Abstract
Current therapeutic strategies for ischemic stroke fall short of the desired objective of neurological functional recovery. Therefore, there is an urgent need to develop new methods for the treatment of this condition. Exosomes are natural cell-derived vesicles that mediate signal transduction between cells under physiological and pathological conditions. They have low immunogenicity, good stability, high delivery efficiency, and the ability to cross the blood–brain barrier. These physiological properties of exosomes have the potential to lead to new breakthroughs in the treatment of ischemic stroke. The rapid development of nanotechnology has advanced the application of engineered exosomes, which can effectively improve targeting ability, enhance therapeutic efficacy, and minimize the dosages needed. Advances in technology have also driven clinical translational research on exosomes. In this review, we describe the therapeutic effects of exosomes and their positive roles in current treatment strategies for ischemic stroke, including their anti-inflammation, anti-apoptosis, autophagy-regulation, angiogenesis, neurogenesis, and glial scar formation reduction effects. However, it is worth noting that, despite their significant therapeutic potential, there remains a dearth of standardized characterization methods and efficient isolation techniques capable of producing highly purified exosomes. Future optimization strategies should prioritize the exploration of suitable isolation techniques and the establishment of unified workflows to effectively harness exosomes for diagnostic or therapeutic applications in ischemic stroke. Ultimately, our review aims to summarize our understanding of exosome-based treatment prospects in ischemic stroke and foster innovative ideas for the development of exosome-based therapies.
Keywords: blood–brain barrier, electroacupuncture, engineering, exercise, exosomes, ischemic stroke, mesenchymal stem cells, microglia, neuroprotection, stents
Introduction
Stroke is a devastating illness and the second leading cause of death and disability worldwide. Characterized by the interruption of blood supply to the brain, stroke is further categorized into two types: ischemic and hemorrhagic (Flores and Tang, 2024; Li et al., 2024; Xie et al., 2024). With recent substantial advances in treatment modalities, a growing number of patients survive after a stroke. However, many drugs fail to reach the central nervous system (CNS) because of the protective strength of the blood–brain barrier (BBB). Furthermore, many survivors suffer from severe neurological sequelae, such as cognitive impairment, hemiparesis, and aphasia, which may be related to the lack of effective CNS-targeted drugs to restrict or repair the development of neuroinflammation, immune response, and vascular injury in the brain after stroke (Ishii et al., 2012; Doyle et al., 2015; Cuartero et al., 2019). Although various guidelines recommend intravenous thrombolysis and mechanical thrombectomy as effective treatment methods, their application is limited by their narrow therapeutic windows. Additionally, these methods can lead to serious complications, e.g., cerebral hemorrhage and ischemia/reperfusion injury (Zerna et al., 2018; Powers et al., 2019). Thus, there is an urgent need to improve the ability of drugs to pass through the BBB and reduce the severity of existing treatment complications.
Exosomes are a subspecies of extracellular vesicles with diameters ranging from 40 to 160 nm. They were first discovered by researchers observing transferrin receptor fate during the maturation of sheep reticulocytes into red blood cells, and were named “exosomes” in 1987 (Pan and Johnstone, 1983; Johnstone et al., 1987; Kalluri and LeBleu, 2020). Subsequently, exosomes were found to be released by various organs, tissues, and cells in the human body. Exosomes can be widely detected in the blood, saliva, ascites, and cerebrospinal fluid (Lässer et al., 2011; Huang et al., 2021).
Although exosomes have been studied for more than 30 years, their biological functions remain unclear. Exosomes, as natural nanomaterials, can deliver information between cells via proteins, lipids, nucleic acids, and other substances (Kalluri and LeBleu, 2020). Compared with liposomes or synthesized nanomaterials, exosomes have low immunogenicity, good stability, and a high delivery efficiency. More importantly, they can cross the BBB, a stringent barrier composed of brain microvascular endothelial cells, pericytes, astrocytes, the basilar membrane, and tight junctions. The BBB strictly regulates the transport of substances between the blood and the brain, protecting the CNS from harm but preventing many drugs from entering the CNS. An increasing number of studies have explored the mechanisms by which exosomes pass through the BBB (Banks et al., 2020; Abdelsalam et al., 2023). Joshi and Zuhorn (2021) found that exosomes derived from C17.2 neural stem cells (NSCs) interact with brain endothelial cells through heparan sulfate proteoglycans to ultimately cross the BBB via endocytosis. Furthermore, exosomes play important roles in post–ischemic stroke (IS) treatment by regulating inflammation, apoptosis, and neurovascular regeneration (Venkat et al., 2018). Although exosomes have a natural brain-homing ability, animal experiments have proven that their targeting ability is inefficient, and numerous engineering techniques have been used to modify exosomes to enhance their targeting efficiency (Khan et al., 2021).
In this review, we summarize the pathological mechanisms of IS, the biogenesis and biochemical composition of exosomes, and exosome isolation methods. We focus on the potential role of exosomes in regulating the harmful microenvironment at lesion sites and in promoting neural regeneration in IS. We discuss the role of exosomes in exercise, remote ischemic conditioning, and electroacupuncture, as well as the application of exosomes for drug delivery and intravascular stents. Finally, we investigate the strategies used to engineer exosomes for targeted delivery and the administration routes in IS. This review will provide a comprehensive understanding of the therapeutic role of exosomes in IS via a combination of basic science and clinical medicine research findings with the aim of promoting further significant progress in exosome-based next-generation therapeutic platforms for IS.
Search Strategy
In this narrative review, we utilized the PubMed database to retrieve relevant articles published from inception to December 19, 2023. The literature search was conducted with a series of search words (MeSH Terms) and various combinations to improve the specificity and sensitivity of the search: exosomes [MeSH Terms], stroke [MeSH Terms], ischemic stroke [MeSH Terms], inflammation [MeSH Terms], neurogenesis [MeSH Terms], exercise [MeSH Terms], electroacupuncture [MeSH Terms], stents [MeSH Terms], and engineering [MeSH Terms]. We excluded articles unrelated to our topic, and mainly selected articles from the past 5 years to showcase the latest research progress in this field.
A timeline of literature sources on exosomes associated with IS is shown in Figure 1.
Figure 1.
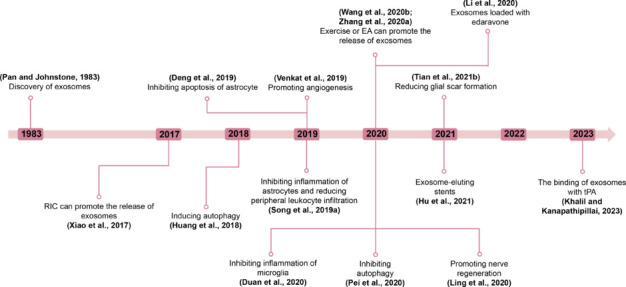
Timeline of literature sources associated with exosomes and ischemic stroke.
Exosomes, first discovered in 1983, have emerged as crucial mediators of intercellular communication. Their potential therapeutic effects on IS have been extensively studied in recent years. These effects include anti-inflammatory, anti-apoptotic, autophagy regulatory, angiogenic, neurogenic, and glial scar formation reduction activities. Researchers have investigated various therapeutic strategies, including exercise (Wang et al., 2020b), RIC (Xiao et al., 2017), and EA (Zhang et al., 2020a). These methods aim to stimulate the release of exosomes and thereby exert neuroprotective effects. As our understanding of exosomes deepens, they have been increasingly engineered for drug delivery purposes. For instance, the integration of edaravone with exosomes was reported in 2020, followed by tPA in 2023. Additionally, Hu et al. (2021) introduced an innovative exosome-eluting stent that offers a promising new avenue for IS therapy. Created with Microsoft PowerPoint 2021. EA: Electroacupuncture; RIC: remote ischemic conditioning; tPA: tissue plasminogen activator.
Pathogenesis of Ischemic Stroke
IS triggered by the insufficient blood supply to the brain caused by vascular blockage, leading to localized brain tissue ischemia, hypoxic necrosis, and subsequent neurological deficits (Virani et al., 2021). The brain is a highly metabolically active organ and, despite its small size, can consume a quarter of the energy supply of the body. When ischemia and hypoxia occur in the brain, a series of harmful cascade reactions are induced in the ischemic area, including mitochondrial dysfunction, calcium overload, reactive oxygen species (ROS) generation, increased excitatory neurotransmitters, and the infiltration of inflammatory cells, ultimately resulting in brain cell apoptosis and death (Figure 2).
Figure 2.
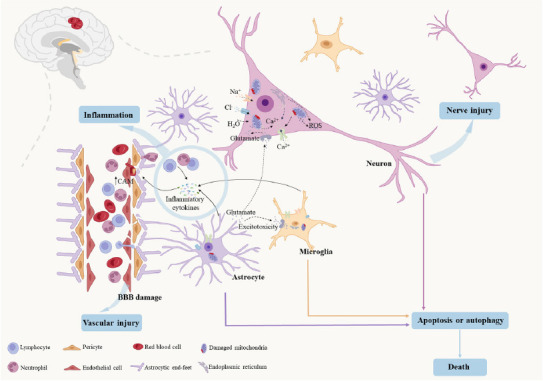
The pathological mechanism of ischemic stroke.
Ischemia and hypoxia induce mitochondrial dysfunction, calcium overload, ROS production, excitatory neurotransmitter generation, and inflammatory cell infiltration, which lead to brain cell apoptosis and death. Upward arrows indicate an increase, and downward arrows indicate a decrease. Created with Microsoft PowerPoint 2021. BBB: Blood–brain barrier; CAM: cell adhesion molecules; ROS: reactive oxygen species.
Specifically, ischemia and hypoxia lead to mitochondrial dysfunction, which further mediates ATP reduction and the influx of Ca2+ into the intracellular fluid from the mitochondria and endoplasmic reticulum. This process also generates substantial amounts of ROS (Lai et al., 2020). The excessive production of ROS can increase the release of excitatory amino acids such as glutamate. After glutamate enters the cell, Na+ and Ca2+ ions flow into the cell, causing a large number of Cl– and water molecules to enter the cell, leading to swelling or edema (Han et al., 2023). Brain cells release damage-associated molecular patterns following ischemia and hypoxia, initiating an inflammatory cascade. Glial cells (mainly microglia and astrocytes) are activated and release multiple inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, interferon gamma (INF-γ), and IL-1β. Simultaneously, inflammatory mediators stimulate endothelial cells to upregulate the expression of cell adhesion molecules (CAM) (Mizuma and Yenari, 2017). Subsequently, numerous neutrophils and lymphocytes from the periphery infiltrate the brain tissue, where they continuously secrete inflammatory cytokines, causing further damage to the BBB (Shigemoto-Mogami et al., 2018; Berchtold et al., 2020). The biochemical changes caused by focal cerebral ischemia lead to a never-ending vicious cycle, ultimately leading to neuronal death. Notably, the lack of oxygen, glucose, and ATP in the core infarction area is irreversible. However, there is a transitional zone (ischemic penumbra) between the ischemic core and normal tissue, and tissue damage in this zone is reversible (Lipton, 1999; Iadecola and Anrather, 2011; Puyal et al., 2013; Hankey, 2017). Therefore, developing an effective method to protect and rescue cells within the ischemic penumbra is crucial.
An Overview of Exosomes
Biogenesis of exosomes
Exosomes are a class of extracellular vesicles (EV) with diameters ranging from 40 to 160 nm that can be secreted by most cell types (Kalluri and LeBleu, 2020). EV are mainly classified based on their biogenesis and are divided into three subtypes: exosomes, apoptotic bodies, and microvesicles (He et al., 2018; Zhou et al., 2022b). In contrast to apoptotic bodies and microvesicles, which are formed via budding from the cell membrane, exosomes are generated by the endolysosomal pathway (Buratta et al., 2020; Dassler-Plenker et al., 2020). The researchers that conceptualized the mechanism of exosome biogenesis drew inspiration from early studies examining the secretion of transferrin receptors by maturing reticulocytes. Over time, this understanding has gradually deepened, as evidenced by contributions from Johnstone (1992) and Record et al. (2011).
The typical process of exosome biogenesis involves the following steps. First, the plasma membrane invaginates to form a cup-like early sorting endosome (ESE) that contains some special proteins from the cell surface and extracellular milieu (van Niel et al., 2018; Mathieu et al., 2019). The trans-Golgi network and the endoplasmic reticulum promote the formation of ESEs that gradually mature into multivesicular bodies (MVBs), which are formed via the inward budding of the endosomal membrane and contain numerous intraluminal vesicles (ILVs) (Ibrahim and Marban, 2016; Dai et al., 2020). Later, these ILV-rich MVBs undergo a degradative pathway via fusion with autophagosomes and lysosomes. Alternatively, they release exosomes into the extracellular space by fusing with the plasma membrane (Mashouri et al., 2019; Kalluri and LeBleu, 2020; Han et al., 2022).
Biochemical composition of exosomes
The composition of exosomes confers exosome-based nanoplatforms with special biological properties able to affect neighboring or distant cells. Therefore, a comprehensive understanding of their biochemical compositions is essential. According to the latest exosome database (http://www.exocarta.org), exosomes can comprise 9769 proteins, 1116 lipids, and 2838 micro (mi)RNAs. These substances play key roles in physiological and pathological processes by participating in various signaling pathways. The exosome proteome shows that membrane-trafficking–related proteins, such as tetraspanin proteins (CD63, CD9, and CD81), are recruited to exosomes via apoptosis-linked gene 2-interacting protein X and endosomal-sorting complex required for transport-dependent pathways (Baietti et al., 2012; Juan and Furthauer, 2018; Larios et al., 2020). In addition, exosomes are enriched in tumor susceptibility gene 101, the heat shock proteins Hsp60 and Hsp90, and integrins (Zhang et al., 2017; Thery et al., 2018). Kamerkar et al. (2017) reported that the presence of CD47 on exosomes facilitates their escape from phagocytosis via monocytes, which prolongs their half-life in circulation, providing new insights into exosome-based nanotechnology for advanced therapies. The lipid content of exosomes encompasses cholesterol, phosphatidylserine, and glycosphingolipids (Skotland et al., 2019; Fayyazpour et al., 2023). However, the proportions of these components in exosomes differ from those in the parent cells. These components are conducive to the rigidity and stability of exosomes, ensuring that the exosomal cargo is not easily degraded. MiRNAs, important posttranscriptional mediators of gene expression that act by regulating a vast array of biological processes, are also enriched in exosomes (Yu et al., 2016; Dai et al., 2020; Miao et al., 2021). For example, exosomal miR-146a-5p can suppress abnormal ocular neovascularization by downregulating the AKT/ERK signaling pathway (Pan et al., 2023). It is clear that a better understanding of the biochemical compositions of exosomes can increase the versatility of exosome-based nanoplatforms used in different therapeutic modes.
Exosome isolation techniques
It is important that researchers obtain a deeper knowledge of different exosome isolation techniques (Table 1), as harvesting exosomes with high purity and yield will contribute to the development of exosome-based diagnostic and therapeutic nanotechnologies. Ultracentrifugation, precipitation, and ultrafiltration are widely used methods for exosome isolation (Yang et al., 2020; Zhu et al., 2020; Tiwari et al., 2021). Based on a report on current and emerging techniques for exosome isolation, ultracentrifugation is the most widely used method (> 80%); however, it is time-consuming and requires large sample volumes (Street et al., 2012). New precipitation-based commercial products are rapidly emerging and are characterized by their ease of use; however, an obvious limitation is their low purity (Diaz et al., 2018). Although ultrafiltration is faster than other methods and does not rely on expensive equipment, the contaminating proteins are difficult to remove (Sidhom et al., 2020). With the rapid development of technology, several novel exosome isolation approaches have been designed with improved efficiency. Lee et al. (2015) designed an advanced acoustic nanofilter system that can purify exosomes in a contact-free manner, and Rho et al. (2013) have developed a magnetic nanosensor that analyzes erythrocyte-derived exosomes. From a clinical perspective, the goal of designing exosome-based delivery systems is to prevent or cure diseases through rational clinical translation. Therefore, more effort should be devoted to finding or designing more convenient and efficient isolation approaches that provide high-purity exosomes to further meet clinical requirements.
Table 1.
Summary of exosome-processing techniques
| Techniques | Time (min) | Initial volume | Purity | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Ultracentrifugation | 480 | ≤ 25 mL | High | Widely used; suitable for preparing large-volume samples | Expensive equipment; unstable recovery; exosome damage | Tauro et al., 2012; Lobb et al., 2015 |
| Precipitation | Overnight or 30–120 | ≤ 10 mL | Low | High yield; simple; fast; cheap | Not suitable for plasma | Ludwig et al., 2018 |
| Ultrafiltration | 130 | ≤ 15 mL | Low | Simple; efficiency; used on a large scale | Blockage of membranes | Liga et al., 2015 |
| Acoustic nanofilter | < 30 | 50 μL | High | Simple; fast; adjustable | Complex operation; excessive sample loss | Lee et al., 2015; Shirejini and Inci, 2022 |
| Magnetic nanosensor | < 30 | < 200 μL | Unknown | Portable; fast | Lack of ability to analyze individual exosomes | Rho et al., 2013 |
The purity of exosomes was measured by comparing the ratio of exosome counts to protein concentration. High purity: Ratios of > 3 × 1010 particles per μg protein (P/μg); low purity: ratios of 2 × 109 to 2 × 1010 P/μg (Webber and Clayton, 2013).
Therapeutic Effect of Exosomes on Ischemic Stroke
Exosomes have enormous potential in the treatment of stroke (Figure 3). Their biological functions can be used to treat stroke in two main ways: (1) direct effects, whereby specific proteins on the surface of exosomes recognize the receptors of target cells to mediate biological signal transduction; and (2) indirect effects, when exosomes exert bioactive effects by delivering inherent substances (such as proteins and nucleic acids) after internalization by target cells. Exosomes participate in a series of restorative activities after stroke, including anti-inflammation, anti-apoptosis, autophagy-regulation, angiogenesis, neurogenesis, and glial scar formation reduction (Table 2).
Figure 3.
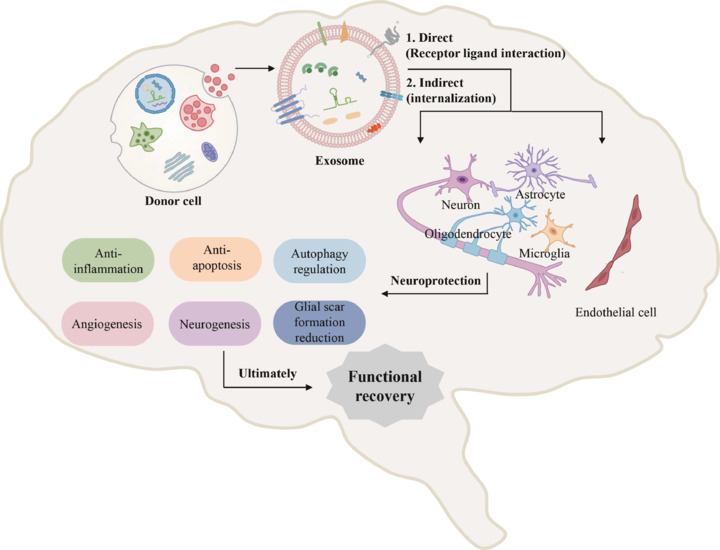
The neuroprotective mechanisms of exosomes after ischemic stroke.
Exosomes have key anti-inflammation, anti-apoptosis, autophagy-regulation, angiogenesis, neurogenesis, and glial scar formation reduction effects via direct or indirect communication with cells in the ischemic area and contribute to functional recovery after stroke. Created with Microsoft PowerPoint 2021.
Table 2.
Potential therapeutic effects of exosomes in ischemic stroke
| Functions | Sources | Target cells | Agents | Mechanisms | References |
|---|---|---|---|---|---|
| Anti-inflammation | Hypoxic preconditioned MSCs | Microglia | miR-216a-5p | Suppress TLR4/NK-kB and activate the PI3K/AKT signaling pathway | Liu et al., 2020 |
| hUMSCs | Microglia | miR-146a-5p | Downregulate IRAK1/TRAF6 pathway | Zhang et al., 2021 | |
| Cortical neurons | Astrocytes | miR-181c-3p | Downregulate CXCL1 | Song et al., 2019a | |
| Astrocytes | Microglia | lncRNA 4933431K23Rik | Upregulate the expression of Smad7 | He et al., 2023b | |
| MSCs | Gut microbiota | miR-150-3p | Unknown | Sun and Xu, 2023 | |
| MSCs | Leukocytes | Unknown | Unknown | Wang et al., 2020a, 2022 | |
| Anti-apoptosis | NSCs | Neurons | miR-150-3p | Regulate CASP2 | Luo et al., 2022 |
| Microglia | Neurons | miR-124 | Downregulate USP14 | Song et al., 2019b | |
| BMSCs | Neurons | miR-150-5p | Repress TLR5 | Li et al., 2022 | |
| BMSCs | Oligodendrocytes | miR134 | Downregulate caspase-8 | Xiao et al., 2019 | |
| BMSCs | Astrocytes | miR-138-5p | Downregulate LCN2 | Deng et al., 2019 | |
| MSCs | Microglia | miR-26a-5p | Downregulate CDK6 | Cheng et al., 2021 | |
| Induce autophagy | Astrocytes | Neurons | Nampt | Activate AMPK/mTOR signaling pathway | Deng et al., 2022 |
| ADMSCs | Nerve cells | Unknown | Increase PEDF content | Huang et al., 2018 | |
| Inhibit autophagy | Astrocytes | Neurons | miR-190b | Regulate Atg7 | Pei et al., 2020 |
| ADMSCs | Neurons | miR-25-3p | Downregulate the p53/BNIP3 signaling pathway | Kuang et al., 2020 | |
| Angiogenesis | BMSCs | Microvessels | miR-21-5p | Increase the expression of VEGF, VEGFR2, Ang-1, and Tie-2 | Hu et al., 2022 |
| Brain endothelial cells | Endothelial cells | miR-126-3p | Unknown | Venkat et al., 2019 | |
| Neurogenesis | USC-Exos | NSCs | miR-26a | Downregulate HDAC6 | Ling et al., 2020 |
| MSCs | Neurons | miR-17-92 | Activate the PI3K/Akt/mTOR pathway | Xin et al., 2021 | |
| Glial scar formation reduction | Microglia | Astrocytes | miR-124 | Downregulate the expression of STAT3 | Tian et al., 2021b |
| Microglia | Astrocytes | Unknown | Unknown | Xin et al., 2023 |
ADMSCs: Adipose-derived mesenchymal stem cells; BMSCs: bone marrow mesenchymal stromal cells; HDAC6: histone deacetylase 6; hUMSCs: human umbilical cord mesenchymal stem cells; MSCs: mesenchymal stem cells; NSCs: neural stem cells; PEDF: pigment epithelium–derived factor; USC-Exos: exosomes from human urine-derived stem cells; VEGF: vascular endothelial growth factor.
Exosomes with anti-inflammatory effects
Inflammation is an important post-IS important event involving multiple cell types and cytokines and warrants significant attention. Although neuroinflammation contributes to remodeling and repair at particular stages after stroke (Jayaraj et al., 2019; Lambertsen et al., 2019), long-lasting and uncontrolled inflammation can cause secondary brain damage, such as BBB disruption, neuronal damage, and vascular aging. Therefore, the control of inflammation is a pivotal goal in stroke treatment.
Microglia are the primary inflammatory cells that initially infiltrate the IS site (Wolf et al., 2017). Exosomes can exert anti-inflammatory effects by regulating the release of bioactive substances from microglia. For example, miR-146a-5p-enriched mesenchymal stem cell (MSC)–derived exosomes decrease the levels of pro-inflammatory mediators (such as monocyte chemoattractant protein-1 and inducible nitric oxide synthase) released by microglia by targeting interleukin-1 receptor-associated kinase 1 and nuclear factor of activated T cells 5 (Duan et al., 2020). Additionally, MSC-derived exosomal miR-145 decreased the level of pro-inflammatory factors released by microglia in oxygen–glucose deprivation/reperfusion-stimulated BV2 cells (Zhou et al., 2022a). In addition to the products of microglia, the inflammatory cytokines released by astrocytes play essential roles in IS-related neuroinflammation and provide promising therapeutic targets. Song et al. (2019a) found that cortical neuron–derived exosomes downregulated the expression of CXCL1 and inflammatory cytokines in astrocytes via miR-181c-3p, reducing inflammation after IS.
Activated microglia and astrocytes release chemokines that recruit peripheral immune cells to the damaged brain tissue. Infiltrating immune cells further activate the inflammatory cascade, resulting in the activation of more microglia and astrocytes, and ultimately leading to neuronal cell death (Alsbrook et al., 2023). Therefore, in addition to directly inhibiting the activation of microglia and astrocytes, it is crucial to reduce peripheral immune cell infiltration of brain tissue with anti-inflammatory treatments for stroke. Song et al. (2019a) found that cortical neurogenic exosomes containing miRNA-181c-3p downregulated the expression of CXCL1 in astrocytes and further reduced the number of peripheral immune cells infiltrating the brain tissue. Recently, Wang et al. (2020a, 2022) demonstrated that MSC-derived exosomes had a neuroprotective role in the ischemic brain tissue of young and aged mice by reducing the infiltration of various types of leukocytes, especially neutrophils, monocytes, and macrophages. Although the exact mechanism is not fully understood, the ability of exosomes to regulate immune responses and reduce peripheral inflammatory cell infiltration has opened up new avenues for the exploration of therapeutic strategies against ischemic brain injury.
The gut microbiota and their metabolites are important components of the peripheral immune system. As the gut is home to tens of thousands of symbiotic bacteria that constantly expose the gastrointestinal immune system to bacterial antigens, the gastrointestinal tract is one of the largest immune cell compartments in the human body. A growing body of evidence demonstrates that the gut microbiota can affect the brain through the brain–gut axis, and gut dysbiosis can also occur after IS. After stroke, a decrease in the number and diversity of beneficial bacteria is usually accompanied by an increase in the number and types of opportunistic bacteria, and this gut dysbiosis exacerbates neuroinflammation and immune activity. Gastrointestinal motility was reported to decrease significantly 6 hours after middle cerebral artery occlusion (MCAO) in a mouse model, and this was accompanied by intestinal epithelial necrosis and shedding, with these features worsening further after 24 hours (Xu et al., 2012; Liu et al., 2017). Simultaneously, intestinal lymphocytes can infiltrate the brain and exacerbate ischemic damage. Benakis et al. (2016) found that infarct volume decreased by (60 ± 6)% in an amoxicillin/clavulanate-treated MCAO mouse model with antibiotic-sensitive flora compared with antibiotic-treated mice carrying resistant flora. Moreover, exosomes derived from human placental mesenchymal stem cells were reported to improve myocardial infarction by modulating the gut microenvironment (Yang et al., 2022), which provides a new direction for the development of exosomes for stroke treatment. Recently, Sun and Xu (2023) suggested that miR-150-3p from MSC-derived exosomes can regulate the abundance and diversity of the intestinal microbiota, including Proteobacteria and Acinetobacter. They then performed fecal microbiota transplantation and observed that gut microbiota-mediated MSC-derived exosomes attenuated neuroinflammation and apoptosis. Therefore, exosomes could serve as a promising anti-inflammatory therapy for stroke through the regulation of the gut microenvironment.
Exosomes with anti-apoptosis and autophagy-regulating effects
After ischemia, the insufficient blood supply to brain tissue leads to a decrease in oxygen, glucose, and ATP levels, ultimately leading to cell death over time. In the core infarction area, oxygen, glucose, and ATP deficiencies are irreversible and result in cell necrosis. However, the ischemic penumbra is reversible and develops slowly, lasting for several hours, providing the time and opportunity to rescue cells and limit infarct volume (Lipton, 1999; Iadecola and Anrather, 2011; Puyal et al., 2013; Hankey, 2017). Therefore, salvaging cells in the penumbra has become a key topic in current clinical treatment discussions.
Brain cells in the penumbra include neurons, oligodendrocytes, astrocytes, and microglia. Accumulating evidence shows that exosomes derived from cells can inhibit the apoptosis of brain cells and help alleviate ischemia/reperfusion injury in cerebral ischemia models.
The apoptotic pathways of cells include the intrinsic pathway mediated by mitochondria and the extrinsic pathway mediated by receptors, both of which are controlled by a set of cystine-aspartate proteases (caspases). Recently, Luo et al. (2022) revealed that NSC-derived exosomes (NSC-Ex) are enriched with miR-150-3p, and the authors screened them for the target gene caspase-2 (CASP2). After an MCAO mouse model received NSC-Ex or miR-150-3p treatment, the level of anti-apoptotic Bcl-2 increased while the levels of proapoptotic Bax and caspase-3 decreased. NSC-Ex inhibited neuronal apoptosis and reduced the infarction area in the MCAO mouse model (Vigneswara and Ahmed, 2019; Luo et al., 2022). Exosomes derived from BMSCs inhibit oligodendrocyte apoptosis by downregulating the activity and expression of caspase-8 via miR-134, which may be a novel potential therapeutic target for IS (Xiao et al., 2019). Deng et al. (2019) showed that neutrophil gelatinase-associated lipocalin (LCN2) is involved in apoptosis and serves as a target gene of miR-138-5p. BMSC-derived exosomal miR-138-5p inhibited astrocyte apoptosis by downregulating LCN2, Bax, and caspase-3 levels. MiR-26a-5p is a newly discovered miRNA in exosomes that plays a significant role in a number of diseases, and miR-26a-5p inhibits apoptosis in cardiomyocytes (Xing et al., 2020). Furthermore, Cheng et al. (2021) demonstrated that miR-26a-5p secreted by MSC-derived exosomes can prevent microglial apoptosis and alleviate brain damage by downregulating CDK6 (the G1 cell-cycle kinase).
Autophagy is a cellular catabolic process that involves the recycling and degradation of intracellular components such as damaged organelles, misfolded proteins, and intracellular pathogens (Noh et al., 2023). The process is highly regulated and dynamic and plays a crucial role in maintaining cellular homeostasis and in adaptations to various stress conditions. Increasing amounts of evidence suggest that autophagy is activated in various cell types, including neurons and glial cells, emphasizing the essential role of autophagy in IS (Wang et al., 2018). Furthermore, the moderate induction of autophagy contributes to increased cell viability and decreased infarct size (Carloni et al., 2010; Dai et al., 2017; Lv et al., 2017; Song et al., 2017; Yan et al., 2019). “Nampt,” also known as nicotinamide phosphoribosyltransferase, plays an important role in the regulation of autophagy during cerebral ischemic injury. Nampt expression is also significantly increased in exosomes derived from oxygen–glucose deprivation/reoxygenation-stimulated astrocytes (OGD/R-ADEXs), and Nampt secreted by OGD/R-ADEXs can induce autophagy by activating AMPK and inhibiting the phosphorylation of mTOR, thereby ameliorating acute IS (Deng et al., 2022). Therefore, the moderate induction of autophagy may be a new strategy for stroke treatment. However, autophagy is a double-edged sword in neuronal survival after IS. Uncontrolled autophagy can result in the breakdown of important cellular organelles and lead to an imbalance in cellular degradation and recycling mechanisms that ultimately result in cell death (He et al., 2012).
Exosomes also play crucial roles in inhibiting autophagy in IS. For example, astrocyte-derived exosomes inhibit autophagy via miR-190b-targeting of the autophagy-related gene 7, thereby inhibiting apoptosis and playing a neuroprotective role (Pei et al., 2020). Kuang et al. (2020) showed that the exosomal regulation of overactivated post-stroke autophagic activity contributes to neuroprotection. They found that exosomes derived from adipose-derived mesenchymal stem cells (ADMSCs) transferred miR-25-3p to target cells and suppressed autophagy by inhibiting the p53/BNIP3 signaling pathway (Kuang et al., 2020). Therefore, exosomes play crucial roles in regulating autophagy in the stroke microenvironment.
Exosomes with angiogenesis effects
Angiogenesis contributes to the recovery of neurological function by enhancing the metabolic capacity of damaged neurons, assisting in neuronal remodeling and the migration of NSCs and clearing necrotic brain tissue fragments (Ergul et al., 2012a). Angiogenesis is a complex process involving interactions among a series of important factors, including VEGF, VEGFR2, Ang-1, and Tie-2. When VEGF binds to VEGFR2, it triggers downstream signaling pathways, promoting endothelial cell proliferation and migration and ultimately forming new vasculature (Viallard and Larrivée, 2017). However, owing to the lack of support from pericytes and the extracellular matrix around blood vessels, the newly formed vasculature is not sufficiently stable. To further promote the maturation of these newly formed vessels, interaction between Ang-1 and Tie-2 is required (Babaei et al., 2003). Hu et al. (2022) showed that exosomes derived from BMSCs can increase the expression of VEGF, VEGFR2, Ang-1, and Tie-2 in the ischemic penumbra and increase microvessel density via miR-21-5p. Similarly, after the intravenous administration of endothelial cell-derived exosomes to a mouse model of type 2 diabetes mellitus stroke, the density of brain microvessels and the formation of capillaries increased, and this effect was mediated by miR-126-3p (Venkat et al., 2019). However, the excessive expression of pro-angiogenic factors, such as VEGF and HIF-1, can disrupt tight junction proteins, leading to increased BBB permeability and an increased risk of adverse events (Zhang et al., 2000; Yan et al., 2012). Therefore, further exploration is needed into the capacity of exosomes to maintain angiogenic homeostasis.
Exosomes with neurogenesis effects
After IS, axonal growth becomes limited, and the number of axons around the infarcted area decreases. Axonal remodeling is a key step in the spontaneous amelioration of neurological recovery (Ueno et al., 2012). Exosomes, especially those derived from stem cells, play important roles in the development and regeneration of axons, owing to their multiple differentiation potential and plasticity. MSC-derived exosomes enriched with miR-17-92 can promote axonal growth and myelination by activating the PI3K/Akt/mTOR pathway and contribute to neurological recovery after stroke (Xin et al., 2021). However, sources of the most well-studied stem cells, such as bone marrow mesenchymal stromal cells (BMSCs) and ADMSCs, are limited, and isolation of these cells requires invasive methods, representing a significant obstacle in their clinical applications. Recently, researchers made significant breakthroughs in this area by successfully isolating a new type of stem cell from urine. The exosomes produced by urine-derived stem cells (USCs) promoted nerve regeneration via the miR-26a/HDAC6 axis (Ling et al., 2020). Therefore, urine can provide a new and convenient source of exosomes for the treatment of IS. In addition to stem cell–derived exosomes, Jin et al. (2023) found that the surface protein HepaCAM of astrocyte exosomes can induce axonal growth via a unique surface contact mechanism. Furthermore, Zhang et al. (2020b) found that exosomes derived from ischemic cerebral endothelial cells promoted axonal growth by increasing the expression of miRNAs and target proteins in receptor neurons. Therefore, exosomes have potential utility in neurogenesis and promoting neurological recovery after IS.
Exosomes with glial scar formation reduction effects
The recovery of neurological function depends not only on the survival and regeneration of neurons, but also on glial cells. Many glial cells proliferate and form dense glial scars in the area surrounding a stroke. However, scars are the main obstacles to axonal regrowth (Cregg et al., 2014; Zhang et al., 2018). Therefore, promoting glial degradation and inhibiting glial formation are beneficial for neurological recovery. Signal transducer and activator of transcription 3 (STAT3) plays a key role in astrogliosis and promotes the expression of glial fibrillary acidic protein (GFAP), a marker of proliferative astrocytes. Therefore, STAT3 may serve as an intervention target in stroke. Microglia-derived exosomes are enriched with miR-124, which has been reported to decrease the expression of STAT3 and GFAP and further suppress the proliferation and migration of astrocytes both in vitro and in vivo (Tian et al., 2021b). Furthermore, Xin et al. (2023) confirmed that hypoxic microglia–derived exosomes can inhibit astrocyte proliferation and limit the formation of glial scars. Therefore, using exosomes may be an effective way to reduce glial scar formation.
Role of Exosomes in Current Therapies for Ischemic Stroke
With the gradual improvements in our understanding of the various effects of exosomes in IS, such as their anti-inflammation, anti-apoptotic, autophagy-regulation, angiogenesis, neurogenesis, and glial scar formation reduction activities, researchers are increasingly interested in exploring related novel therapeutic methods. Owing to the unsatisfactory results of traditional drug therapies, an increasing number of researchers have started to pay attention to non-drug therapies such as exercise, remote ischemic conditioning (RIC), and electroacupuncture. However, these have not been widely applied, as we lack a solid theoretical foundation for their efficacy. In recent years, we have discovered that these therapies can exert various therapeutic effects through exosomes, providing new insights and ideas for IS therapy (Figure 4).
Figure 4.
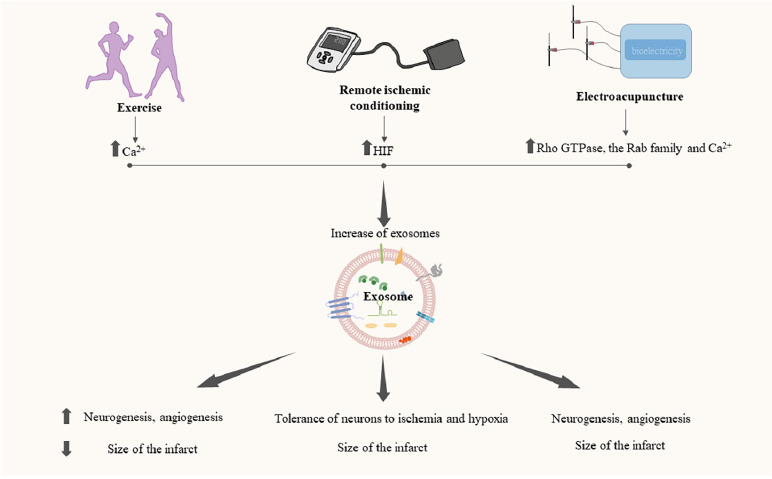
Role of exosomes in exercise, remote ischemic conditioning, and electroacupuncture.
Exercise, remote ischemic conditioning, and electroacupuncture can promote the release of exosomes and achieve various therapeutic effects through exosomes. Created with Microsoft PowerPoint 2021. Upward arrows indicate an increase, and downward arrows indicate a decrease. HIF: Hypoxia inducible transcription factor.
Exercise
Exercise is an effective non-pharmacological anti-stroke method, and the American Heart Association explicitly recommends exercise as part of stroke prevention and treatment strategies (Winstein et al., 2016). Appropriate exercise can reduce oxidative damage, neuroinflammation, and cell death after IS (Austin et al., 2014) and can contribute to reducing the size of the infarction, inducing angiogenesis, and promoting neurogenesis (Li et al., 2021).
Exosomes are a crucial factor in the connection between exercise and stroke protection. Recent studies have found that the release of exosomes is closely related to an increase of Ca2+ influx (Savina et al., 2003; Kumar et al., 2020). During exercise, the motor neurons that innervate skeletal muscle fibers are stimulated, promoting the release of Ca2+ from the sarcoplasmic reticulum into the cytoplasm. Therefore, it is thought that, after exercise, the body rapidly releases exosomes into the circulatory system to transmit information to tissues, and experiments have shown that appropriate levels of exercise can increase exosome levels in the bloodstream of healthy individuals (Whitham et al., 2018). Exercise interventions before or after stroke have been described as increasing the exosome content of the serum and brain tissue of MCAO rats or mice and exerting neuroprotective effects via exosomes (Wang et al., 2020b; Li et al., 2021).
Endothelial cell damage is a crucial pathogenic outcome of IS (Ergul et al., 2012b). Ma et al. (2018) found that exercise increased the levels of exosomes and miR-126 in healthy mice and subsequently protected endothelial cells from injury through the miR-126/SPRED1/VEGF pathway. Later, Wang et al. (2020b) conducted a study into the impact of a 4-week exercise intervention in mice before MCAO. They found that appropriate exercise prior to stroke increased the levels of exosomes and miR-126 in both the serum and brain tissues of MCAO mice. They also observed that exercise reduced neurological deficits, decreased infarct volume, increased microvessel density, and promoted neurogenesis. The mechanism underlying these effects may have been related to the increased release of miR-126 from exosomes. Therefore, exosomes could improve rehabilitation plans and enhance the prospects of stroke treatment.
In another study, Li et al. (2021) observed that, compared with simple exercise, a combination therapy of exosomes and exercise increased the levels of brain tissue exosomes, number of synapses, and quantity of synaptic plasticity-associated proteins in MCAO mice, and also reduced the size of the infarct. This study further validated the fact that exercise exerts a beneficial effect through exosomes and raised the possibility that combining exosomes with exercise therapy could be a new direction for stroke treatment.
Remote ischemic conditioning
Although exercise has proven to be a highly effective means of stroke prevention and treatment, it often leads to low patient participation. RIC is a clinical regimen used to improve tolerance to ischemia and hypoxia by activating endogenous protective mechanisms. This is achieved by repeatedly and briefly occluding and reperfusing the blood flow in distant limbs, ultimately protecting target organs and tissues (He et al., 2023a). Compared with exercise, RIC passivity results in higher compliance and thus improves the recovery rate of IS patients, making it a very attractive choice of intervention to replace or assist exercise therapy after stroke. Multiple large-scale clinical trials have been conducted on RIC in IS patients, and promising results have been achieved (Chen et al., 2022; Blauenfeldt et al., 2023). Geng et al. (2021) have identified many overlapping mechanisms between RIC and exercise in terms of their neuroprotective impacts.
Considering that exosomes can penetrate the BBB for delivery to the brain, they represent potential transporters of the neuroprotective signals of RIC from the periphery to the CNS. Seventy-five differentially expressed exosome proteins have been found between RIC and non-RIC groups of healthy individuals, among which theobromine, apolipoprotein A1, and hemopexin are associated with neuroprotective effects after stroke (Du et al., 2023). This discovery has increased researchers’ confidence in the important role of exosomes in RIC-mediated brain protection, and several studies have been conducted to verify this (Li et al., 2019; Cui et al., 2020). For example, Li et al. (2019) suggested that, compared with exosomes extracted from non-RIC-treated mice, exosomes extracted from RIC-treated mice can better attenuate infarct volume and improve neurological symptoms in MCAO mice. Additionally, the exosomes released by RIC-treated mice contain higher levels of hypoxia inducible transcription factor 1 (HIF-1α) and can transfer HIF-1α to ischemic brain tissue or upregulate HIF-1α in damaged brain cells to exert neuroprotective effects. Cui et al. (2020) found that exosomes released by vascular endothelial cells after RIC are rich in miR-126, which reduces LINE-1 and Alu methylation levels by targeting DNMT3B and thereby improving neuronal tolerance to ischemia and hypoxia. RIC is also conducive to exosome release (Xiao et al., 2017), which may involve the activation of HIF signaling pathway by hypoxia (Bister et al., 2020). Therefore, exosomes play crucial roles in RIC-mediated brain protection and could provide novel mechanisms of RIC treatment in IS.
Electroacupuncture
Acupuncture has been used to treat various diseases for thousands of years. Electroacupuncture (EA) is a modern form of acupuncture that involves the application of a small electrical current, similar to human bioelectricity, via fine needles for specific acupoint stimulation. EA has been recommended by the World Health Organization as an alternative or complementary therapy for the prevention and treatment of IS (Ulloa et al., 2017; Wang et al., 2023a). EA promotes neurogenesis and angiogenesis, alleviates functional neurological deficits, and reduces infarct size.
EA is a common form of electrical stimulation that can increase the release of exosomes by affecting Rho GTPase, Rab family members, and the level of Ca2+ (Fukuta et al., 2020; Debbi et al., 2022). Zhang et al. (2020a) showed that the exosome content in the peri-infarct area increases in IS after EA stimulation. Simultaneously, EA can increase the content of miR-146b in exosomes, thereby promoting the differentiation of NSCs and achieving neurogenesis. Xu et al. (2022) found that EA therapy exerted angiogenic effects in an MCAO rat model by activating the HIF-1/VECF/Notch1 signaling pathway through exosomal miR-210. These findings provide a new theoretical basis for the clinical application of EA and key evidence for the benefits of acupuncture in the field of modern medicine.
Exosome-based Therapies for Ischemic Stroke
Current clinical therapeutic methods for IS typically include drug therapy (such as thrombolytic and neuroprotective drugs) and intravascular stent therapy. However, these therapies have limited efficacy and inevitable side effects. Over the years, studies have shown that exosomes have promising prospects for clinical application (Khalil and Kanapathipillai, 2023; Rehman et al., 2023). It is likely that further explorations of combinations of exosomes and clinical therapies for IS will contribute to reducing the side effects of existing drug and stent therapies, as well as to the development of more innovative and effective therapeutic methods, providing more appropriate clinical therapy options for IS patients (Figure 5).
Figure 5.
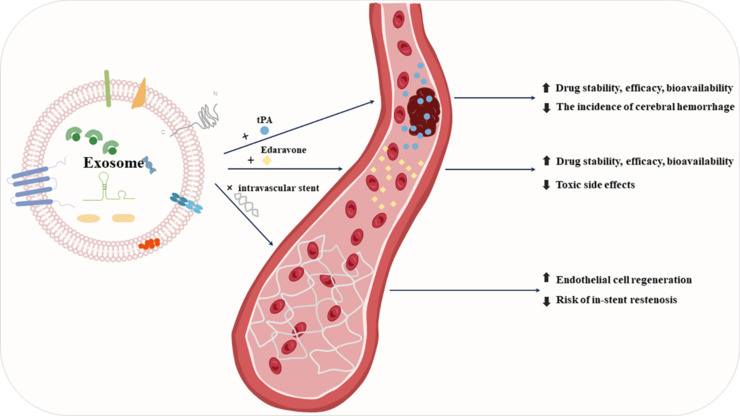
Exosome-based therapies for ischemic stroke.
Combining exosomes with drugs can improve their stability, efficiency, and bioavailability and reduce side effects. When combined with stents, exosomes can promote endothelial growth and reduce the risk of in-stent restenosis. Created with Microsoft PowerPoint 2021. Upward arrows indicate an increase, and downward arrows indicate a decrease. tPA: Tissue plasminogen activator.
Roles of exosomes in drug delivery
Exosomes are cell-secreted vesicles that can carry miRNAs, proteins, nucleic acids, lipids, and other substances to transmit information between cells. In addition, various phospholipid and protein components that enrich the lumen and surface of exosomes can help achieve drug loading, thereby improving drug stability, efficacy, and bioavailability, and reducing toxic side effects (Li et al., 2020). Compared with other synthetic nanomaterials, exosomes have low immunogenicity, good stability, and a long half-life, which endow them with a promising ability to serve as safe and effective drug carriers (Rehman et al., 2023).
Thrombolytic drugs
Owing to the high probability that thrombosis will lead to IS, current stroke guidelines recommend tissue plasminogen activator (tPA) as one of the most effective drugs for IS treatment (Powers et al., 2019). tPA converts plasminogen into plasmin by binding to, and ultimately degrading, fibrin, and it can penetrate the BBB and infiltrate the brain parenchyma, where it may cause intracerebral hemorrhage (Tanne et al., 2002; Su et al., 2008). Therefore, ideally, tPA should be delivered to the thromboembolic site without reaching the brain parenchyma. To realize the accurate delivery of tPA to the thrombus site and extend its half-life, researchers have attempted to use exosomes as carriers. Recently, Khalil and Kanapathipillai (2023), developed an exosome-coated tPA nanoformulation for thrombolytic therapy. Exo-tPA showed higher stability than free tPA, and the activity of tPA encapsulated in exosomes was unaffected by intervention with plasminogen activator inhibitors. In addition, the conjugated binding between exosomes and tPA leads to a larger particle size, meaning fewer tPA can penetrate the brain parenchyma, reducing the incidence of cerebral hemorrhage.
Neuroprotective drug
For patients in whom the thrombolysis time window has been missed, the use of neuroprotective agents is recommended to slow disease progression in clinical settings. After ischemia and hypoxia, brain lesion sites release substantial amounts of oxygen free radicals, leading to further damage to neural function. Edaravone is a clinically mature neuroprotective drug that eliminates oxygen free radicals, inhibits cell peroxides, and reduces cell damage (Enomoto et al., 2019). However, its application is limited by its short half-life, difficulty in crossing the BBB, low bioavailability, and potential to damage the kidneys at high doses. To address these issues, Li et al. (2020) loaded edaravone into macrophage-derived exosomes (Exo + Edv) and found that Exo + Edv improved the bioavailability of edaravone, reduced neuronal damage and the infarct size, and provided neuroprotective effects. Plasma-derived exosomes have a natural brain-targeting ability mediated through interactions between transferrin receptors in brain endothelial cells and the transferrin expressed on exosomes (Qu et al., 2018). Gao et al. (2021) loaded edaravone into plasma-derived exosomes; compared with free edaravone, edaravone-loaded exosomes showed improved brain targeting, enhanced safety and bioavailability, and their effects reduced the infarct area in the acute stage of stroke.
The therapeutic efficacy of thrombolytic- and neurotrophic-drug–loaded exosomes has been preliminarily confirmed. However, the potential adverse reactions of patients to exosomes in clinical treatment remain unclear, and large-scale clinical trials are required to verify their feasibility. Though, overall, exosome-based drug delivery systems show promise in the treatment of IS.
Role of exosomes in intravascular stent treatment
Intravenous thrombolysis and mechanical thrombectomy are highly recommended treatment methods for IS, but their application is limited because of the short treatment time windows (Powers et al., 2018; Jadhav et al., 2021). In patients with large-vessel occlusion, re-occlusion can still occur, even after thrombolysis or thrombectomy, because of the presence of narrowed blood vessels. The main reason for mechanical thrombectomy failure is the presence of intracranial atherosclerotic stenosis (Hurford et al., 2020). Therefore, intravascular stenting is an optional therapeutic strategy for patients with IS. After the implantation of bare metal stents into the blood vessels, smooth muscle cells proliferate and migrate to the injured site, causing in-stent restenosis (Alfonso et al., 2014). To address this issue, drug-eluting stents have been developed that have reduced the occurrence of in-stent restenosis. However, eluted drugs can also delay endothelial cell coverage of the stent, delay arterial healing, and increase the possibility of late-stage vascular restenosis (Hu et al., 2021). Therefore, there is a need to find new eluting drugs for intravascular stents.
Exosomes are promising eluting drug candidates owing to their low immunogenicity, stability, and inherent bioactivity. For example, exosomes derived from human umbilical cord mesenchymal stem cells can exert anti-inflammatory effects through miR146a-5p (Zhang et al., 2021); while MSC-derived exosomal let-7b-5p and miR-23a-3p can target genes related to vascular repair and generation (Beltrami et al., 2017; Ferguson et al., 2018). MSC-derived exosomes also contain many proteins related to angiogenesis, such as collagen α1 and fibronectin (Anderson et al., 2016). Based on the above functions, exosomes have been considered as potential stent coatings for the treatment of IS. Recently, Hu et al. (2021) developed a bioresponsive exosome-eluting stent, and to prevent the premature depletion of exosomes during treatment, they added modified biodegradable “linkers” between the exosomes and the stent that can respond to ROS. Under ischemia-reperfusion injury, the large amount of ROS generated can accelerate the release of exosomes from the stent, which then reach the damaged site through blood vessels. Compared with drug-eluting stents, exosome-eluting stents accelerate endothelial cell regeneration and reduce the risk of in-stent restenosis (Hu et al., 2021). Thus, emerging exosome nanotechnologies are expected to improve stent implantation.
Exosomes originate from cells and have a phospholipid bilayer structure similar to that of the cell membrane. Therefore, when exosomes are covalently coupled to the surface of a metal stent, they can effectively disguise its foreign properties to prevent immune system attacks. Additionally, exosomes contain multiple active substances with anti-inflammatory, anti-apoptotic, and angiogenic properties, and the various phospholipid and protein components that are abundant in the lumen and surface of exosomes are important foundations for drug loading. Therefore, multiple drugs can be loaded onto stents through exosomes, achieving complementary therapeutic effects while expanding the blood vessels.
Engineering Strategies for Exosomal Targeted Delivery in Ischemic Stroke
Novel exosome-based strategies are emerging for the treatment of CNS diseases. However, the ability of pristine exosomes to target the ischemic lesion sites remains insufficient. Thus, improving the specificity of exosomes by targeting specific cell types at ischemic lesion sites has become a key step in optimizing their therapeutic effects.
The classic method of exosome targeting is to fuse the peptides of specific cells to the membrane of exosomes via genetic engineering. For example, neuron-specific rabies viral glycoprotein (RVG), which specifically binds to the acetylcholine receptor, was fused to the lysosome-associated membrane glycoprotein 2b of dendritic cell-derived exosomes, resulting in their targeted accumulation within neurons. After intravenous injection of RVG-infused exosomes loaded with GAPDH short interfering (si)RNA, specific gene knockdown was observed in neurons, but not in the liver, spleen, kidney, or other organs (Alvarez-Erviti et al., 2011). Similar approaches have been subsequently used to deliver different siRNAs and miRNAs to lesion sites in IS (Yang et al., 2017; Kim et al., 2019). Furthermore, a recombinant targeting peptide containing the arginine-glycine-aspartic acid (RGD)-4C peptide (ACDCRGDCFC) fused to the C1 and C2 domains of lactadherin, which can bind to phosphatidylserine on the exosome membrane, has been formulated. These engineered exosomes also display the ability to targeting lesional regions of the ischemic brain (Tian et al., 2021a). Although genetic engineering is a highly feasible strategy for modifying exosomes, expressing the targeted ligand on the exosome membrane may affect the original bioactivity of other membrane proteins (Salunkhe et al., 2020).
In another targeting strategy involving both chemical and physical methods, the cycle (Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)], with high affinity for the integrin αvβ3 of the cerebral vascular endothelial cells in ischemic lesion sites, was conjugated to the MSC-derived exosome surface using click chemistry. After intravenous injection of the above surface-functionalized exosomes into a transient MCAO mice model, immunofluorescence and near-infrared fluorescence imaging displayed an obvious enrichment of the exosomes at ischemic lesion sites, and the exosomes were also found in microglia, neurons, and astrocytes (Tian et al., 2018). Additionally, given that mannose has selective binding affinity for the mannose receptor expressed on microglia, researchers have designed mannose-conjugated exosomes that display excellent microglia-targeting properties in IS (Liu et al., 2023). Compared with genetic engineering, chemical modification is more cost-effective and suitable for large-scale production. However, there is a potential risk that chemical modifications may inactivate exosome surface proteins during the process (Liang et al., 2021).
Magnetic navigation has also been used in IS-therapy studies (Lin et al., 2017; Kim et al., 2020). To enhance the targeting ability of exosomes, iron oxide nanoparticles have been loaded into exosomes, resulting in the accumulation of magnetic nanovesicles in the brain under the guidance of an external magnetic field (Kim et al., 2020). With the applied magnetic field, the delivery efficiency of therapeutic exosomes can be improved, and the retention time within target organs can be prolonged. However, because of the structural complexity of the human body, magnetic navigation may not be a highly efficient for all organs (Zhuo et al., 2021). These studies have conferred superior targeting capabilities upon exosomes via ingenious modifications or nanoengineering to improve their therapeutic efficacy. The exploration and development of more suitable targeting strategies has become an inevitable trend among researchers aiming to maximize the therapeutic potential of exosomes.
Administration Routes of Exosomes
Several methods of exosome administration have reached preclinical and clinical trial stages. The intravenous injection of exosomes is the most universally accepted technique (Ortega et al., 2020; Guo et al., 2023; Ma et al., 2023; Ran et al., 2023). However, the main limitation of this is the pulmonary first-pass effect; exosomes also enrich the liver, kidney, and spleen, indicating that the number of exosomes in the lesion site of the brain is insufficient (Wiklander et al., 2015). Intra-arterial injection can result in their higher accumulation but may lead to the formation of intra-arterial and cerebral microvascular emboli (Cui et al., 2015). Although some more invasive methods, such as intracerebral and intrathecal injections, result in a larger number of exosomes reaching the lesion sites, the risk of infection is increased. The intranasal delivery route is increasingly garnering attention because of its tendency to lead to satisfactory brain accumulation, offering a significant advantage over intravenous administration (Donega et al., 2013). Of note, intranasally delivered exosomes can cross the BBB, a phenomenon supported by in vivo neuroimaging (Perets et al., 2018). However, no studies have comparatively analyzed the functional efficiency of different administration methods in MCAO mouse models. To further improve the distribution of exosomes to targeted sites in the brain, more effort should be made to modify exosomes by bioengineering and overcome the disadvantages of traditional administration methods.
Limitations
Currently, our understanding of exosomes remains in its nascent stages, and numerous limitations need to be addressed. One major hurdle is the inefficiency of currently available time-consuming isolation techniques, which hinder the commercialization and clinical application of exosomes. A significant challenge lies in the overlap in particle size between exosomes (ranging from 40 to 160 nm) and another subclass of EVs known as microvesicles (100–1000 nm), as there is a lack of an efficient isolation methods to separate these particles. In addition, different isolation approaches may affect the characterization (such as morphology and zeta potential) of samples. Cell culture conditions and engineering modifications may also affect the biochemical and biophysical features of exosomes. Because of the heterogeneous nature of exosomes, there are uncertainties regarding batch-to-batch variations. Furthermore, there have been concerns raised about potential functional compromises resulting from genetic engineering or chemical/physical alterations of exosomes. Additionally, our fundamental knowledge and understanding of exosome biology remains inadequate and preliminary, particularly regarding the mechanism of exosome internalization and their pharmacokinetic and pharmacodynamic performance in vivo. Exosomes are considered promising biomarkers because they contain proteins and other biomolecules, such as DNA, miRNA, long non-coding RNA, and circular RNA, and various biosensors have been developed for their detection; however, the sensitivity of these methods is often hindered by the presence of a large number of nucleic acids or cells in samples.
Conclusion and Future Prospectives
IS the primary cause of long-term disability and mortality worldwide. Its complex pathogenesis and pathological microenvironment have led to a lack of effective therapies in clinical practice. In recent decades, researchers have made great advances in increasing our understanding of the biogenesis, biochemical composition, and biological functions of exosomes. Accumulating evidence shows that exosomes play key roles in the protection and restoration of stroke and are considered potential therapeutic strategies.
In this review, we reviewed the exosome biogenesis, biochemical composition, and current isolation techniques, which may promote a better understanding of exosomes among clinical researchers. We have summarized what is known about the multiple therapeutic efficacies of exosomes in IS, such as anti-inflammation, anti-apoptosis, autophagy-regulation, angiogenesis, nerve regeneration, and glial scar formation reduction. Additionally, we described how exosomes are closely related to the effects of current IS therapies such as exercise, RIC, and electroacupuncture, which may help clinical researchers further understand these therapies. Studies on exosome-based drug delivery systems and exosome-eluting stents also provide new insights for clinical researchers. The rapid development of nanotechnology has advanced the evolution of engineered exosomes, which can effectively improve targeting ability, enhance therapeutic efficacy, and reduce the required drug dose.
Our review has recognized the close link between basic science and clinical medicine research that has helped to provide a more comprehensive view of exosomes. More importantly, this paper may encourage interdisciplinary research and promote the development of exosomes-based therapies for IS. In current experimental studies in IS, researchers are developing many exosome-based therapies that show a good safety and therapeutic benefits in preclinical animal models, attributed to their high biocompatibility and their enrichment in therapeutic cargoes (Song et al., 2019b; Wu et al., 2023). Therefore, exosomes show vast potential for clinical translation. Recently, Aruna Bio, Inc. (a pioneer in the development of neural exosome-based therapeutics) announced that the U.S. Food and Drug Administration has cleared their exosome product as an Investigational New Drug for their Phase 1b/2a IS clinical trial. Moreover, several ongoing clinical trials have registered with ClinicalTrial.gov, and we are looking forward to their research results. For example, the clinical trial NCT03384433 aims to evaluate the therapeutic efficacy of MSC-derived exosomes in patients with IS. In another clinical trial (NCT06138210), human induced pluripotent stem cell–derived exosomes are being used in an evaluation of safety and preliminary efficacy. Additionally, clinical trial NCT05370105 is assessing the prognostic power of exosomes in patients with IS. Given the lack of clinical evidence in this field, the therapeutic efficacy and safety of exosomes need further exploration.
To enhance our understanding of exosomes and explore their potential application in the treatment of IS, it is imperative that we focus on developing more precise and versatile isolation techniques, along with improved downstream purification methods, to obtain high-quality exosomes suitable for effective therapeutic use. The detailed characterization of exosomes, both quantitative and qualitative, is also crucial. To facilitate this, advanced technologies are needed, along with the design of unified workflows and standards for exosome isolation and characterization, including morphology, diameter, zeta potential, and markers. Samples that do not meet these standards may require additional processing steps to improve their purity. Furthermore, the stability of exosomes is a key consideration. Storage conditions, such as 4°C or –80°C temperatures, can affect exosome properties, with the potential loss of proteomic content. Therefore, exploring optimal storage conditions is also essential. Collaborative efforts among nanotechnologists, basic scientists, biomedical scientists, and those researching exosome-based therapies for IS will help to continuously innovate and revolutionize traditional therapeutic approaches.
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 82071291 (to YY), 82301464 (to HM); the Norman Bethune Health Science Center of Jilin University, No. 2022JBGS03 (to YY); a grant from Department of Science and Technology of Jilin Province, Nos. YDZJ202302CXJD061 (to YY), 20220303002SF (to YY); a grant from Jilin Provincial Key Laboratory, No. YDZJ202302CXJD017 (to YY); Talent Reserve Program of First Hospital of Jilin University, No. JDYYCB-2023002 (to ZNG).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editor: Song LP; T-Editor: Jia Y
Data availability statement:
Not applicate..
References
- Abdelsalam M, Ahmed M, Osaid Z, Hamoudi R, Harati R. Insights into exosome transport through the blood-brain barrier and the potential therapeutical applications in brain diseases. Pharmaceuticals (Basel) 2023;16:571. doi: 10.3390/ph16040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- Alsbrook DL, Di Napoli M, Bhatia K, Biller J, Andalib S, Hinduja A, Rodrigues R, Rodriguez M, Sabbagh SY, Selim M, Farahabadi MH, Jafarli A, Divani AA. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. 2023;23:407–431. doi: 10.1007/s11910-023-01282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Anderson JD, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res. 2014;87:8–15. doi: 10.1016/j.neures.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 2020;21:4407. doi: 10.3390/ijms21124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami C, Besnier M, Shantikumar S, Shearn AI, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, Emanueli C. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther. 2017;25:679–693. doi: 10.1016/j.ymthe.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold D, Priller J, Meisel C, Meisel A. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol. 2020;30:1208–1218. doi: 10.1111/bpa.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister N, Pistono C, Huremagic B, Jolkkonen J, Giugno R, Malm T. Hypoxia and extracellular vesicles: a review on methods, vesicular cargo and functions. J Extracell Vesicles. 2020;10:e12002. doi: 10.1002/jev2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauenfeldt RA, et al. Remote ischemic conditioning for acute stroke: the resist randomized clinical trial. JAMA. 2023;330:1236–1246. doi: 10.1001/jama.2023.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, Emiliani C. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21:2576. doi: 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Chen HS, et al. Effect of remote ischemic conditioning vs usual care on neurologic function in patients with acute moderate ischemic stroke: the RICAMIS randomized clinical trial. JAMA. 2022;328:627–636. doi: 10.1001/jama.2022.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Chen X, Wang Y, Cheng W, Zuo X, Tang W, Huang W. MSCs-derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. 2021;27:67. doi: 10.1186/s10020-021-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero MI, de la Parra J, Pérez-Ruiz A, Bravo-Ferrer I, Durán-Laforet V, García-Culebras A, García-Segura JM, Dhaliwal J, Frankland PW, Lizasoain I, Moro M. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J Clin Invest. 2019;129:1536–1550. doi: 10.1172/JCI120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Liu N, Chang Z, Gao Y, Bao M, Xie Y, Xu W, Liu X, Jiang S, Liu Y, Shi R, Xie W, Jia X, Shi J, Ren C, Gong K, Zhang C, Bade R, Shao G, Ji X. Exosomal MicroRNA-126 from RIPC serum is involved in hypoxia tolerance in sh-sy5y cells by downregulating DNMT3B. Mol Ther Nucleic Acids. 2020;20:649–660. doi: 10.1016/j.omtn.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LL, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, Janowski M, Walczak P, Boltze J, Lukomska B, Jolkkonen J. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SH, Chen T, Li X, Yue KY, Luo P, Yang LK, Zhu J, Wang YH, Fei Z, Jiang XF. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic Biol Med. 2017;108:345–353. doi: 10.1016/j.freeradbiomed.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Dassler-Plenker J, Kuttner V, Egeblad M. Communication in tiny packages: exosomes as means of tumor-stroma communication. Biochim Biophys Acta Rev Cancer. 2020;1873:188340. doi: 10.1016/j.bbcan.2020.188340. [DOI] [PubMed] [Google Scholar]
- Debbi L, Guo S, Safina D, Levenberg S. Boosting extracellular vesicle secretion. Biotechnol Adv. 2022;59:107983. doi: 10.1016/j.biotechadv.2022.107983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. doi: 10.1186/s13036-019-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Duan R, Ding W, Gu Q, Liu M, Zhou J, Sun J, Zhu J. Astrocyte-derived exosomal nicotinamide phosphoribosyltransferase (Nampt) ameliorates ischemic stroke injury by targeting AMPK/mTOR signaling to induce autophagy. Cell Death Dis. 2022;13:1057. doi: 10.1038/s41419-022-05454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz G, Bridges C, Lucas M, Cheng Y, Schorey JS, Dobos KM, Kruh-Garcia NA. Protein digestion, ultrafiltration, and size exclusion chromatography to optimize the isolation of exosomes from human blood plasma and serum. J Vis Exp. 2018;13:57467. doi: 10.3791/57467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donega V, van Velthoven CT, Nijboer CH, van Bel F, Kas MJ, Kavelaars A, Heijnen CJ. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8:e51253. doi: 10.1371/journal.pone.0051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Quach LN, Solé M, Axtell RC, Nguyen TV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci. 2015;35:2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Qiu R, Chen L, Chen Y, Zhong Z, Li P, Fan F, Cheng Y. Identification of serum exosomal metabolomic and proteomic profiles for remote ischemic preconditioning. J Transl Med. 2023;21:241. doi: 10.1186/s12967-023-04070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Wang F, Cao J, Wang C. Exosomes derived from microRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. 2020;14:3143–3158. doi: 10.2147/DDDT.S255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M, Endo A, Yatsushige H, Fushimi K, Otomo Y. Clinical effects of early edaravone use in acute ischemic stroke patients treated by endovascular reperfusion therapy. Stroke. 2019;50:652–658. doi: 10.1161/STROKEAHA.118.023815. [DOI] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets. 2012;12:148–158. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazpour P, Fayyazpour A, Abbasi K, Vaez-Gharamaleki Y, Zangbar MS, Raeisi M, Mehdizadeh A. The role of exosomes in cancer biology by shedding light on their lipid contents. Pathol Res Pract. 2023;250:154813. doi: 10.1016/j.prp.2023.154813. [DOI] [PubMed] [Google Scholar]
- Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8:1419. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J, Tang J. Role of N-formyl peptide receptor 2 in germinal matrix hemorrhage: an intrinsic review of a hematoma resolving pathway. Neural Regen Res. 2024;19:350–354. doi: 10.4103/1673-5374.379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta T, Nishikawa A, Kogure K. Low level electricity increases the secretion of extracellular vesicles from cultured cells. Biochem Biophys Rep. 2020;21:100713. doi: 10.1016/j.bbrep.2019.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Wang Q, Lee H, Huber C, Wills M, Elkin K, Li F, Ji X, Ding Y. Remote ischemic postconditioning vs. physical exercise after stroke: an alternative rehabilitation strategy? Mol Neurobiol. 2021;58:3141–3157. doi: 10.1007/s12035-021-02329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wang F, Hu Y, Luo Y, Wei Y, Xu K, Zhang H, Liu H, Bo L, Lv S, Sheng S, Zhuang X, Zhang T, Xu C, Chen X, Su J. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4:100881. doi: 10.1016/j.xcrm.2022.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Pan J, Li F, Zhao L, Shi Y. A novel brain targeted plasma exosomes enhance the neuroprotective efficacy of edaravone in ischemic stroke. IET Nanobiotechnol. 2021;15:107–116. doi: 10.1049/nbt2.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang JH, Zhang SJ. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21:207. doi: 10.1186/s12943-022-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Song Y, Rocha M, Shi Y. Ischemic brain edema: emerging cellular mechanisms and therapeutic approaches. Neurobiol Dis. 2023;178:106029. doi: 10.1016/j.nbd.2023.106029. [DOI] [PubMed] [Google Scholar]
- Hankey GJ. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Ma Y, Fang C, Deng Z, Wang F, Qu Y, Yin M, Zhao R, Zhang D, Guo F, Yang Y, Chang J, Guo ZN. Remote ischemic conditioning attenuates blood-brain barrier disruption after recombinant tissue plasminogen activator treatment via reducing PDGF-CC. Pharmacol Res. 2023;187:106641. doi: 10.1016/j.phrs.2022.106641. [DOI] [PubMed] [Google Scholar]
- He S, Wang C, Dong H, Xia F, Zhou H, Jiang X, Pei C, Ren H, Li H, Li R, Xu H. Immune-related GTPase M (IRGM1) regulates neuronal autophagy in a mouse model of stroke. Autophagy. 2012;8:1621–1627. doi: 10.4161/auto.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Huang Y, Liu Y, Zhang X, Wang Q, Liu Y, Ma X, Long X, Ruan Y, Lei H, Gan C, Wang X, Zou X, Xiong B, Shu K, Lei T, Zhang H. Astrocyte-derived exosomal lncRNA 4933431K23Rik modulates microglial phenotype and improves post-traumatic recovery via SMAD7 regulation. Mol Ther. 2023;31:1313–1331. doi: 10.1016/j.ymthe.2023.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Hu X, Li L, Fang Y, Yang Y, Gu J, Xu J, Chu L. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of miR-21-5p. Biomolecules. 2022;12:883. doi: 10.3390/biom12070883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li Z, Shen D, Zhu D, Huang K, Su T, Dinh PU, Cores J, Cheng K. Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. 2021;5:1174–1188. doi: 10.1038/s41551-021-00705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Xiao C, Zhang L, Li L, Luo J, Chen L, Hu X, Zheng H. Bioinformatic analysis of exosomal microRNAs of cerebrospinal fluid in ischemic stroke rats after physical exercise. Neurochem Res. 2021;46:1540–1553. doi: 10.1007/s11064-021-03294-1. [DOI] [PubMed] [Google Scholar]
- Huang X, Ding J, Li Y, Liu W, Ji J, Wang H, Wang X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371:269–277. doi: 10.1016/j.yexcr.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. 2020;19:413–421. doi: 10.1016/S1474-4422(20)30079-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A, Marban E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol. 2016;78:67–83. doi: 10.1146/annurev-physiol-021115-104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Asai T, Oyama D, Fukuta T, Yasuda N, Shimizu K, Minamino T, Oku N. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81–87. doi: 10.1016/j.jconrel.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Desai SM, Jovin TG. Indications for mechanical thrombectomy for acute ischemic stroke: current guidelines and beyond. Neurology. 2021;97:S126–S136. doi: 10.1212/WNL.0000000000012801. [DOI] [PubMed] [Google Scholar]
- Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Chen X, Tian Y, Jarvis R, Promes V, Yang Y. Astroglial exosome HepaCAM signaling and ApoE antagonization coordinates early postnatal cortical pyramidal neuronal axon growth and dendritic spine formation. Nat Commun. 2023;14:5150. doi: 10.1038/s41467-023-40926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM. The jeanne manery-fisher memorial lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70:179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- Joshi BS, Zuhorn IS. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood-brain barrier model. Eur J Neurosci. 2021;53:706–719. doi: 10.1111/ejn.14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil S, Kanapathipillai M. Exosome-coated tPA/catalase nanoformulation for thrombolytic therapy. Bioengineering (Basel) 2023;10:177. doi: 10.3390/bioengineering10020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H, Pan JJ, Li Y, Zhang Z, Yang GY. Native and bioengineered exosomes for ischemic stroke therapy. Front Cell Dev Biol. 2021;9:619565. doi: 10.3389/fcell.2021.619565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Kim TJ, Kang L, Kim YJ, Kang MK, Kim J, Ryu JH, Hyeon T, Yoon BW, Ko SB, Kim BS. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim G, Hwang DW, Lee M. Delivery of high mobility group box-1 siRNA using brain-targeting exosomes for ischemic stroke therapy. J Biomed Nanotechnol. 2019;15:2401–2412. doi: 10.1166/jbn.2019.2866. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Zheng X, Zhang L, Ai X, Venkataramani V, Kilic E, Hermann DM, Majid A, Bähr M, Doeppner TR. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles. 2020;10:e12024. doi: 10.1002/jev2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Tang Q, Müller SA, Gao P, Mahlstedt D, Zampagni S, Tan Y, Klingl A, Bötzel K, Lichtenthaler SF, Höglinger GU, Koeglsperger T. Fibroblast growth factor 2-mediated regulation of neuronal exosome release depends on VAMP3/cellubrevin in hippocampal neurons. Adv Sci (Weinh) 2020;7:1902372. doi: 10.1002/advs.201902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Lin P, Chen M, Zhang Y, Chen J, Zheng M, Liu J, Du H, Chen R, Pan X, Liu N, Chen H. Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol. 2020;34:101503. doi: 10.1016/j.redox.2020.101503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219:e201904113. doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–2327. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ke C, Su Y, Wan C. Exercise intervention promotes the growth of synapses and regulates neuroplasticity in rats with ischemic stroke through exosomes. Front Neurol. 2021;12:752595. doi: 10.3389/fneur.2021.752595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhao L, Shi Y, Liang J. Edaravone-loaded macrophage-derived exosomes enhance neuroprotection in the rat permanent middle cerebral artery occlusion model of stroke. Mol Pharm. 2020;17:3192–3201. doi: 10.1021/acs.molpharmaceut.0c00245. [DOI] [PubMed] [Google Scholar]
- Li X, Bi T, Yang S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered. 2022;13:3030–3043. doi: 10.1080/21655979.2021.2012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Feng S, Xu Q, Zhang X, Xiong X, Gu L. Ferroptosis and endoplasmic reticulum stress in ischemic stroke. Neural Regen Res. 2024;19:611–618. doi: 10.4103/1673-5374.380870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ren C, Li H, Jiang F, Wang L, Xia C, Ji X. Role of exosomes induced by remote ischemic preconditioning in neuroprotection against cerebral ischemia. Neuroreport. 2019;30:834–841. doi: 10.1097/WNR.0000000000001280. [DOI] [PubMed] [Google Scholar]
- Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liga A, Vliegenthart AD, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15:2388–2394. doi: 10.1039/c5lc00240k. [DOI] [PubMed] [Google Scholar]
- Lin BL, Zhang JZ, Lu LJ, Mao JJ, Cao MH, Mao XH, Zhang F, Duan XH, Zheng CS, Zhang LM, Shen J. Superparamagnetic iron oxide nanoparticles-complexed cationic amylose for in vivo magnetic resonance imaging tracking of transplanted stem cells in stroke. Nanomaterials (Basel) 2017;7:107. doi: 10.3390/nano7050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X, Zhang G, Xia Y, Zhu Q, Zhang J, Li Q, Niu X, Hu G, Yang Y, Wang Y, Deng Z. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J Cell Mol Med. 2020;24:640–654. doi: 10.1111/jcmm.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hao Y, Huang Z, Shi Y, Su C, Zhao L. Modulation of microglial polarization by sequential targeting surface-engineered exosomes improves therapy for ischemic stroke. Drug Deliv Transl Res. 2023;14:418–432. doi: 10.1007/s13346-023-01408-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo S, Kou L, Tang C, Huang R, Pei Z, Li Z. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci Lett. 2017;658:165–170. doi: 10.1016/j.neulet.2017.08.061. [DOI] [PubMed] [Google Scholar]
- Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AK, et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J Extracell Vesicles. 2018;7:1528109. doi: 10.1080/20013078.2018.1528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Ye G, Liu Y, Huang D, Luo Q, Chen W, Qi Z. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci Lett. 2022;779:136635. doi: 10.1016/j.neulet.2022.136635. [DOI] [PubMed] [Google Scholar]
- Lv B, Li F, Han J, Fang J, Xu L, Sun C, Hua T, Zhang Z, Feng Z, Jiang X. Hif-1α overexpression improves transplanted bone mesenchymal stem cells survival in rat MCAO stroke model. Front Mol Neurosci. 2017;10:80. doi: 10.3389/fnmol.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wang J, Liu H, Chen Y, Ma X, Chen S, Chen Y, Bihl JI, Yang YI. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc. 2018;50:2024–2032. doi: 10.1249/MSS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- Ma T, Chen S, Wang J, Liang S, Chen M, Liu Q, Zhang Z, Liu G, Yang Y, Hu Y, Xie J. Enhanced osteolysis targeted therapy through fusion of exosomes derived from m2 macrophages and bone marrow mesenchymal stem cells: modulating macrophage polarization. Small. 2023;20:e2303506. doi: 10.1002/smll.202303506. [DOI] [PubMed] [Google Scholar]
- Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Miao C, Wang X, Zhou W, Huang J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res. 2021;169:105680. doi: 10.1016/j.phrs.2021.105680. [DOI] [PubMed] [Google Scholar]
- Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol. 2017;8:467. doi: 10.3389/fneur.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, McCullough LD, Moruno-Manchon JF. Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome. Neural Regen Res. 2023;18:31–37. doi: 10.4103/1673-5374.340406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A, Martinez-Arroyo O, Forner MJ, Cortes R. Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics. 2020;13:3. doi: 10.3390/pharmaceutics13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Pan T, Wu Y, Zhang X, Wang J, Wang X, Gu Q, Xu C, Fan Y, Li X, Xie P, Liu Q, Hu Z. Lens epithelial cell-derived exosome inhibits angiogenesis in ocular pathological neovascularization through its delivery of miR-146a-5p. FASEB J. 2023;37:e23192. doi: 10.1096/fj.202301020RR. [DOI] [PubMed] [Google Scholar]
- Pei X, Li Y, Zhu L, Zhou Z. Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle. 2020;19:906–917. doi: 10.1080/15384101.2020.1731649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets N, Hertz S, London M, Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism. 2018;9:57. doi: 10.1186/s13229-018-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50:e344–418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Puyal J, Ginet V, Clarke PG. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- Ran N, Li W, Zhang R, Lin C, Zhang J, Wei Z, Li Z, Yuan Z, Wang M, Fan B, Shen W, Li X, Zhou H, Yao X, Kong X, Feng S. Autologous exosome facilitates load and target delivery of bioactive peptides to repair spinal cord injury. Bioact Mater. 2023;25:766–782. doi: 10.1016/j.bioactmat.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. doi: 10.1016/j.biomaterials.2022.121949. [DOI] [PubMed] [Google Scholar]
- Rho J, Chung J, Im H, Liong M, Shao H, Castro CM, Weissleder R, Lee H. Magnetic nanosensor for detection and profiling of erythrocyte-derived microvesicles. ACS Nano. 2013;7:11227–11233. doi: 10.1021/nn405016y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunkhe S, Dheeraj, Basak M, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J Control Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Sato K. Activated microglia disrupt the blood-brain barrier and induce chemokines and cytokines in a rat in vitro model. Front Cell Neurosci. 2018;12:494. doi: 10.3389/fncel.2018.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirejini SZ, Inci F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol Adv. 2022;54:107814. doi: 10.1016/j.biotechadv.2021.107814. [DOI] [PubMed] [Google Scholar]
- Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21:6466. doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60:9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Zhang TT, Chen JL, Xia YF, Qin ZH, Waeber C, Sheng R. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 2017;8:e2912. doi: 10.1038/cddis.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Zhang X, Chen R, Miao J, Wang L, Cui L, Ji H, Liu Y. Cortical neuron-derived exosomal microRNA-181c-3p inhibits neuroinflammation by downregulating CXCL1 in astrocytes of a rat model with ischemic brain injury. Neuroimmunomodulation. 2019;26:217–233. doi: 10.1159/000502694. [DOI] [PubMed] [Google Scholar]
- Song Y, Li Z, He T, Qu M, Jiang L, Li W, Shi X, Pan J, Zhang L, Wang Y, Zhang Z, Tang Y, Yang GY. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910–2923. doi: 10.7150/thno.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Xu G. Mesenchymal stem cell-derived exosomal miR-150-3p affects intracerebral hemorrhage by regulating TRAF6/NF-κB axis, gut microbiota and metabolism. Stem Cell Rev Rep. 2023;19:1907–1921. doi: 10.1007/s12015-023-10541-1. [DOI] [PubMed] [Google Scholar]
- Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002;105:1679–1685. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Thery C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Tian T, Cao L, He C, Ye Q, Liang R, You W, Zhang H, Wu J, Ye J, Tannous BA, Gao J. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11:6507–6521. doi: 10.7150/thno.56367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Zhang F, Qiu Y, Wang S, Li F, Zhao J, Pan C, Tao Y, Yu D, Wei W. Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells. Nat Biomed Eng. 2021;5:968–982. doi: 10.1038/s41551-021-00764-3. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. 2021;85:e13367. doi: 10.1111/aji.13367. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, Liu XS, Zhang Y, Roberts C, Zhang ZG. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43:2221–2228. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol Med. 2017;23:1103–1120. doi: 10.1016/j.molmed.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Venkat P, Chen J, Chopp M. Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab. 2018;38:2165–2178. doi: 10.1177/0271678X18782789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Cui C, Chopp M, Zacharek A, Wang F, Landschoot-Ward J, Shen Y, Chen J. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke. 2019;50:2865–2874. doi: 10.1161/STROKEAHA.119.025371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- Vigneswara V, Ahmed Z. Pigment epithelium-derived factor mediates retinal ganglion cell neuroprotection by suppression of caspase-2. Cell Death Dis. 2019;10:102. doi: 10.1038/s41419-019-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- Wang C, Börger V, Sardari M, Murke F, Skuljec J, Pul R, Hagemann N, Dzyubenko E, Dittrich R, Gregorius J, Hasenberg M, Kleinschnitz C, Popa-Wagner A, Doeppner TR, Gunzer M, Giebel B, Hermann DM. Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke. 2020;51:1825–1834. doi: 10.1161/STROKEAHA.119.028012. [DOI] [PubMed] [Google Scholar]
- Wang C, Börger V, Mohamud Yusuf A, Tertel T, Stambouli O, Murke F, Freund N, Kleinschnitz C, Herz J, Gunzer M, Popa-Wagner A, Doeppner TR, Giebel B, Hermann DM. Postischemic neuroprotection associated with anti-inflammatory effects by mesenchymal stromal cell-derived small extracellular vesicles in aged mice. Stroke. 2022;53:e14–e18. doi: 10.1161/STROKEAHA.121.035821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Xu SY, Lv HQ, Zhang C, Peng YJ. Electroacupuncture inhibits ferroptosis induced by cerebral ischemiareperfusion. Curr Neurovasc Res. 2023;20:346–353. doi: 10.2174/1567202620666230623153728. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu H, Chen S, Zhang W, Chen Y, Yang Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp Neurol. 2020;330:113325. doi: 10.1016/j.expneurol.2020.113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163-164:98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Z, Mo F, Chen-Mayfield TJ, Saini A, LaMere AM, Hu Q. Chemically engineering cells for precision medicine. Chem Soc Rev. 2023;52:1068–1102. doi: 10.1039/d2cs00142j. [DOI] [PubMed] [Google Scholar]
- Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27:237–251.e234. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- Wu Y, Huang X, Tan Z, Zang J, Peng M, He N, Zhang T, Mai H, Xu A, Lu D. FUS-mediated HypEVs: neuroprotective effects against ischemic stroke. Bioact Mater. 2023;29:196–213. doi: 10.1016/j.bioactmat.2023.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X, Gao Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–1209. doi: 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xiao Y, Geng F, Wang G, Li X, Zhu J, Zhu W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem. 2019;120:2109–2118. doi: 10.1002/jcb.27519. [DOI] [PubMed] [Google Scholar]
- Xie X, Wang L, Dong S, Ge S, Zhu T. Immune regulation of the gut-brain axis and lung-brain axis involved in ischemic stroke. Neural Regen Res. 2024;19:519–528. doi: 10.4103/1673-5374.380869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Liu Z, Buller B, Li Y, Golembieski W, Gan X, Wang F, Lu M, Ali MM, Zhang ZG, Chopp M. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab. 2021;41:1131–1144. doi: 10.1177/0271678X20950489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Pan Y, Wei W, Tatenhorst L, Graf I, Popa-Wagner A, Gerner ST, Huber S, Kilic E, Hermann DM, Bähr M, Huttner HB, Doeppner TR. Preconditioned extracellular vesicles from hypoxic microglia reduce poststroke AQP4 depolarization, disturbed cerebrospinal fluid flow, astrogliosis, and neuroinflammation. Theranostics. 2023;13:4197–4216. doi: 10.7150/thno.84059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X, Guo S, Zhang G, Liu Y, Bi S, Wang X, Lu Q. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz J Med Biol Res. 2020;53:e9106. doi: 10.1590/1414-431X20199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Zeng CL, Ni SM, Peng YJ. The angiogenesis effects of electro-acupuncture treatment via exosomal miR-210 in cerebral ischemia-reperfusion rats. Curr Neurovasc Res. 2022;19:61–72. doi: 10.2174/1567202619666220321115412. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhu Y, Chuai J. Changes in serum ghrelin and small intestinal motility in rats with ischemic stroke. Anat Rec (Hoboken) 2012;295:307–312. doi: 10.1002/ar.21490. [DOI] [PubMed] [Google Scholar]
- Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, Zhu X, Won MH, Bo P, Su P. Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol. 2019;78:157–171. doi: 10.1093/jnen/nly119. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Z, Shi H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci. 2012;69:115–128. doi: 10.1007/s00018-011-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–3707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang T, Zhang X, Zhang H, Yan N, Zhang G, Yan R, Li Y, Yu J, He J, Jia S, Wang H. Exosomes derived from human placental mesenchymal stem cells ameliorate myocardial infarction via anti-inflammation and restoring gut dysbiosis. BMC Cardiovasc Disord. 2022;22:61. doi: 10.1186/s12872-022-02508-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Odenthal M, Fries JW. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci. 2016;17:2028. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerna C, Thomalla G, Campbell BCV, Rha JH, Hill MD. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. 2018;392:1247–1256. doi: 10.1016/S0140-6736(18)31874-9. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, Zhu ZJ, Flores R, Wen Y, Gong X, Liu Q, Li YP. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8:589. doi: 10.1038/s41467-017-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wu Y, Xie F, Zhong Y, Wang Y, Xu M, Feng J, Charish J, Monnier PP, Qin X. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018;25:1503–1516. doi: 10.1038/s41418-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Jin T, Wang L, Liu W, Zhang Y, Zheng Y, Lin Y, Yang M, He X, Lin H, Chen L, Tao J. Electro-acupuncture promotes the differentiation of endogenous neural stem cells via exosomal microRNA 146b after ischemic stroke. Front Cell Neurosci. 2020;14:223. doi: 10.3389/fncel.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qin Y, Chopp M, Li C, Kemper A, Liu X, Wang X, Zhang L, Zhang ZG. Ischemic cerebral endothelial cell-derived exosomes promote axonal growth. Stroke. 2020;51:3701–3712. doi: 10.1161/STROKEAHA.120.031728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F, Han J, Sun H, Ouyang Q, Hua S, Lv B, Hua T, Liu Z, Cai Y, Zou Y, Tang Y, Jiang X. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging (Albany NY) 2021;13:3060–3079. doi: 10.18632/aging.202466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhou J, Teng H, Yang H, Qiu J, Li X. MiR-145 enriched exosomes derived from bone marrow-derived mesenchymal stem cells protects against cerebral ischemia-reperfusion injury through downregulation of FOXO1. Biochem Biophys Res Commun. 2022;632:92–99. doi: 10.1016/j.bbrc.2022.09.089. [DOI] [PubMed] [Google Scholar]
- Zhou M, Li YJ, Tang YC, Hao XY, Xu WJ, Xiang DX, Wu JY. Apoptotic bodies for advanced drug delivery and therapy. J Control Release. 2022;351:394–406. doi: 10.1016/j.jconrel.2022.09.045. [DOI] [PubMed] [Google Scholar]
- Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ, Hu BY, Qin W, Zou TT, Fu Y, Shen XT, Zhu WW, Geng Y, Lu L, Jia HL, Qin LX, Dong QZ. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13:152. doi: 10.1186/s13045-020-00987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Z, Wang J, Luo Y, Zeng R, Zhang C, Zhou W, Guo K, Wu H, Sha W, Chen H. Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles. Acta Biomater. 2021;134:13–31. doi: 10.1016/j.actbio.2021.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicate..


