (eg, participant illness).
Primary outcome
Six-minute walk test (6MWT)
The 6MWT will be conducted at weeks 1, 8 and 34. Change in 6-min walk distance between baseline and week 8 is the primary outcome for the study. Change in the pain-free walking distance during the 6MWT is a secondary outcome measure.
As per standard procedures, a course of 30 metres length is marked out in a covered area at least 2 metres in width, with a cone at each end.70 Chairs are also placed every 10 metres along the course so that participants can sit and rest during the test if needed. Participants will be asked to walk up and down the course for 6 min and to complete as many laps and cover as much distance as possible in that time. Participants will be asked to indicate to the test supervisor when the onset of claudication occurs and then to rate the severity of their claudication/discomfort using a hand signal at the completion of each lap (ie, every 60 metres) using the claudication rating scale.64 During the test, heart rate will be continually monitored with a heart rate monitor and recorded at the end of each lap. During the test, participants can stop walking and rest if their claudication pain becomes intolerable; however, the timing continues and participants are requested to resume walking as soon as possible. At the end of the test, the number and timing of any rest breaks, the time and distance to the onset of claudication (pain-free walking distance) and the total distance walked (6-min walk distance) are recorded. At the end of the test, participants will be asked to provide a rating of their general exertion using the modified rate of perceived exertion Borg Scale.63
Walking capacity measured during the 6MWT has been chosen as the primary outcome as it has excellent test-retest reliability (interclass correlation coefficient=0.970, 95% CI 0.950 to 0.981, n=173),71 and it correlates strongly with a range of relevant clinical outcomes including physical activity,72 patient-reported outcomes, as well as cardiovascular morbidity and mortality associated with PAD.7 Based on this strong reliability, a reported advantage of the 6MWT for clinical trials is that there is no learning effect.73 Nonetheless, participants will be familiarised with the 6MWT prior to the baseline assessment in the current study. This approach is consistent with recommended practice and reporting of performance outcomes for clinical trials in patients with PAD.74 The minimal clinically important difference (MCID) for the 6-min walk distance has been established for people with and without PAD. Based on the change in the 6-min walk distance with exercise therapy and the corresponding change in reported physical function, the MCID thresholds are 12 metres (small effect), 32 metres (moderate effect) and 34 metres (large effect).75
Secondary outcome measures
Graded treadmill walking test
The graded treadmill walking test including measures of maximum walking time and pain-free walking time will be performed at weeks 1 and 8. The Gardner-Skinner protocol will be used, which was specifically developed for the assessment of walking capacity in patients with PAD.76 77 The treadmill will start at 3.2 km/h at a 0% incline, and then every 2 min, the gradient of the treadmill will increase by 2%. Adjustments will be made to the treadmill protocol using standardised procedures for participants who are unable to maintain the 3.2 km/h treadmill speed. The treadmill test will be conducted and supervised by an exercise physiologist, a cardiac technician and a medical doctor. During the test, participants will be monitored with a continuous 12-lead ECG, and heart rate and blood pressure will be measured and recorded at the end of each stage (ie, every 2 min). At the end of the test, participants will be asked to rate the severity of their claudication pain in each leg using the claudication scale and to provide a rating of their general exertion using the modified rate of perceived exertion Borg scale.63 The MCID values for small, moderate and large changes in maximum treadmill walking time after supervised exercise training are 121, 141 and 241 (seconds), respectively, in patients with PAD.75
Cardiorespiratory fitness
Cardiorespiratory fitness (VO2peak) will be assessed during the graded treadmill walking test at weeks 1 and 8. Cardiorespiratory fitness is a strong predictor of CVD and all-cause mortality rates in patients with PAD.78 79 Oxygen uptake (O2) will be continuously measured with a portable O2 system (K5, COSMED, Italy) and a breath-by-breath gas exchange and ventilation face mask. VO2peak will be determined as the highest 15-s average during the final 60 s of peak exercise.
Quality of life
Disease-specific quality of life will be assessed using the intermittent claudication questionnaire (ICQ) at weeks 1, 8 and 34. The ICQ is a self-administered tool consisting of 16 items that focus on limitations imposed by claudication while performing various tasks, such as walking specific distances or performing activities of daily living.80 The instrument is scored by summing the patient responses to individual items, which are all equally weighted and transformed to a 0 to 100 composite score, where 0 is the best score. The composite score will be calculated and used as the outcome for analysis.
Self-reported walking capacity
Self-reported walking capacity will be assessed using the walking impairment questionnaire (WIQ) at weeks 1, 8 and 34. The WIQ is a PAD-specific measure of self-reported difficulty during walking with three domains: walking distance, walking speed and stair climbing.81 Each domain is scored on a scale from 0 to 100 (100 indicating the best possible score). A small, moderate and large MCID for each of the three WIQ domain scores are: 6, 14 and 23 for walking distance; 4, 11 and 18 for walking speed; and 6, 15 and 23 for stair climbing, respectively.75
Physical activity levels
Objectively assessed physical activity. Free living physical activity levels will be objectively assessed using a GT9XActiGraph accelerometer (ActiGraph, Pensacola, FL, USA) at weeks 1, 8 and 34. Participants will be instructed to wear the device on their non-dominant wrist for 7 full days at each assessment point.82 At the end of the recording period, the accelerometer is removed by the participant and returned to the research team (in person or by reply-paid delivery) for data upload, quality assurance and analysis. The ActiGraph accelerometer will be initialised to collect raw data at 100 Hz.83 The in-built inclinometer will also enable the assessment of body position (ie, sitting/lying vs standing). At each assessment period, a minimum wear-time criteria of 4 days and 600 min per day will be applied.84 The ActiLife software (V.6.13.5; AcriGraph LLC) will be used to process the raw data to create 60-s epochs.83 The data will be processed using the Choi algorithm within the ActiLife software to define wear and non-wear minutes.85 The primary outcome measure of physical activity will be steps per day. Other outcome measures will include sedentary time and time spent (min/day) engaging in light, moderate and vigorous physical activity. During the 7-day monitoring period, participants will also keep a brief daily physical activity diary to record periods of sleep, work, non-wear time and structured exercise that are essential for analysis and cannot be inferred from the monitor data alone. The ActiGraph accelerometer has been reported to be reliable and valid in the assessment of walking, body posture, and sedentary behaviour during free-living activity86,88 and when used in patients with PAD.89,91 The MCID values for small, moderate and large changes in total daily steps after supervised exercise training are 569, 1423 and 2277 (steps/day), respectively, in patients with PAD.92
Self-reported physical activity. Self-reported physical activity levels will be assessed using the International Physical Activity Questionnaire for elderly (IPAQ-E) at weeks 1, 8 and 34. The IPAQ-E is a self-reported questionnaire which has been validated for use for individuals over the age of 65.93 The IPAQ-E consists of questions about frequency (days per week) and time (minutes per day) spent sitting, walking and performing physical activities of moderate and vigorous intensity. All self-reported activity domains (sitting, walking, moderate and vigorous physical activities) have been reported to positively correlate with corresponding variables objectively assessed by accelerometers.93
Exploratory outcomes
Ankle to brachial blood pressure index
The ankle-brachial index (ABI) of both legs will be measured at weeks 1, 8 and 34. After resting in a supine position for 10 min, brachial and ankle blood pressures will be measured. Brachial blood pressures will be measured in both arms using an automated blood pressure monitor.94 Systolic blood pressure of the dorsalis pedis artery and posterior tibial artery at the left and right ankles will also be measured using a manual cuff sphygmomanometer and handheld 5–7 MHz Doppler ultrasound probe. The average of the closest two recordings at each artery will be recorded. The ABI for each leg will be calculated by dividing the higher dorsalis pedis artery or posterior tibial artery value by the highest brachial artery value obtained from either side.59
Brachial artery flow-mediated dilation
Brachial artery flow-mediated dilation (FMD) will be measured in response to a reactive hyperaemia test (cuff occlusion) at weeks 1, 8 and 34. Brachial artery FMD is an independent predictor of cardiovascular events in patients with PAD.95 As per standard procedures,96 brachial artery FMD will be measured with participants in the supine position after 10 min of rest. This measurement will involve a rapid inflation of a pressure cuff positioned at the forearm. A 10-MHz multifrequency linear array probe, attached to a high-resolution ultrasound machine (Terason, Burlington, USA), will be used to image the brachial artery (2 cm proximal to the elbow). The ultrasound settings will be optimised for each individual and will be kept constant between all assessments. Continuous Doppler velocity will also be obtained using the ultrasound at an insolation angle of 60°. Following baseline assessments, reactive hyperaemia will be induced by inflating the cuff to 200 mm Hg for 5 min. Artery diameter and flow recordings will resume 30 s before cuff deflation and continue for 3 min thereafter.97 Brachial artery FMD will be expressed as a relative change (percent change) in peak arterial diameter from baseline (pre-cuff inflation) to post-cuff deflation. The analysis of the brachial artery FMD will be undertaken using a continuous edge-detection and wall-tracking software.
Arterial stiffness
Arterial stiffness outcomes incorporate measures of AIx and carotid-femoral artery PWV and will be assessed at weeks 1, 8 and 34. Arterial stiffness is an independent predictor of CVD and all-cause mortality rates in patients with PAD.98 99 After resting in the supine position for 10 min, brachial artery pulse waves will be obtained by partially inflating a cuff over the right brachial artery using a SphygmoCor XCEL system (AtCor Medical Pty Ltd, Sydney, Australia) and following standard guidelines.100 101 The brachial waveforms will be used to generate central aortic pressure waveforms and to determine AIx, which is the ratio of wave reflection amplitude relative to central pulse pressure. For the PWV assessment, the carotid-femoral PWV will be measured using the applanation tonometry technique. A handheld tonometer probe (AtCor Medical Pty Ltd, Sydney, Australia) will be held against the skin surface over the right carotid artery to obtain carotid-artery pulse waves, and a pressure cuff will be placed around the right upper thigh to record femoral artery pulse waves. The distance from the carotid site above the suprasternal notch to the proximal edge of a thigh cuff over the femoral artery will be measured using a tape measure over the body area. The carotid and femoral pulse waves will be recorded simultaneously, and the femoral pulse wave requires the thigh cuff to be partially inflated. The PWV will then be automatically calculated as the ratio of the distance between the pulse measuring sites to the time delay between the carotid and femoral pulse waves. PWV will be recorded as the average of triplicate measurements.
Biomarkers of cardiovascular disease (CVD) risk
Biomarkers of CVD risk will be assessed at weeks 1 and 34. The most recent blood test (within 8 weeks of baseline and within 8 weeks of follow-up visit) will be retrieved from the medical records of each participant. Biomarkers of CVD risk will include total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein and haemoglobin A1c levels.
Sample size calculations
Sample size calculations were conducted for the primary outcome 6-min walk distance and the secondary outcome maximal walking time during the graded treadmill walking test.
Six-minute walk test (6MWT)
Previous studies that assessed the effects of postrevascularisation exercise therapy indicated a potential effect of 53.2 m with an SD of 81 m for the 6-min walk distance.56 102 This would provide a medium effect size of 0.65. To establish this effect from baseline to week 8 with 80% power and an alpha 0.05, 30 participants would be required in each group. Allowing for 10% dropout, 33 participants will be recruited in each group (total n=66).
Graded treadmill walking test
A previous study that assessed the effects of postrevascularisation exercise therapy indicated a potential effect of 5 min and 46 s with an SD of 6 min and 13 s for maximal walking time during the graded treadmill walking test.37 This would provide a large effect size of 0.89. To establish this effect from baseline to week 8 with 80% power and an alpha 0.05, 17 participants would be required in each group (total n=34). As this outcome of maximal walking time during the treadmill test is a secondary outcome, participants will be given the option to opt out of performing this test during the trial.
Statistical analysis
Data analysis will follow the CONSORT statement for randomised-controlled trials.69 All data collected will be deidentified and coded throughout the trial. The data collected will remain coded for participant confidentiality purposes. Baseline data for the two groups will be provided using counts and percentages and means and standard deviations (or non-parametric equivalents) for categorical variables. Furthermore, tables will show the outcome measures at weeks 8 and 34 and percent changes from baseline.
The primary analysis will be performed based on the intention-to-treat principle, where all participants will be analysed as per their allocation, regardless of the treatment they received. Non-adherence will be assessed through per-protocol analyses. Per protocol analysis will primarily include participants that attend at least 70% of the supervised CR exercise sessions (ie, nine exercise sessions overall) during the 6-week intervention period. The total number of supervised exercise sessions completed will be included in the analysis as a covariate.
Statistical analyses will be conducted using the IBM SPSS software (SPSS Inc, Chicago, IL). The data will be tested for normality using the Shapiro-Wilk test and will be considered normally distributed when p>0.05. Analyses will be conducted using analysis of variance for repeated measures. The primary comparison will be change in 6-min walk distance from baseline to postintervention (week 8) in the CR versus the usual care group. Additional analyses will be performed from baseline to 6-month follow-up timepoint (week 34). As required, confounding variables (including comorbidities, age, sex, smoking behaviour, medications) will be adjusted for using analysis of covariance. In all analyses, p<0.05 will be considered statistically significant. Post hoc analysis will be performed when a significant effect is present.
Data management
All data collected during the study will be coded and stored for a minimum of 15 years. Prospective participants will initially be assigned a screening number, and on consent into the study, they will be assigned a participant identification code. A coding log will be maintained and kept in a secure location (hard copy in locked cabinet and electronic copy on password protected file) in accordance with the International Council on Harmonisation Good Clinical Practice guidelines, the study data management plan and the data security policy of the University of the Sunshine Coast. The only personnel who will have access to participants’ individual identity are the principal investigator (CDA) and authorised project staff. Access to the coding log would only occur in the case where further medical history information is required in relation to a specific participant, in cases of emergency (eg, to identify and contact next of kin) or during the investigation of any events (eg, serious adverse event).
All individual participant information will be de-identified in the reporting of data and resulting publications or presentations to fully protect the confidentiality of participants. Participants will be informed in the PICF that information or reports from the study will be prepared and will be submitted for publication. Participant information will normally be presented as group data. If necessary, information obtained from specific individuals may be presented; however, names will not be used to identify the individuals. Participants will only be identified in such publications by an identification number and possibly their age and gender.
Adverse events
Information on all adverse events (study-related and non-study-related) will be recorded immediately in the trial adverse event report form and in the appropriate case report form for the relevant participant. All clearly related signs, symptoms and abnormal procedural results will be recorded. For all recorded adverse events, the principal investigator or delegate will determine the adverse event’s causality to the intervention and the severity or intensity of the event. The clinical course of each event will be followed until resolution, stabilisation or until it has been determined that the study intervention or participation is not the cause. All logged events will be summarised and reported to the participant’s general practitioner and the relevant human research ethics committees and governance agencies as part of the reporting requirements.
Ethics and dissemination
This study has received ethics approval from the Human Research Ethics Committees (HREC) of Queensland Health Metro North Hospital and Health Service (94155) and the University of the Sunshine Coast (S231914). Any protocol amendments will be submitted to the aforementioned HREC for approval. Findings from this study will be disseminated in peer-reviewed journals and through national and international conference presentations.
supplementary material
Footnotes
Funding: This work is supported by a Sunshine Coast Health Institute (SCHI) Collaborative Seed Grant Scheme (Grant ID number: 2020-01) awarded to CDA and TS (Principal Investigators). KF is supported by a collaborative University of the Sunshine Coast and Sunshine Coast Hospital and Health Service scholarship as well as a PhD Top-Up Scholarship Award from the Queensland Cardiovascular Research Network (QCVRN). The aforementioned funders had no input in the design of the study and will have no role in the collection, management, analysis and interpretation of the data or decision to submit this work for publication.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-089203).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Collaborators: The following are members of the Saving Legs & Lives Trial Group: Kim Greaves, Norman Morris, Fraser D. Russell, Meegan A. Walker, Mathew J. Summers, Jill O’Donnell, Karl Schulze, Rebecca J. Magee, Daniel McGlade, Vivienne Moult, Amanda Shepherd, Michael D. Shanahan, Tuppence Richman, Caitlin Coppock, Helen J. Rodgers, Ashley R. Samarasinghe, Damien P. Kerley, Lisa M. Polowyi.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Contributor Information
Krist Feka, Email: krist.feka@research.usc.edu.au.
Pankaj Jha, Email: pankaj.jha@health.qld.gov.au.
Michelle Aust, Email: michelle.aust@health.qld.gov.au.
Joseph J. Scott, Email: jscott4@usc.edu.au.
Mia Schaumberg, Email: mschaum1@usc.edu.au.
Tony Stanton, Email: Tony.Stanton@health.qld.gov.au.
Christopher D. Askew, Email: caskew@usc.edu.au.
Saving Legs & Lives Trial Group, Email: kfeka@usc.edu.au.
Saving Legs & Lives Trial Group:
Kim Greaves, Norman Morris, Fraser D. Russell, Meegan A. Walker, Mathew J. Summers, Jill O’Donnell, Karl Schulze, Rebecca J. Magee, Daniel McGlade, Vivienne Moult, Amanda Shepherd, Michael D. Shanahan, Tuppence Richman, Caitlin Coppock, Helen J. Rodgers, Ashley R. Samarasinghe, Damien P. Kerley, and Lisa M. Polowyi
References
- 1.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–30. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.Gardner AW, Montgomery PS, Scott KJ, et al. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. 2007;46:1208–14. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med Lond Engl. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G, Belch JJF, Baumgartner I, et al. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis. 2020;293:94–100. doi: 10.1016/j.atherosclerosis.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Reid CM, Ademi Z, Nelson MR, et al. Outcomes from the REACH Registry for Australian general practice patients with or at high risk of atherothrombosis. Med J Aust. 2012;196:193–7. doi: 10.5694/mja11.10731. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Greenland P, Tian L, et al. Association of 6-Minute Walk Performance and Physical Activity With Incident Ischemic Heart Disease Events and Stroke in Peripheral Artery Disease. J Am Heart Assoc. 2015;4:e001846. doi: 10.1161/JAHA.115.001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner AW, Addison O, Katzel LI, et al. Association between Physical Activity and Mortality in Patients with Claudication. Med Sci Sports Exerc. 2021;53:732–9. doi: 10.1249/MSS.0000000000002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordanstig J, Behrendt CA, Baumgartner I, et al. European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2023;S1078-5884:00741–4. doi: 10.1016/j.ejvs.2023.08.067. [DOI] [PubMed] [Google Scholar]
- 10.Regensteiner JG, Hargarten ME, Rutherford RB, et al. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiol Open Access. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 11.Fakhry F, Fokkenrood HJ, Spronk S, et al. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;3:CD010512. doi: 10.1002/14651858.CD010512.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Je HG, Kim BH, Cho KI, et al. Correlation between Patient-Reported Symptoms and Ankle-Brachial Index after Revascularization for Peripheral Arterial Disease. Int J Mol Sci. 2015;16:11355–68. doi: 10.3390/ijms160511355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devine EB, Alfonso-Cristancho R, Yanez ND, et al. Effectiveness of a Medical vs Revascularization Intervention for Intermittent Leg Claudication Based on Patient-Reported Outcomes. JAMA Surg. 2016;151:e162024. doi: 10.1001/jamasurg.2016.2024. [DOI] [PubMed] [Google Scholar]
- 14.Perkins JMT, Collin J, Creasy TS, et al. Reprinted article Exercise training versus angioplasty for stable claudication. Long and medium term results of a prospective, randomised trial. Eur J Vasc Endovasc Surg. 2011;42 Suppl 1:S41–5. doi: 10.1016/j.ejvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Hattum ES, Tangelder MJD, Lawson JA, et al. The quality of life in patients after peripheral bypass surgery deteriorates at long-term follow-up. J Vasc Surg. 2011;53:643–50. doi: 10.1016/j.jvs.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Wann-Hansson C, Hallberg IR, Risberg B, et al. Health-related quality of life after revascularization for peripheral arterial occlusive disease: long-term follow-up. J Adv Nurs. 2005;51:227–35. doi: 10.1111/j.1365-2648.2005.03499.x. [DOI] [PubMed] [Google Scholar]
- 17.Djerf H, Millinger J, Falkenberg M, et al. Absence of Long-Term Benefit of Revascularization in Patients With Intermittent Claudication: Five-Year Results From the IRONIC Randomized Controlled Trial. Circ Cardiovasc Interv. 2020;13:e008450. doi: 10.1161/CIRCINTERVENTIONS.119.008450. [DOI] [PubMed] [Google Scholar]
- 18.Mustapha JA, Finton SM, Diaz-Sandoval LJ, et al. Percutaneous Transluminal Angioplasty in Patients With Infrapopliteal Arterial Disease: Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2016;9:e003468. doi: 10.1161/CIRCINTERVENTIONS.115.003468. [DOI] [PubMed] [Google Scholar]
- 19.Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e10–33. doi: 10.1161/CIR.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 20.Askew CD, Parmenter B, Leicht AS, et al. Exercise & Sports Science Australia (ESSA) position statement on exercise prescription for patients with peripheral arterial disease and intermittent claudication. J Sci Med Sport. 2014;17:623–9. doi: 10.1016/j.jsams.2013.10.251. [DOI] [PubMed] [Google Scholar]
- 21.Tew GA, Harwood AE, Ingle L, et al. The bases expert statement on exercise training for people with intermittent claudication due to peripheral arterial disease. Sport Exerc Sci. 2018 [Google Scholar]
- 22.Aboyans V, Ricco JB, Bartelink M, et al. ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the european society for vascular surgery (esvs): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesendorsed by. the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) 2017;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 23.Parmenter BJ, Dieberg G, Smart NA. Exercise Training for Management of Peripheral Arterial Disease: A Systematic Review and Meta-Analysis. Sports Med. 2015;45:231–44. doi: 10.1007/s40279-014-0261-z. [DOI] [PubMed] [Google Scholar]
- 24.Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12:CD000990. doi: 10.1002/14651858.CD000990.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–80. [PubMed] [Google Scholar]
- 26.Gardner AW, Katzel LI, Sorkin JD, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–62. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 27.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–15. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 28.Parmenter BJ, Dieberg G, Phipps G, et al. Exercise training for health-related quality of life in peripheral artery disease: A systematic review and meta-analysis. Vasc Med. 2015;20:30–40. doi: 10.1177/1358863X14559092. [DOI] [PubMed] [Google Scholar]
- 29.Lanzi S, Calanca L, Berchtold A, et al. Improvement in 6-Minute Walking Distance after Supervised Exercise Training Is Related to Changes in Quality of Life in Patients with Lower Extremity Peripheral Artery Disease. J Clin Med. 2021;10:3330. doi: 10.3390/jcm10153330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg. 2012;55:1346–54. doi: 10.1016/j.jvs.2011.11.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parmenter BJ, Raymond J, Dinnen P, et al. High-intensity progressive resistance training improves flat-ground walking in older adults with symptomatic peripheral arterial disease. J Am Geriatr Soc. 2013;61:1964–70. doi: 10.1111/jgs.12500. [DOI] [PubMed] [Google Scholar]
- 32.Tsai JC, Chan P, Wang CH, et al. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. J Intern Med. 2002;252:448–55. doi: 10.1046/j.1365-2796.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 33.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–74. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whipple MO, Burt MA, Pergolski AL, et al. Uptake and outcomes of supervised exercise therapy for peripheral artery disease: The importance of vascular medicine specialists at a large midwestern health care system during the first 5 years of CMS reimbursement. Vasc Med. 2024;29:112–9. doi: 10.1177/1358863X231215246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fakhry F, Spronk S, van der Laan L, et al. Endovascular Revascularization and Supervised Exercise for Peripheral Artery Disease and Intermittent Claudication: A Randomized Clinical Trial. JAMA. 2015;314:1936–44. doi: 10.1001/jama.2015.14851. [DOI] [PubMed] [Google Scholar]
- 36.Badger SA, Soong CV, O’Donnell ME, et al. Benefits of a supervised exercise program after lower limb bypass surgery. Vasc Endovascular Surg. 2007;41:27–32. doi: 10.1177/1538574406296209. [DOI] [PubMed] [Google Scholar]
- 37.Kruidenier LM, Nicolaï SP, Rouwet EV, et al. Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. J Vasc Interv Radiol JVIR. 2011;22:961–8. doi: 10.1016/j.jvir.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Menêses AL, Ritti-Dias RM, Parmenter B, et al. Combined Lower Limb Revascularisation and Supervised Exercise Training for Patients with Peripheral Arterial Disease: A Systematic Review of Randomised Controlled Trials. Sports Med. 2017;47:987–1002. doi: 10.1007/s40279-016-0635-5. [DOI] [PubMed] [Google Scholar]
- 39.Mazari FAK, Khan JA, Carradice D, et al. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. Br J Surg. 2012;99:39–48. doi: 10.1002/bjs.7710. [DOI] [PubMed] [Google Scholar]
- 40.Pandey A, Banerjee S, Ngo C, et al. Comparative Efficacy of Endovascular Revascularization Versus Supervised Exercise Training in Patients With Intermittent Claudication. JACC: Cardiovascular Interventions. 2017;10:712–24. doi: 10.1016/j.jcin.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Dua A, Gologorsky R, Savage D, et al. National assessment of availability, awareness, and utilization of supervised exercise therapy for peripheral artery disease patients with intermittent claudication. J Vasc Surg. 2020;71:1702–7. doi: 10.1016/j.jvs.2019.08.238. [DOI] [PubMed] [Google Scholar]
- 42.Harwood AE, Pymer S, Ibeggazene S, et al. Provision of exercise services in patients with peripheral artery disease in the United Kingdom. Vascular. 2022;30:874–81. doi: 10.1177/17085381211035259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makris GC, Lattimer CR, Lavida A, et al. Availability of Supervised Exercise Programs and the Role of Structured Home-based Exercise in Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2012;44:569–75. doi: 10.1016/j.ejvs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Supervia M, Turk-Adawi K, Lopez-Jimenez F, et al. Nature of Cardiac Rehabilitation Around the Globe. E Clin Med. 2019;13:46–56. doi: 10.1016/j.eclinm.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin B-J, Arena R, Haykowsky M, et al. Cardiovascular Fitness and Mortality After Contemporary Cardiac Rehabilitation. Mayo Clin Proc. 2013;88:455–63. doi: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Dibben GO, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: a meta-analysis. Eur Heart J. 2023;44:452–69. doi: 10.1093/eurheartj/ehac747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tegegne TK, Rawstorn JC, Nourse RA, et al. Effects of exercise-based cardiac rehabilitation delivery modes on exercise capacity and health-related quality of life in heart failure: a systematic review and network meta-analysis. Open Heart. 2022;9:e001949. doi: 10.1136/openhrt-2021-001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018;20:1735–43. doi: 10.1002/ejhf.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahden S, Ngo V, Hoskin J, et al. Inclusion of People With Peripheral Artery Disease in Cardiac Rehabilitation Programs: A Pan-Canadian Survey. Heart Lung Circ. 2021;30:1031–43. doi: 10.1016/j.hlc.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Siercke M, Jørgensen LP, Missel M, et al. Cardiovascular Rehabilitation Increases Walking Distance in Patients With Intermittent Claudication. Results of the CIPIC Rehab Study: A Randomised Controlled Trial. Eur J Vasc Endovasc Surg. 2021;62:768–76. doi: 10.1016/j.ejvs.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Devrome AN, Aggarwal S, McMurtry MS, et al. Cardiac rehabilitation in people with peripheral arterial disease: A higher risk population that benefits from completion. Int J Cardiol. 2019;285:108–14. doi: 10.1016/j.ijcard.2019.02.070. [DOI] [PubMed] [Google Scholar]
- 52.Ambrosetti M, Temporelli PL, Faggiano P, et al. Lower extremities peripheral arterial disease among patients admitted to cardiac rehabilitation: the THINKPAD registry. Int J Cardiol. 2014;171:192–8. doi: 10.1016/j.ijcard.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Jeger RV, Rickenbacher P, Pfisterer ME, et al. Outpatient rehabilitation in patients with coronary artery and peripheral arterial occlusive disease. Arch Phys Med Rehabil. 2008;89:618–21. doi: 10.1016/j.apmr.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 54.Tam MC, Longenecker CT, Chow C, et al. Occult peripheral artery disease is common and limits the benefit achieved in cardiac rehabilitation. Vasc Med. 2016;21:130–6. doi: 10.1177/1358863X15625370. [DOI] [PubMed] [Google Scholar]
- 55.Anghel R, Adam CA, Mitu O, et al. Cardiac Rehabilitation and Mortality Risk Reduction in Peripheral Artery Disease at 6-Month Outcome. Diagnostics (Basel) 2022;12:1500. doi: 10.3390/diagnostics12061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sami F, Ranka S, Lippmann M, et al. Cardiac rehabilitation in patients with peripheral arterial disease after revascularization. Vascular. 2021;29:350–4. doi: 10.1177/1708538120945530. [DOI] [PubMed] [Google Scholar]
- 57.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verdicchio C, Freene N, Hollings M, et al. A Clinical Guide for Assessment and Prescription of Exercise and Physical Activity in Cardiac Rehabilitation. A CSANZ Position Statement. Heart Lung Circ. 2023;32:1035–48. doi: 10.1016/j.hlc.2023.06.854. [DOI] [PubMed] [Google Scholar]
- 59.Sanderson B, Askew C, Stewart I, et al. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. J Vasc Surg. 2006;44:119–27. doi: 10.1016/j.jvs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 60.Wood RE, Sanderson BE, Askew CD, et al. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clin Sci. 2006;111:401–9. doi: 10.1042/CS20060151. [DOI] [PubMed] [Google Scholar]
- 61.Tew GA, Humphreys L, Crank H, et al. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vasc Med. 2015;20:348–57. doi: 10.1177/1358863X15577857. [DOI] [PubMed] [Google Scholar]
- 62.Astley CM, Beleigoli A, Tavella R, et al. Assessing the quality of cardiac rehabilitation programs by measuring adherence to the Australian quality indicators. BMC Health Serv Res. 2022;22:267. doi: 10.1186/s12913-022-07667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borg GAV. Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise. 1982;14:377. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Martinez CA, Carmeli E, Barak S, et al. Changes in pain-free walking based on time in accommodating pain-free exercise therapy for peripheral arterial disease. J Vasc Nurs. 2009;27:2–7. doi: 10.1016/j.jvn.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13:496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes 2016. Med J Aust. 2016;205:128–33. doi: 10.5694/mja16.00368. [DOI] [PubMed] [Google Scholar]
- 67.Australian Government Department of Health and Aged Care . Australian Government; 2021. Physical activity and exercise guidelines for all australians.https://www.health.gov.au/topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians#order-the-guidelines Available. [Google Scholar]
- 68.Australian Government Department of Health and Aged Care The australian dietary guidelines. 2019. https://www.health.gov.au/resources/publications/the-australian-dietary-guidelines Available.
- 69.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 70.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 71.Golledge J, Yip L, Fernando ME, et al. Relationship between requirement to stop during a six-minute walk test and health-related quality of life, physical activity and physical performance amongst people with intermittent claudication. Ann Vasc Surg. 2021;76:363–9. doi: 10.1016/j.avsg.2021.03.038. [DOI] [PubMed] [Google Scholar]
- 72.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–9. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–7. doi: 10.1016/j.jvs.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birkett ST, Harwood AE, Caldow E, et al. A systematic review of exercise testing in patients with intermittent claudication: A focus on test standardisation and reporting quality in randomised controlled trials of exercise interventions. PLoS One. 2021;16:e0249277. doi: 10.1371/journal.pone.0249277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med. 2018;23:349–57. doi: 10.1177/1358863X18762599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 77.Gardner AW, Skinner JS, Vaughan NR, et al. Comparison of three progressive exercise protocols in peripheral vascular occlusive disease. Angiol Open Access. 1992;43:661–71. doi: 10.1177/000331979204300806. [DOI] [PubMed] [Google Scholar]
- 78.Leeper NJ, Myers J, Zhou M, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–33. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harber MP, Kaminsky LA, Arena R, et al. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog Cardiovasc Dis. 2017;60:11–20. doi: 10.1016/j.pcad.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Chong PFS, Garratt AM, Golledge J, et al. The intermittent claudication questionnaire: a patient-assessed condition-specific health outcome measure. J Vasc Surg. 2002;36:764–71. [PubMed] [Google Scholar]
- 81.Regensteiner J, Steiner J, Panzer R, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2 [Google Scholar]
- 82.Hildebrand M, Van Hees VT, Hansen BH, et al. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Med Sci Sports Exerc. 2014;46:1816–24. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 83.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017;47:1821–45. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Airlie J, Forster A, Birch KM. An investigation into the optimal wear time criteria necessary to reliably estimate physical activity and sedentary behaviour from ActiGraph wGT3X+ accelerometer data in older care home residents. BMC Geriatr. 2022;22:136. doi: 10.1186/s12877-021-02725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi L, Ward SC, Schnelle JF, et al. Assessment of Wear/Nonwear Time Classification Algorithms for Triaxial Accelerometer. Med Sci Sports Exerc. 2012;44:2009–16. doi: 10.1249/MSS.0b013e318258cb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chron Dis. 2012;9:E26. [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly LA, McMillan DG, Anderson A, et al. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13:5. doi: 10.1186/1756-6649-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McDermott MM, Spring B, Berger JS, et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease. JAMA. 2018;319:1665. doi: 10.1001/jama.2018.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hernandez H, Myers SA, Schieber M, et al. Quantification of Daily Physical Activity and Sedentary Behavior of Claudicating Patients. Ann Vasc Surg. 2019;55:112–21. doi: 10.1016/j.avsg.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerage AM, Correia M de A, Oliveira PML de, et al. Physical Activity Levels in Peripheral Artery Disease Patients. Arq Bras Cardiol. 2019;113:410–6. doi: 10.5935/abc.20190142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardner AW, Montgomery PS, Wang M, et al. Minimal clinically important differences in daily physical activity outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med. 2022;27:142–9. doi: 10.1177/1358863X211072913. [DOI] [PubMed] [Google Scholar]
- 93.Hurtig-Wennlöf A, Hagströmer M, Olsson LA. The International Physical Activity Questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr. 2010;13:1847–54. doi: 10.1017/S1368980010000157. [DOI] [PubMed] [Google Scholar]
- 94.Montgomery PS, Gardner AW. Comparison of Three Blood Pressure Methods Used for Determining Ankle/Brachial Index in Patients with Intermittent Claudication. Angiol Open Access. 1998;49:723–8. doi: 10.1177/000331979804901003. [DOI] [PubMed] [Google Scholar]
- 95.Brevetti G, Silvestro A, Schiano V, et al. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 96.Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–47. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 97.Bailey TG, Birk GK, Cable NT, et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am J Physiol Heart Circ Physiol. 2012;303:H533–8. doi: 10.1152/ajpheart.00272.2012. [DOI] [PubMed] [Google Scholar]
- 98.Kals J, Lieberg J, Kampus P, et al. Prognostic Impact of Arterial Stiffness in Patients with Symptomatic Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2014;48:308–15. doi: 10.1016/j.ejvs.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang M-H, Yoo J-K, Kim H-K, et al. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens. 2014;28:475–81. doi: 10.1038/jhh.2013.144. [DOI] [PubMed] [Google Scholar]
- 101.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertens Dallas Tex. 1979;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sandberg A, Cider Å, Jivegård L, et al. Test-retest reliability, agreement, and minimal detectable change in the 6-minute walk test in patients with intermittent claudication. J Vasc Surg. 2020;71:197–203. doi: 10.1016/j.jvs.2019.02.056. [DOI] [PubMed] [Google Scholar]