Abstract
The primary mechanism of secondary injury after cerebral ischemia may be the brain inflammation that emerges after an ischemic stroke, which promotes neuronal death and inhibits nerve tissue regeneration. As the first immune cells to be activated after an ischemic stroke, microglia play an important immunomodulatory role in the progression of the condition. After an ischemic stroke, peripheral blood immune cells (mainly T cells) are recruited to the central nervous system by chemokines secreted by immune cells in the brain, where they interact with central nervous system cells (mainly microglia) to trigger a secondary neuroimmune response. This review summarizes the interactions between T cells and microglia in the immune-inflammatory processes of ischemic stroke. We found that, during ischemic stroke, T cells and microglia demonstrate a more pronounced synergistic effect. Th1, Th17, and M1 microglia can co-secrete pro-inflammatory factors, such as interferon-γ, tumor necrosis factor-α, and interleukin-1β, to promote neuroinflammation and exacerbate brain injury. Th2, Treg, and M2 microglia jointly secrete anti-inflammatory factors, such as interleukin-4, interleukin-10, and transforming growth factor-β, to inhibit the progression of neuroinflammation, as well as growth factors such as brain-derived neurotrophic factor to promote nerve regeneration and repair brain injury. Immune interactions between microglia and T cells influence the direction of the subsequent neuroinflammation, which in turn determines the prognosis of ischemic stroke patients. Clinical trials have been conducted on the ways to modulate the interactions between T cells and microglia toward anti-inflammatory communication using the immunosuppressant fingolimod or overdosing with Treg cells to promote neural tissue repair and reduce the damage caused by ischemic stroke. However, such studies have been relatively infrequent, and clinical experience is still insufficient. In summary, in ischemic stroke, T cell subsets and activated microglia act synergistically to regulate inflammatory progression, mainly by secreting inflammatory factors. In the future, a key research direction for ischemic stroke treatment could be rooted in the enhancement of anti-inflammatory factor secretion by promoting the generation of Th2 and Treg cells, along with the activation of M2-type microglia. These approaches may alleviate neuroinflammation and facilitate the repair of neural tissues.
Keywords: brain, immune, inflammation, interaction, ischemic stroke, mechanism, microglia, neuron, secondary injury, T cells
Introduction
Stroke is a devastating cerebrovascular event that occurs due to an interruption of cerebral blood flow. Such events are classified as either ischemic stroke or hemorrhagic stroke. In the past, the brain was thought to be an immune-privileged organ protected by the blood–brain barrier (Halder et al., 2023; Wei et al., 2023; Yue and Hoi, 2023; Singh et al., 2024). Recent studies however have revealed that cerebral ischemia/reperfusion injury can compromise the integrity of the blood–brain barrier and induce endogenous signaling and peripheral antigen activation (Goldmann and Prinz, 2013; Qiu et al., 2021; Candelario-Jalil et al., 2022). Subsequently, there is a massive infiltration of peripheral immune cells into the site of injury, in which both innate and adaptive immune responses are involved, leading to full-blown neuroinflammation. The inflammatory response in the brain after ischemic stroke may accelerate the formation of ischemic damage, affecting neuronal death and neural tissue regeneration and leading to secondary damage (Bayraktutan, 2019). The neuroinflammatory response after cerebral ischemia is characterized by the activation of microglia and astrocytes and an increase in inflammasomes. Microglia are the predominant immune cells in the central nervous system (CNS) and the first line of immune defense in the CNS (Lambertsen et al., 2019). As the first immune cells to be activated after ischemic stroke, microglia in the ischemic core and penumbra are induced to aggregate by Toll-like receptor (TLR)-mediated nuclear factor kappa-B (NF-κB) pathways within minutes. Activated microglia remove cellular debris and release inflammatory mediators while expressing major histocompatibility complex II (MHC II), which functions to present antigens to other cells (Abellanas et al., 2019).
After the onset of ischemic stroke, peripheral blood immune cells, including myeloid dendritic cells, lymphocytes, monocytes/macrophages, and neutrophils, are recruited by the chemokines secreted by immune cells in the brain; they migrate into the brain within 1 day after stroke, peaking at 3 days after stroke, and persist until day 7 after stroke. Peripheral T cells can re-edit their gene expression profiles, upregulate the expression of chemokine receptors and adhesion molecules, and promote their migration toward inflammatory tissues while enhancing their ability to cross the blood–brain barrier. They thus enter the CNS to interact with CNS cells and trigger a secondary neuroimmune response (Yoshida et al., 2022).
After ischemic stroke, microglia can promote the infiltration of T cells into the nervous system by means of antigen presentation. This interaction can induce T cell differentiation toward two distinct subpopulations: pro-inflammatory and anti-inflammatory cells. Additionally, peripheral T cells entering the CNS can regulate the function of resident microglia. It is worth exploring how these two immune cells influence the course of the inflammatory response through their mutual interactions. Therefore, the aim of this paper is to explore the mechanisms of interaction between T cells and microglia and classify them into pro-inflammatory and anti-inflammatory directions. This understanding can guide future therapeutic approaches for ischemic stroke, enabling the development of targeted drugs to block the pro-inflammatory links between T cells and microglia while promoting their anti-inflammatory mechanisms. The glial cell line-derived neurotrophic factor (GDNF) secreted by microglia during inflammation suppression can promote the repair and regeneration of neurons and therefore play a neuroprotective role. The regeneration of brain tissue is an important manifestation of ischemic stroke repair. Therefore, the information covered in this review is closely connected to nerve regeneration and provides a theoretical support for future treatments of ischemic stroke.
Search Strategy and Selection Criteria
To conduct this narrative review, the literature was searched using a combination of the following terms: “microglia” AND “ischemic stroke,” “T cell” AND “ischemic stroke,” “Th1 cell” AND “microglia” OR “ischemic stroke,” “Th17 cell” AND “microglia” OR “ischemic stroke,” “Treg” AND “microglia” OR “ischemic stroke,” “immune-inflammatory” AND “microglia” AND “ischemic stroke,” and “immune-inflammatory” AND “T cell” AND “ischemic stroke.” The PubMed database was used to search for relevant articles published between January 2000 and April 2023. Articles that provided information on the classification of T cells and microglia, their physiological functions, interactions between T cells and microglia in ischemic stroke, and their role in immunoinflammation were included in this review to explore the interactions among T cells and microglia in immune inflammation in ischemic stroke. A timeline of findings on the roles of microglia and T cells in ischemic stroke provided in the literature is presented in Figure 1.
Figure 1.
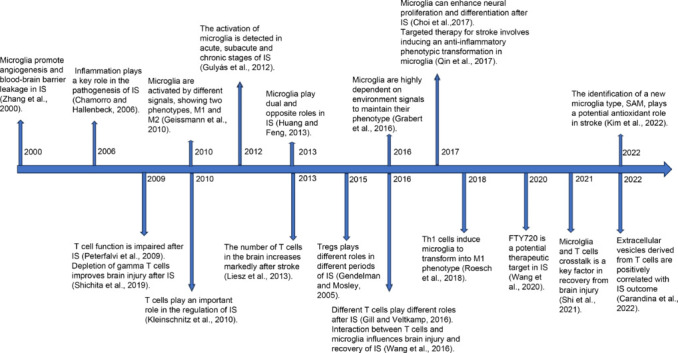
Timeline showing the roles of microglia and T cells in ischemic stroke described in the literature.
SAM: Stroke-associated microglia; IS: ischemic stroke.
Although microglia were discovered by Franz Nissl (Ziebell et al., 2012) and F. Robertson in the late 19th century through immunohistochemical experiments, they were first named “microglia” in the 1920s. In the century that followed their discovery, their important role in encephalopathy has been gradually explored. Huang and Feng (2013) reported that microglia play important roles in ischemic stroke. Microglia can promote angiogenesis and blood–brain barrier (BBB) leakage during (Zhang et al., 2000) and enhance neuronal proliferation and differentiation after ischemic stroke (Choi et al., 2017). Under various signal stimulations, the two phenotypes of M1 and M2 were shown to interact and regulate the treatment and prognosis of ischemic stroke patients (Geissmann et al., 2010). By promoting the transformation of microglia to an anti-inflammatory phenotype, they can be targeted in treatments (Qin et al., 2017). In 2022, a novel cell type, stroke-associated microgila, was identified and reported to potentially play an antioxidant role in stroke (Kim et al., 2022).
T cells, essential immune cells in the human body, are found as a wide range of cellular species and have complex functions. They play significant regulatory roles in ischemic stroke, with different T cell types playing distinct roles at different stages (Gill and Veltkamp, 2016). For instance, regulatory T cells (Tregs) are considered the primary neuroprotective regulators in ischemic stroke (Gendelman and Mosley, 2015). Th1 cells promote microglial phenotypic conversion (Roesch et al., 2018), while the depletion of γ T cells mitigates brain damage following ischemic stroke (Shichita et al., 2009).
Peripheral Immune Cells: T lymphocytes
Introduction to T cells
T lymphocytes, or T cells, originate from bone marrow and mature in the thymus (Adu-Berchie et al., 2023). Upon reaching maturity, they are distributed via the bloodstream and settle in thymus-dependent areas of peripheral immune organs. They can be recirculated via lymphatics, peripheral blood, and tissue fluids to execute functions such as cellular immunity and immunomodulation. T cells play pivotal roles in the adaptive immune system (Qin et al., 2020), and execute important roles in the crosstalk between the innate and adaptive immune systems (Ponsaerts et al., 2021). Within the body, T cells are constantly renewed, and various subpopulations at different developmental stages or functions can coexist simultaneously. The taxonomic nomenclature of T cells is therefore highly complex.
Classification of T cells
Based on differences in their T cell antigen receptors (TCRs), T lymphocytes can be classified into αβ T lymphocytes and γδ T lymphocytes. The TCR is a specific receptor that allows T cells to recognize and bind foreign antigens, serving as a unique identifier for T cells. The receptor is a heterodimer composed of two distinct peptide chains. The TCR of αβ T lymphocytes consists of a heterodimer of α and β chains, while that of γδ T lymphocytes comprises a heterodimer of γ and δ chains. In adult peripheral blood, 95% of T cells belong to the αβ T lymphocyte category, with only a small fraction belonging to γδ T lymphocytes. However, the role of the latter should not be overlooked. Furthermore, αβ T lymphocytes can be further subclassified into CD4 T cells and CD8 T cells based on their surface expression of differentiation antibody population cluster of differentiation molecules. In peripheral lymphoid tissues, CD4 T cells account for approximately 65% of T cells. The CD4 T cell TCR recognition of antigens is MHC class II molecule-restricted, i.e., it is stimulated when CD4 T cells bind to antigenic MHC class II molecules expressed on the surface of antigen-presenting cells (APCs) (Lu, 1995).
The primary role of αβ T lymphocytes is to promote the proliferation and differentiation of B cells, T cells, and other immune cells. This assistance is crucial for both humoral immunity and cellular immunity. CD8 T cells, which account for approximately 35% of T cells, have TCR recognition that is MHC class I molecule-restricted. These CD8 T cells play significant roles in immune defense and immune surveillance. They eliminate intracellular infections and malignant cells by secreting cytokines and directly killing infected cells, and this process is crucial in providing long-term protective immunity. CD8 T cells are classified further into cytotoxic T cells (TC) and suppressor T cells (TS) based on their functions (Zhang et al., 2021) CD4 T cells, however, are categorized into conventional helper T cells (Th) and Tregs (Hedrick, 2002). Conventional Th cells are further divided into Th1 cells, Th2 cells, Th9 cells, Th17 cells, and T follicular helper cells (Tfh), depending on their cytokine secretion patterns (Stockinger et al., 2006; Zhu et al., 2010; Crotty, 2011; Lei et al., 2021), and these subsets possess distinct markers and functions.
Functions of Th1 cells
The surface markers of Th1 cells include αβTCR, CD3, CD4, interleukin (IL)-12 receptor (R), interferon gamma (IFN-γ)R, and C–X–C motif chemokine receptor 3. The major transcription factors for Th1 cell differentiation are T-box expressed in T cells (T-bet), signal transducer and activator of transcription (STAT)4, and STAT1, which promote the expression of specific genes in Th1 cells (Watanabe et al., 2020). The major effector molecules secreted by Th1 cells include IFN-γ, IL-2, and lymphotoxin alpha. These molecules promote protective immunity against intracellular pathogens by secreting IFN-γ, which induces macrophage activation and the upregulation of inducible nitric oxide synthase (iNOS), leading to intracellular pathogen death (Gocher et al., 2022).
Functions of Th2 cells
The surface markers of Th2 cells include αβTCR, CD3, CD4, IL-4R, IL-33R, C–C motif chemokine receptor 4, IL-17R B, chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells, and others. The major transcription factors for Th2 cell differentiation are GATA-binding protein 3, STAT6, differentiated embryo-chondrocyte expressed gene 2, and macrophage activation factor (Zhang et al., 2017). Additionally, IFN regulatory factor 4 is a transcription factor involved in Th2 cell differentiation (Williams et al., 2013). These transcription factors promote the expression of specific genes in Th2 cells. The major effector molecules secreted by Th2 cells are IL-4, IL-5, IL-13, and IL-10. These cytokines play crucial roles in promoting humoral immune responses and host defenses against extracellular parasites; however, they also contribute to allergic reactions and asthma (Altin et al., 2012).
Functions of Th9 cells
The surface markers of Th9 cells encompass αβTCR, CD3, and CD4. The key transcription factor driving Th9 cell differentiation is the Spi-1 proto-oncogene (PU.1), which facilitates the expression of specific genes in these cells. The predominant effector molecules secreted by Th9 cells are IL-9 and IL-10. These cytokines primarily contribute to the host’s defense against extracellular parasites. Additionally, Th9 cells produce IL-10, which can exacerbate allergic inflammation. However, given that this Th9 subpopulation has been recently characterized, its involvement in other inflammatory conditions remains unclear (Khokhar and Purohit, 2024).
Functions of Th17 cells
The surface markers of Th17 cells include αβTCR, CD3, CD4, IL-23R, C–C motif chemokine receptor 6, IL-1R, and CD161 (humans only). The major transcription factors for Th17 cell differentiation are retinoic acid receptor-related orphan receptor γt, STAT3, and related orphan receptor A, which promote the expression of Th17-cell–specific genes. The main effector molecules secreted by Th17 cells are IL-17A, IL-17F, IL-21, IL-22, and C–C motif chemokine 20. These cytokines are primarily distributed on mucosal surfaces and play crucial roles in promoting protective immunity against extracellular bacteria and fungi. Additionally, they contribute to the treatment of autoimmune and inflammatory diseases (Wang et al., 2014).
Functions of Tfh cells
The surface markers of Tfh cells include αβTCR, CD3, CD4, C–X–C motif chemokine receptor 5, signaling lymphocytic activation molecule, OX40L (CD252), CD40 ligand, inducible T cell costimulator (ICOS), IL-21R, and programmed cell death-1 (PD-1). The pivotal transcription factor for Tfh cell differentiation is B-cell lymphoma 6, facilitating the expression of specific genes in these cells. The primary effector molecule secreted by Tfh cells is IL-21, which plays a crucial role in information transfer during B-cell differentiation. Specifically, IL-21 facilitates the activation of B cells, promotes the formation of germinal centers and immunoglobulin class switching, and sustains prolonged humoral immune responses (Crotty, 2014; Jogdand et al., 2016).
Functions of cytotoxic T cells and suppressor T cells
TC cells function by recognizing target cells expressing specific antigens, establishing contact with them, and releasing cytotoxic agents such as perforins, granzymes, and granzyme hemolysins. These substances form transmembrane pore channels, ultimately leading to the lysis and death of the target cells. Alternatively, TC cells can induce apoptosis by manipulating the Fas–Fas ligand system within the target cells. Conversely, TS cells suppress the activity of Th cells, indirectly inhibiting B cell differentiation and the cytotoxic function of TC cells. This leads to negative regulation of both humoral and cellular immunity (Utzschneider et al., 2016; Prasad Singh et al., 2020).
Functions of γδ T cells
γδ T cells are the initial T cell population to emerge in the thymus. However, their relative abundance diminishes with the emergence and growth of αβ T cells. In adulthood, γδ T cells constitute 1%–5% of peripheral blood T cells (Kou et al., 2022), whereas in specific regions of the intestine, they can constitute up to 30% of all T cells (Mayassi and Jabri, 2018). Furthermore, γδ T cells are extensively present in epithelial-dense tissues that form the inner and outer surfaces of the body, including the germinal tract, intestinal epithelium, and the epidermis and dermis of the skin (Alves de Lima et al., 2020). γδ T cells express intracellular TLR2, TLR17, the central TLR junction molecule MyD54, as well as TLR55 (Schafer et al., 2012); they are characterized by the expression of γδ TCR and CD3 molecules on their surface, and their main secreted effector molecules include IFN-γ, IL-17A, IL-17F, and IL-22. Cells producing IFN-γ and IL-17A are distinct populations that can be distinguished by the expression of CD27 and C–C motif chemokine receptor 6 on their surface. γδ T cells possess both anti-inflammatory and pro-inflammatory functions and contribute to both innate and adaptive immunity. Their recognition of innate immunity is affected by the expression of TLRs. The surface phenotypes and functions of T cell subpopulations are summarized in Table 1.
Table 1.
Surface phenotypes of T cell subsets and their functions
| T cell categorization | Surface phenotype | Function |
|---|---|---|
| αβT | CD3, αβTCR | – |
| CD4+ αβ T | CD3, αβTCR, CD4 | – |
| Th1 | CD3, αβTCR, CD4, CXCR3, CCR5, IFN-γ, IL-12R | Th1 cells are involved in cellular immune responses as well as defenses against pathogenic microbial infections, including promotion of proliferation and differentiation of cytotoxic T cells and phagocytosis by macrophages. |
| Th2 | CD3, αβTCR, CD4, CCR3, CCR4, IFN-γ, IL-4R, IL-17R, IL-33R | Th2 cells are involved in the humoral immune response and in defending against parasitic infections, including promoting the proliferation and differentiation of B cells into plasma cells that produce antibodies. |
| Th9 | CD3, αβTCR, CD4 | Th9 cells are associated with tumor progression, may exert both antitumor and pro-tumor effects, and promote the development and progression of certain inflammatory diseases. |
| Th17 | CD3, αβTCR, CD4, CCR6, CD161, IL-1R, IL-23R | Th17 cells are involved in the inflammatory response and defense against extracellular pathogenic microorganisms, especially in the intestinal and respiratory mucosa. |
| Treg | CD3, αβTCR, CD4, CD25, CD39, CD73, CD103 | Treg is a class of cells that can negatively regulate immune responses, and its most prominent function is to maintain self-tolerance and immune homeostasis. |
| Tfh | CD3, αβTCR, CD4, CD84, CD126, CXCR5, OX40L, CD40L, PD1 | Tfh aids B-cell survival and proliferation in the germinal center and promotes B-cell differentiation to plasma cells, antibody class switching, and antibody affinity maturation. |
| CD8+ αβ T | CD3, αβTCR, CD8, CCR7, IL-7R | CD8+ αβ T cells specifically recognize antigenic peptide-MHC class I molecular complexes and thus kill target cells, including cells infected by intracellular pathogens and tumor cells. |
| γδT | CD3, γδTCR | γδT has both anti-inflammatory and pro-inflammatory functions, both intrinsic and adaptive immunity, and its recognition of intrinsic immunity is influenced by the expression of TLRs. |
CCR: Chemokine receptor; CD: cluster of differentiation; CD40L: CD40 ligand; CDL: cluster of differentiation 126; CXCR: C–X–C motif chemokine receptor 3; CXCR5: C–X–C motif chemokine receptor 5; IFN-γ: interferon-γ; IL-17R: interleukin 17 receptor; OX40L: OX40 ligand; PD1: programmed death-1; TCR: T cell antigen receptor; Tfh: T follicular helper cells; Th: helper T cell; Treg: regulatory cells.
T cell infiltration into the brain after ischemic stroke
After ischemic stroke, innate immune cells are activated, and T cells are induced to infiltrate the brain tissue. There are three main routes of infiltration: the blood-brain barrier, choroid plexus, and soft meningeal infiltration (Benakis et al., 2018). Following stroke, immune cells accumulate near the epithelial cells of the blood–brain barrier and secrete inflammatory factors that disrupt its integrity, allowing T cells to infiltrate the ischemic brain tissue. The choroid plexus is a structure that produces cerebrospinal fluid, and after ischemic stroke, there is a significant increase in chemokine expression in the injured brain tissue. T cells can migrate from the choroid plexus to the brain parenchyma along a chemokine gradient (Zhang et al., 2021b). As for the soft meningeal infiltration pathway, fluorescent cell-tracking experiments have shown that labeled intestinal γδ-T cells accumulate in the soft meninges in the early stages of stroke (Benakis et al., 2016), but the specific pathway of their migration to the meninges remains unclear and requires further investigation.
A study by Feng et al. (2017) demonstrated that T cells begin to infiltrate ischemic brain tissue as early as 24 hours after stroke and can remain in the brain tissue until post-ischemia week 7. Different types of T cells infiltrate the brain at different times and are simultaneously regulated by the varying effects of various cytokines and chemokines (Zhang et al., 2021b). CD4 T cells infiltrate the infarcted area on the first day and increase in numbers significantly thereafter, peaking on the seventh day. Some subsets of CD4 T cells continue to infiltrate the infarcted area for 7 days after acute ischemic stroke (Stubbe et al., 2013; Miró-Mur et al., 2020). Among the various subtypes of CD4 T cells, Th1 and Th17 cells have been demonstrated to increase in numbers within the first 3 days and decrease on day 7, whereas the opposite trend is observed for Th2 cells (Yu et al., 2022). CD8 T cells infiltrate the brain tissue within a few hours of stroke, and as the first invading lymphocyte, they significantly increase within the first 3 days after a stroke (Miró-Mur et al., 2020). In addition, only a small amount of Treg infiltration occurs during the acute phase of ischemic stroke (Shi et al., 2021), but Treg cell infiltration increases significantly from day 7 onward and can persist for more than 5 weeks (Stubbe et al., 2013; Xu et al., 2013). Studies have also shown that γδ-T cells can be observed in ischemic brain tissue within 24 hours of stroke, and their secretion of the major effector molecule IL-17 peaks on the third day (Shichita et al., 2009; Dong et al., 2022).
Distinct functions of various T cells after ischemic stroke
It is being increasingly recognized that the interactions between ischemic stroke and T cells are complex and multifaceted, with different types of T cells playing distinct roles after ischemic stroke. These interactions have important implications for effective brain function during the disease and recovery process (Selvaraj and Stowe, 2017).
Th1 cells
A key feature of the Th1 cell response is the production of IFN-γ, which has been associated with a delayed immune response to stroke. Blockade of the IFN-γ signaling pathway has been shown to reduce neurodegeneration following middle cerebral artery occlusion and decrease the expression of the inflammatory chemokine IFN-inducible protein 10 (Seifert et al., 2014). IFN-γ is also a crucial activator of microglia, significantly enhancing their number and morphology (Kaya et al., 2022). As CD4 T cells are depleted over time after a stroke, IFN-γ levels decrease, ultimately leading to improved behavioral outcomes (Harris et al., 2020).
Th2 cells
The Th2 cell response is characterized by the release of various IL cytokines, such as IL-10. IL-10 exerts anti-inflammatory effects by downregulating various pathways, including NF-κB in activated B cells. IL-10 has also been shown to reduce infarct volume and exhibit immunomodulatory effects in the CNS. Furthermore, evidence showed that BBB integrity improved in a cerebral ischemia/reperfusion model using hydrogen sulfide donors, and this was accompanied by enhanced IL-10 expression and reduced NF-κB nuclear translocation (Wang et al., 2011).
Tregs
Tregs are considered major neuroprotective modulators in the nervous system (Zhang et al., 2021b). During the early phase of ischemic stroke, Tregs curb the systemic inflammatory immune response and the activation of peripheral effector T cells (Teffs). They also restore the integrity of the BBB and promote neuronal recovery. Additionally, Tregs influence the trafficking of peripheral immune cells, primarily by inhibiting the elevation of IL-6 and tumor necrosis factor-α (TNF-α) in the blood (Asseman et al., 1999; Qin et al., 2020).
During the later stages after ischemic stroke, Tregs trigger apoptosis in Teffs by promoting the secretion of granzymes and perforin (Gondek et al., 2005). Simultaneously, Tregs can reduce microglia/monocyte activation through the release of anti-inflammatory cytokines, such as IL-4, IL-10, and transforming growth factor-β (TGF-β), in the post-stroke environment (Liesz et al., 2009; Liu et al., 2020). Furthermore, Tregs can inhibit reactive oxygen species (ROS) synthesis and release from microglia and regulate IL-6 and STAT3 pathways in microglia and astrocytes via amphiphysin, thereby exerting neuroprotective effects (Ito et al., 2019). Crucially, Tregs are involved in immunomodulatory processes during ischemic stroke, expressing a range of immunosuppressive molecules, such as CD47 (also known as integrin-associated protein), CD39 (also called ectonucleoside triphosphate diphosphohydrolase-1), PD-1, and signal-regulated protein-alpha (Huang et al., 2017).
Lastly, it is important to note that, in Treg-cell-depleted stroke mice, the surface area and volume of microglia were reported to decreased, indicating that the presence of Treg cells can alter the activation status of microglia in the post-stroke brain. Experimental data have shown that genes associated with the anti-inflammatory phenotype, such as arginase-1, fibrinogen-like 2, mannose receptor 1, IL-1R antagonist, and advanced glycation end-product receptor 3, are significantly up-regulated in microglia co-cultured with activated Treg cells. Similarly, genes encoding proteins involved in brain repair, such as chemokine receptor 5, protein-glutamine gamma-glutamyltransferase 2, vascular endothelial growth factor A, fibroblast growth factor 1, and Gdnf, are also up-regulated. This suggests that Treg cells play a role in polarizing microglia toward an anti-inflammatory and repair phenotype, which can have potential therapeutic implications. Additionally, Treg cells and microglia create an osteopontin-rich microenvironment through their interactions. This environment can positively impact microglia responses, oligodendrocyte regeneration, white matter repair in the chronic phase of ischemic stroke, and long-term neurological function and outcomes after cerebral ischemic stroke (Shi et al., 2021).
γδ T cells
The crucial role of γδ T cells in brain tissue injury after ischemic stroke is not performed in isolation, but rather is primarily a result of their release of cytokines, including IL-17A, IL-21, IL-22, and IFN-γ. The primary source of IL-17A in stroke is γδ T cells (Dong et al., 2022). IL-17a downregulates tight junctions (TJs) by inducing the production of ROS, leading to a decrease in TJ molecule expression. IL-17a also elevates matrix metalloproteinase-2, matrix metalloproteinase-3, and matrix metalloproteinase-9 levels in brain microvascular endothelial cells, hydrolyzing TJs and increasing BBB permeability. IL-17A promotes neutrophil infiltration, and the enhanced infiltration of neutrophils further disrupts the integrity of the BBB and promotes neuronal lysis and apoptosis, thereby exacerbating brain injury (Gu et al., 2015; Ruhnau et al., 2017). Additionally, IL-17A promotes the expression of apoptosis-associated proteins, including caspase-3, caspase-9, and BCL2-associated X, and increases the BCL2-associated X/B-cell lymphoma-2 ratio after brain injury, leading to neuronal apoptosis (Li et al., 2017).
IL-21 is strongly up-regulated in the injured brain, and it significantly affects brain injury by upregulating autophagy-related genes in neuronal cells expressing IL-21R. This receptor has been found to exert neuroprotective effects through the Janus tyrosine kinase (JAK)/STAT signaling pathway and the upregulation of caspase-3 (Lee et al., 2016; Weiner and Ducruet, 2016).
IL-22 has protective effects against ischemic stroke, and experiments have shown that the exogenous administration of IL-22 reduces oxidative stress and neuronal apoptosis in affected brain tissue. A small subset of γδ T cells can produce IFN-γ (Wang et al., 2023), which can act on both microglia and the CNS.
γδ T cells play roles in the pathological process of ischemic stroke through their secreted cytokines, which have significant effects on brain tissue injury, neurological deficits, and functional improvement in later stages (Wang et al., 2022). In conclusion, T cells have critical functions in various aspects of ischemic stroke pathology, and the interactions between T cells and microglia, as well as the underlying mechanisms involved in stroke, require further exploration and investigation.
Central Immune Cells–Microglia
Introduction to microglia
Microglia are macrophages that reside within the brain and serve as the immune cells of the CNS. These cells remain in the brain for the duration of an individual’s life, maintaining their numbers and spatial distribution through self-renewal (Huang et al., 2021). Microglia are recognized as the first non-neuronal cells to respond to various acute brain traumas and are the principal component of the brain parenchyma’s defense network (Woodburn et al., 2021). Microglia are analogous to brain sentinels and are very dynamic APCs in the healthy brain (Woodburn et al., 2021). They vigilantly guard the brain, continuously detecting and tracking any disturbances in homeostasis (Borst et al., 2021). To quickly react to changes in the extracellular environment; to do this, mature microglia in the brain utilize a diverse range of surface molecules, allowing them to respond to hormones, neurotransmitters, chemokines, purines, and cytokines. Like other tissue-resident macrophages, microglia express common markers such as CD11b, the surface glycoproteins F4/80 and CD68, the fractalkine receptor CX3CR1, colony-stimulating factor 1 receptor, ionized calcium-binding adapter molecule 1, and the pan-hematopoietic cell marker CD45. However, at steady state, their expression levels are lower than those of perivascular macrophages and blood monocytes (Greter and Merad, 2013). Furthermore, the presence of any deleterious conditions leads to microglia activation, which results in distinct activation phenotypes. There are two main activation phenotypes of microglia: the classical M1 phenotype and the alternative M2 phenotype (Gao et al., 2021). The M2 phenotype primarily secretes anti-inflammatory factors that function to suppress inflammatory responses and promote tissue repair and functional remodeling. Conversely, the M1 phenotype plays a pro-inflammatory role by producing high levels of oxidative metabolites and pro-inflammatory cytokines to initiate a series of inflammatory responses (Lanza et al., 2021). These two phenotypes are not mutually exclusive; they can coexist side by side and transition between one another (Song and Suk, 2017).
Physiologic functions of microglia
Microglia, as specialized phagocytes of the brain (Mallat et al., 2005), play a pivotal phagocytic role in the normal development of the brain. They are responsible for the elimination of surplus synapses, dendrites, axons, myelin, neurons, and neuronal precursors (Schafer et al., 2012; Butler et al., 2021), and thus contribute to the execution of functions needed for a healthy brain and positively impact memory and learning. Furthermore, microglia have the ability to support neurons. Microglia are crucial components of the effective myelin regeneration response and can sustain and nourish neurons by releasing neurotrophic factors (Sen et al., 2022). In addition, they are involved in programmed neuronal death apoptosis, and the clearance of neurons during development (Long et al., 2023), and are crucial for the health and survival of neurons. Under physiological conditions, microglia can secrete trophic factors such as insulin-like growth factor 1, brain-derived neurotrophic factor, and nerve growth factor to provide nutrients to neurons and other cells.
Recent studies have demonstrated the critical roles of microglia in the formation and maturation of neural circuits, particularly during postnatal development (Wake et al., 2011; Paolicelli and Ferretti, 2017; Sun et al., 2023). In the healthy CNS, microglia are located in close proximity to neuronal synapses, maintaining constant contact with neurons and astrocytes. They can engage in direct interactions with presynaptic and postsynaptic components. During brain development, the establishment of neuronal synaptic structures is integral to the maturation of the CNS. Using transmission electron microscopy and two-photon microscopic imaging, Tremblayet al. (2019) revealed that microglia engage in contact with various sites during learning and perception, including dendritic spines, axon terminals, astrocyte protrusions, and synaptic clefts. Microglia phagocytose and prune synaptic components, contributing to synaptic remodeling during sensory processing (Gaire, 2022).
Functions of microglia in pathological conditions
Under pathological conditions, microglia become activated and stimulated to express various surface receptors, mainly scavenger receptors (SRs), e.g., SR class A types A (SRA-A), SR class B types B (SRB-B), CD36, CD68, and other SRs. These receptors are primarily involved in the phagocytosis of heterogeneous molecules on the surface of apoptotic cells, as well as the endocytosis of ligands associated with intercellular adhesion and cell debris. Neuronal injury triggers microglia to migrate to the site of injury by releasing adenosine triphosphate (ATP). The SR binds to ectopically translated phosphatidylserine, and phagocytosis of cellular debris by SRA-A promotes the phagocytosis of Aβ oligomers by microglia as well as astrocytes (Yang et al., 2011) When SRA-A is able to bind to amyloid-β (Aβ) and is activated, CD36 can mediate the phagocytosis of Aβ by inhibiting the prostaglandin E2 pathway (Silverstein and Febbraio, 2009).
Microglia play crucial roles in CNS injury responses. Recombinant purinergic receptor P2Y G-protein–coupled 12 (P2RY12) on microglia is involved in ATP sensing and regulates microglia recruitment following cell injury. Purinergic receptor P2Y13 may contribute to microglia activation and the response to adenosine diphosphate (ADP) sensing during inflammation (Fekete et al., 2018; Jairaman et al., 2022).
Additionally, microglia expressing MHC II molecules can cross-present antigens to regulate T cell responses within the CNS, dysregulation of both interactions may lead to altered phagocytosis of neuronal synapses by microglia, resulting in neurocognitive deficits (Chhatbar and Prinz, 2021).
Various roles of microglia after ischemic stroke
When an ischemic stroke occurs, microglia are activated and quickly migrate to the site of injury, where ischemia, hypoxia, and energy deprivation cause the branching of microglia populations into cells with a hypertrophic amoeba-like morphology. It is increasingly recognized thatthat microglia play a diversity of roles depending on the stage of injury, interactions with other cells, pro- and anti-inflammatory conditions, and the composition of the surrounding cellular environment (Hu et al., 2014; Ma et al., 2017; Qin et al., 2019) In the acute phase, activated microglia secrete cytokines such as sphingosine-1-phosphate (S1P), high-mobility group box 1 protein, S-100 protein beta chain, and chemokine like factor-1. These cytokines bind to membrane receptors such as the cytokine receptors S1P receptor, advanced glycosylation end product-specific receptor, TLR4, IL-4R, and recombinant chemokine C–C motif receptor 4. This triggers pro-inflammatory cell signaling pathway activity via JAK2/STAT1/NF-κB, JAK2/STAT3/NF-κB, and myeloid differentiation factor 88/NF-κB. These signaling cascades initiate an intense inflammatory response (Jiang et al., 2020) and can also lead to endothelial necrosis and disruption of the BBB (Qin et al., 2019). Following the acute phase, the inflammatory response gradually subsides, and the infarcted area of ischemic stroke enters a new phase of tissue healing (Hiu et al., 2016). Microglia contribute to the repair of infarcted tissue, the promotion of cerebral revascularization, and improvements in neurological functions in this later stage (Zhang et al., 2000; Liu et al., 2016), and the mechanisms primarily involve the phagocytosis of cellular debris and secretion of growth factors (De Sousa, 2022). Figure 2 illustrates the effects of distinct subpopulations of T cells and microglia on neurons.
Figure 2.
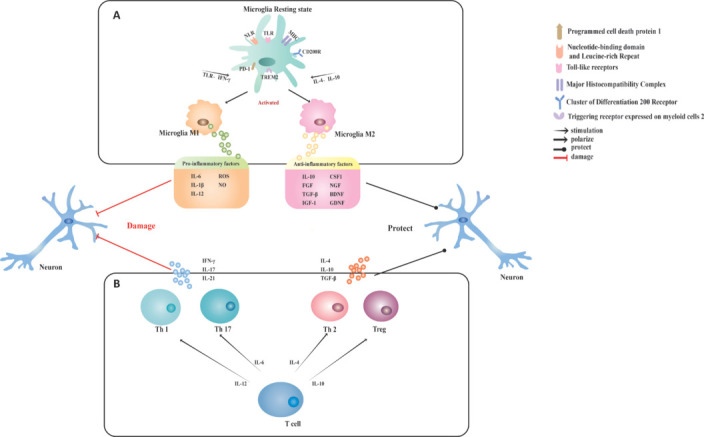
Effects of different T cell and microglia subpopulations on neurons.
(A) Resting microglia are activated to diversify into two types by different stimuli. Stimulation with TLR and IFN-γ activates resting microglia to adopt the M1 type, while stimulation with IL-4 and IL-10 activates resting microglia to form the M2 type. M1-type microglia release various pro-inflammatory cytokines that can damage neural tissue, while M2-type microglia release anti-inflammatory cytokines and trophic factors that promote neural tissue repair. (B) T cells also differentiate into different subpopulations in response to different stimuli. Th1 and Th17 cells secrete pro-inflammatory factors to trigger severe inflammation, while Th2 and Treg cells secrete anti-inflammatory factors to suppress the onset of inflammation. BDNF: Brain-derived neurotrophic factor; CD200R: cluster of differentiation 200 receptor; CSF1: colony-stimulating factor 1; FGF: fibroblast growth factor; GDNF: glial cell line-derived neurotrophic factor; IFN-γ: interferon gamma; IGF-1: insulin-like growth factor 1; IL: interleukin; LRR: leucine-rich repeat; MHC: major histocompatibility complex; NGF: nerve growth factor; NLR: nucleotide-binding domain and leucine-rich repeat; NO: nitric oxide; PD1: programmed cell death protein 1; ROS: reactive oxygen species; TGF-β: transforming growth factor-β; Th: helper T cell; TLR: Toll-like receptors; Treg: regulatory T cells; TREM2: triggering receptor expressed on myeloid cells 2.
Interaction of T Cells with Microglia
Antigen presentation
Antigen processing and presentation play crucial roles in regulating the immune response. These mechanisms process foreign or intracellular antigens and present them to T cells for recognition, ultimately leading to their activation (Kawasaki et al., 2022).
Studies by Almolda et al. (2010, 2011) have shown that two key signals are involved in the antigen presentation mechanism. The first signal is provided by the binding of MHC molecules carrying antigens to TCRs on the surface of T lymphocytes. This interaction confers specificity to the mechanism, as MHC class I molecules are specifically recognized by CD8+ T lymphocytes, while MHC class II molecules bind exclusively to CD4+ T cells (Joffre et al., 2012). Typically, MHC class I molecules present intracellular antigens, such as those produced by viruses, faulty self-molecules, or tumor-associated antigens. In contrast, MHC class II molecules are used to present antigens from extracellular pathogens like bacteria, parasites, and poisons (Elmer and McAllister, 2012). While MHC class I molecules are expressed by all nucleated cells, APCs, such as dendritic cells and macrophages, also possess the necessary processing and delivery mechanisms for presenting external antigens via MHC class II molecules (Almolda et al., 2011). The second signal, known as the co-stimulatory signal, is generated by the binding of different receptors expressed on the surface of APCs and lymphocytes to their respective co-receptors and is antigen-independent. The presence of these co-stimulatory signals is necessary for adequate T cell activation, as TCR binding to MHC without co-stimulation can lead to T cell apoptosis or incompetence (Lanzavecchia, 1997; Kishimoto and Sprent, 1999). Common co-stimulatory molecules include ICOS, CD154, and OX40 (also known as CD134), which bind to their corresponding ligands, B7h3 (also known as CD276), CD40, and OX40L, respectively, delivering stimulatory signals to lymphocytes and induce their activation and proliferation. In contrast, other co-stimulatory molecules, such as PD1, which binds to its receptor PD-L1, trigger inhibitory signals that lead to lymphocyte apoptosis (Nurieva et al., 2009).
Throughout the years, numerous investigations have established the fundamental roles of microglia in the CNS. When the brain is healthy, microglia exist in a dormant state, yet these resting cells are far from inert. Instead, they undergo significant and continuous structural changes, displaying remarkable motility. Microglia use the high motility conferred by cell protrusions to constantly observe and monitor the CNS microenvironment (Catalano et al., 2022). Microglia sense subtle changes in their environment through a variety of surface receptors. When the microenvironment undergoes molecular transformations, such as the appearance of lesions, neurotoxicity, or infection, microglia respond swiftly, transitioning from irregular patrol movements to purposeful movements toward the site of the lesion (Dheen et al., 2007; Hanisch and Kettenmann, 2007).
Infected and damaged cells secrete nucleotides, which microglia sense through their purinergic receptors, causing them to migrate toward the affected cells. Microglia’s perception of damage-associated molecular patterns triggers their activation, enhancing their ability to detect pathogen-associated molecular patterns. At this point, microglia transform into fully functional APCs, significantly increasing MHC II expression and presenting antigens to T cells (Goddery et al., 2021). Microglia possess the unique ability to cross-present antigens, allowing MHC class I molecules to present extracellular antigens, thereby accelerating the immune response process (Moseman et al., 2020). Quantitative analysis of cells using flow cytometry revealed that T cells in the spleen exhibit a strong negative correlation with cells in the brain during the first 3 days of ischemic stroke, indicating there is a large influx of T cells from the spleen into the brain following the event (Liu and Sorooshyari, 2021). This finding also confirms the antigen-presenting role of microglia at the experimental level.
The regulation of antigen presentation by microglia is influenced by multiple factors. In the intact CNS, microglia are maintained in a quiescent state through local interactions between the microglial cell receptor CD200 and its ligand. Healthy neurons can suppress the expression of MHC molecules in microglia as a means to reduce inflammation (Neumann, 2001). In neurodegenerative diseases, microglia can promote the recruitment of CD8+ memory T cells to the aging CNS by releasing C-C motif chemokine ligand 3 and upregulating the expression of vascular cell adhesion molecule and intercellular cell adhesion molecule in brain endothelial cells (Zhang et al., 2022). Both IL-6 and IFN-α contribute to the formation of reactive microglia in the core area and promote antigen presentation in animal models of neurodegenerative and neuroinflammatory diseases (West et al., 2022). During the early stages of neurological injury, activated microglia with a pro-inflammatory phenotype predominate and upregulate the expression of molecules involved in phagocytosis, oxidative damage, antigen presentation, and T cell stimulation. In later stages, microglia in active lesions shift to a phenotypic intermediate between pro-inflammatory and anti-inflammatory activation, and antigen presentation is greatly diminished (Zrzavy et al., 2017).
Secondary neurodegeneration is a chronic neuroinflammatory response to a traumatic brain injury event that occurs in areas distant from, but synaptically connected to, the primary site of damage (Stuckey et al., 2021). Current findings suggest that the inflammatory population at the site of secondary neurodegeneration is a mixture of cells, consisting of resident immune cells (microglia) and cells recruited from the circulation (Jones et al., 2018). Microglia play a key role in the stimulation of the leukocyte adhesion cascade response, which triggers the migration of T cells to the infarct zone (Jones et al., 2018). The secondary neurodegeneration site, like the primary infarcted area, is characterized by pronounced microglia activation. Activated microglia assist in the extravasation of immune cells to thalamic sites in secondary neurodegeneration at a later stage. However, unlike the primary infarct site, the secondary neurodegeneration site is not characterized by extensive and rapid BBB catabolism during stroke (Kluge et al., 2017). Therefore, infiltrating immune cells are not present at the secondary neurodegeneration site in the early stages after stroke. Fourteen days after ischemic stroke, there is a period of intense neuroinflammation at the site of secondary neurodegeneration (Jones et al., 2015). At this point, T cells infiltrate the secondary neurodegeneration site and become the main resident immune cells (Jones et al., 2018).
T cells interact with microglia to promote inflammation
The role of microglia as APCs is to promote the infiltration of T cells into the CNS and hence trigger inflammation. In addition to presenting antigens to T cells, microglia become activated after brain tissue injury and worsen neurological inflammation by secreting various cytokines that interact with subsets of T cells that release pro-inflammatory factors. As the main intrinsic immune cells in the brain, microglia are swiftly activated to take part in the immune response after ischemic stroke, when neurovascular units are damaged. Microglia in the ischemic brain can differentiate into two subpopulations according to the microenvironment: the classically activated microglia (M1 phenotype) and the alternatively activated microglia (M2 phenotype) (Zeng et al., 2022). Within 24 hours of cerebral ischemia, the activated M1 microglia develop in the ischemic brain tissue and commence secreting chemokines and inflammatory cytokines (Schilling et al., 2009; Gombault et al., 2012). These cytokines interact with subsets of Th1 and Th17 cells that infiltrate the CNS and, together, they produce high levels of pro-inflammatory cytokines, exacerbating the inflammatory response. Following ischemic injury, neurons in the hippocampus release zinc ions, which promote the M1-type polarization of microglia and the release of pro-inflammatory factors through the activation of P2X7 receptors (Higashi et al., 2017).
The activation and functional expression of microglia is associated with the involvement of their surface receptors, particularly TLRs, which play pivotal roles in microglia-mediated inflammation. During focal cerebral ischemia, TLR2 and TLR4 engagement triggers microglia activation, leading to the production of pro-inflammatory cytokines and subsequent brain damage (Yenari et al., 2010). Numerous studies have consistently demonstrated an association between TLR4 levels and the severity of ischemia-induced neuronal injury (Yang et al., 2008; Hamanaka and Hara, 2011; Ye et al., 2019). Furthermore, TLR4 serves as a regulator of the interactions between M1 and Th1 cells and influences the immune response following ischemic stroke (Wang et al., 2011). TLR2 is also expressed in T cells, where it promotes Th1 cell function and participates in Th1-mediated responses (Imanishi et al., 2007). M1 microglia themselves produce a variety of pro-inflammatory cytokines and chemokines, including IL-1β, IL-6, TNF-α, CCL2, and C–X–C motif chemokine ligand 10. These pro-inflammatory cytokines can, in turn, further promote the activation of M1 microglia (Yenari et al., 2010). Thus, Th1 cells can also facilitate the activation of M1 microglia by secreting pro-inflammatory factors. Notably, NF-κB is an essential pathway for microglia polarization (Kou et al., 2022).
Pyroptosis, a form of neuroinflammation, is a programmed necrotic cell death process driven by inflammatory vesicles. This process is characterized by the disruption of cell membrane integrity, cell swelling, and the release of intracellular inflammatory contents, leading to an inflammatory response. The signaling pathway of cellular pyroptosis can be divided into the caspase-1-dependent classical pyroptosis pathway and the caspase-4/5/11-dependent nonclassical pyroptosis pathway. In the former, the high expression of marker molecules, such as Nod-like receptor protein 3 (NLRP3) and caspase-1 and its downstream response factor gasdermin D, is a key feature (Kovacs and Miao, 2017). Once activated, caspase-1 exhibits a protein-shearing function that processes inactive IL-1β and IL-18 precursors into their mature forms. Additionally, activated caspase-1 cleaves the gasdermin D protein, leading to cell membrane perforation and the release of cellular contents, ultimately contributing to cellular pyroptosis (Shi et al., 2015). The NLRP3 inflammasome is activated by various factors in response to pathogen invasion, including ROS, K+ efflux, Ca2+ signaling, and mitochondrial malfunction. Additionally, the NF-κB pathway, which is mediated by Toll-like receptors, can initiate the activation process (Wang et al., 2019). In a mouse model of cerebral ischemia, immunofluorescence co-localization experiments revealed that the NLRP3 inflammasome is predominantly expressed in microglia (Yang et al., 2014). Silencing of the NLRP3 gene reduced the release of pro-inflammatory cytokines, attenuating the inflammatory response and tissue damage. Furthermore, NLRP3 modulates the microglia’s polarization state, promoting M1-type microglia activation and enhancing their pro-inflammatory capacity (Yan et al., 2023). An additional subset of human TH17 cells expresses gasdermin E, a membrane-pore-forming molecule. With the aid of the NLRP3 inflammasome–caspase-8–caspase-3 axis, IL-1α is released through the cell membrane pore, triggering a series of pro-inflammatory responses (Chao et al., 2023).
The JAK/STAT signaling pathway is a crucial signal-transduction mechanism that can be influenced by various cytokines and plays a pivotal role in various biological processes, including cell proliferation, differentiation, apoptosis, immunity, and metabolic regulation (Dodington et al., 2018). It has been established that the JAK2/STAT3 signaling pathway is activated during cerebral ischemia, leading to microglia activation and neuronal apoptosis. Cellular experiments have confirmed that the secretion of TNF-α and IL-1β by lipopolysaccharide-induced BV2 microglia can be reduced by downregulating the phosphorylation of the JAK2/STAT3 pathway (Shrivastava et al., 2013). Additionally, downregulating the phosphorylation of the JAK2/STAT3 pathway promotes the polarization of microglia to the anti-inflammatory M2 phenotype, mitigates neuronal apoptosis and degeneration, and alleviates inflammatory responses and brain damage (Hristova et al., 2016). Previous studies have shown that ischemic stroke patients exhibit significantly increased Th17 cells and significantly decreased peripheral regulatory T (Treg) cells (Dolati et al., 2018; Shan et al., 2023), suggesting that an imbalance between peripheral Th17 and Treg cells may underlie the pathogenesis of ischemic stroke (Dolati et al., 2018). IL-17, secreted by Th17 cells, is the primary mediator of the delayed inflammatory response in ischemic stroke (Roy et al., 2005). Upon cerebral ischemia, microglia activate the TLR17/IL-2/IL-23 signaling pathway and secrete IL-17, leading to neuronal injury (Lv et al., 2011). Once Th17 cells enter the CNS, the local APCs, i.e., microglia, stimulate them again, and this causes microglia activation and IL-1, TNF-α, and IL-6 release (Murphy et al., 2010). IL-1β, TNF-α, and IL-17 all activate the JAK2/STAT3 signaling pathway (Boscia et al., 2013; Liddelow et al., 2017; Li et al., 2018). More interestingly, the JAK/STAT pathway not only promotes the transcription and translation of inflammatory cytokines but also regulates the differentiation of T cells into Th17 cells in the spleen, thereby influencing the Th17/Treg balance in the peripheral blood and further inducing inflammatory injury (Chang et al., 2018).
The primary effector cytokine produced by Th1 cells with a pro-inflammatory phenotype is IFN-γ (Dardalhon et al., 2008), which is a precise promoter of M1 microglia activation. These two types of cells and the cytokines they secrete interact to form a closed loop that together promote the progressive development of the inflammatory response.
In a clinical trial, Fu et al. (2014) treated 11 patients withacute ischemic stroke using standard management in combination with fingolimod. The results of contrast-enhanced T1-weighted imaging (CET1-WI) showed a significant reduction in infarct zones and a significant decrease in microvascular permeability in those who received fingolimod compared with the 11 patients who only received standard management. This clinical study demonstrated that fingolimod, acting as an immunosuppressant, can prevent the infiltration of T cells into the brain parenchyma, thereby mitigating the inflammatory response of the nervous system and slowing down the progression of secondary injury after ischemic stroke (Fu et al., 2014). Therefore, fingolimod may be a promising alternative drug for the acute phase of ischemic stroke.
T cells interact with microglia to suppress inflammation
Early research on antigen presentation by microglia revealed that these cells are inefficient T cell activators, thus leading to tolerance or anergy (Ford et al., 1996). Secondary signals that effectively suppress T cell responses, often referred to as T cell “exhaustion,” are elicited by PD-1:PD-L1 interactions. The characteristics of these exhausted phenotypes include decreased proliferative capability, cytokine production, and cytotoxic effector functions. In uninfected mice, PD-L1 was expressed at basal levels on approximately 20% of microglia; however, a week following viral brain infection, increased expression was seen on over 90% of cells (Schachtele et al., 2014). During neuroinflammation, microglia upregulate PD-L1, which binds to PD-1 molecules on the surface of T cells, thereby inhibiting the production of T cell pro-inflammatory factors. This suggests resident neuroglia have a role in limiting CNS pathology (Lokensgard et al., 2015).
Evidence has shown that microglia expressing PD-L1 after middle cerebral artery occlusion can inhibit the pro-inflammatory capacity of T cells and limit the extent of infarction, peripheral inflammatory cell migration, and experimental neurologic injury (Ren et al., 2011). This contrasts with the fundamental role of antigen presentation, which is to inform immune cells of the presence of a pathogen, thereby triggering an immune response. It would be inconsistent for microglia to present antigens to T cells but cause immune response suppression. If this were true, microglia would not be accurately described as APCs. However, subsequent research has revealed that microglia do not suppress the immune effects of all T cells; rather, they induce T cells to differentiate into two distinct subpopulations: pro-inflammatory and anti-inflammatory (Wang et al., 2016).
In post-stroke neuroinflammation, T cell subsets can have beneficial or harmful effects. In particular, it has been demonstrated that the pro-inflammatory Th1 and Th17 subsets of Th cells, as well as IL-17–producing T cells, cause secondary neurotoxicity, leading to infarct expansion and functional decline (Gelderblom et al., 2012). Conversely, Treg cells limit excessive inflammatory responses to the brain infarct by acting as anti-inflammatory and neuroprotective cells (Benakis et al., 2022). MHC class II (+) CD40 (dim) CD86 (dim) IL-10 (+) microglia have also been shown to be powerful inducers of Ag-specific CD4 (+) forkhead box protein P3 (+) in vitro. Microglia with low MHC II expression, co-stimulatory molecules, and high IL-10 expression promote Treg induction in a manner depending on the amount of IFN-γ challenge and the dose of Ag (Ebner et al., 2013). In contrast, Treg cells are key immunomodulators that protect the brain during acute ischemic stroke. They inhibit the overproduction of pro-inflammatory cytokines, regulate the invasion and/or activation of lymphocytes and microglia, and prevent the spread and growth of secondary infarct areas in the ischemic brain (Liesz et al., 2009). In the pathological process of ischemic stroke, the effect of microglia on the induction of T cells is not unidirectional. Single-cell sequencing has shown that, after an ischemic stroke, microglia may express their chemotactic properties in a T cell-dependent manner.
Distinct T cell subsets differently regulate the early activation of microglia after stroke. Th1 cells increase type I interferon signaling in microglia, driving them toward an antigen-immune functional phenotype, while regulatory T cells boost genes related to chemotaxis in microglia (Benakis et al., 2022). Tregs have been demonstrated to mediate the inhibition of M1-like microglial function by suppressing NF-κB activation (Reynolds et al., 2009), which reduces migration, phagocytosis, and the generation of neurotoxic factors. For M2 microglia, the osteopontin derived from Treg cells acts through integrin β1 (ITGβ1) receptors to enhance the repair activity of microglia (Shi et al., 2021). This promotes oligodendrocyte production and white matter regeneration, ultimately rescuing the neurological impairment caused by stroke. Gene set enrichment analysis showed that, after coculture with activated Treg cells, microglia had activated pathways associated with the type II immune response and tissue remodeling. Under Treg cell stimulation, microglia enhance the expression of genes and proteins that are key drivers of oligodendrogenesis, including vascular endothelial growth factor, transglutaminase 2, osteopontin, and arginase-1, proteins that boost the repair of ischemic brain tissue (Zera and Buckwalter, 2021).
Th1 cells release IFN-γ, IL-2, and TNF-β and express the transcription factor T-bet, and thereby promote cell-mediated immune responses and phagocyte-dependent inflammation. In contrast, Th2 cells secrete IL-4, IL-10, and IL-13 and express the transcription factor GATA3. According to experimental results, the adoptive transfer of M2 cells reduced the number of CD4 T-bet Th1 cells and increased the number of CD4 GATA3 Th2 cells. These findings suggest that M2 microglia can shift the local microenvironment from a Th1-dominated state to a Th2-dominated state, thereby facilitating the inhibition of neuroinflammation.
Increasing evidence suggests that modulating microglia/macrophages through the CD47–SIRPα pathway alters phagocytosis. Signal regulatory protein α (SIRPα) is a typical inhibitory immunological receptor that is expressed on myeloid cells such as macrophages, granulocytes, and microglia (Kharitonenkov et al., 1997; van den Berg et al., 2005; Matozaki et al., 2009). At the same time, CD47 is expressed on Treg cells (Zhang et al., 2021a). Binding of CD47 to SIRPα immunoreceptor tyrosine-based inhibitory motifs within the cytoplasmic region of SIRPα, leading to the recruitment of Src homologous phosphatases, e.g., SH2-containing protein tyrosine phosphatase-1 (SHP-1), and SHP-2, in macrophages. In turn, these phosphatases inhibit the accumulation of myosin II at phagocytic synapses and inhibit phagocytosis by microglia as a means of attenuating the inflammatory response (Russ et al., 2018). An experimental study demonstrated that the Treg cell-derived exosome miR-709 can be delivered to microglia and plays a key role in inhibiting microglia focal death, the mechanism of which might be related to the negative regulation of NF-κB–activating protein by miR-709 (Xiong et al., 2022).
In summary, M2 microglia and Treg cells demonstrate positive anti-inflammatory effects during the acute phase of ischemic stroke. Therefore, future scientific research and clinical trials should prioritize investigations into pro-polarization and the agonistic action of interactions between these two cell types. The mechanism of interaction between T cells and microglia is shown in Figure 3. The interactions between microglia and T cells are summarized in Table 2.
Figure 3.
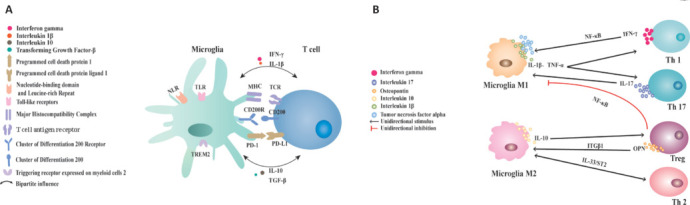
Mechanisms of interaction between T cells and microglia.
(A) The MHC molecules expressed by microglia can be specifically recognized by T cell antigen receptors (TCRs) present on the surface of T lymphocytes. When PD1 binds to its receptor PD-L1, it initiates a suppressive signaling cascade in T cells, ultimately leading to lymphocyte apoptosis. Microglia maintain a quiescent state through local interactions with the CD200 receptor and its corresponding ligand, thus mitigating inflammation. (B) Microglia interact with T cells to regulate the homeostasis of inflammation by secreting various pro- and anti-inflammatory factors. Th1 cells promote the activation of M1 microglia by secreting pro-inflammatory factors, and NF-κB is an essential pathway for microglia polarization. IL-17 can be secreted by Th17 cells and activates microglia, while microglia also secrete IL-17 to re-stimulate Th17 cells. Upon activation, microglia release IL-1, TNF-α, and IL-6, which have the potential to promote the differentiation of Th1 and Th17 cells. Through the suppression of NF-κB activation, Tregs have been demonstrated to mediate the inhibition of M1-like microglia function. Treg cell-derived OPN acts through ITGβ1 receptors to enhance the repair activity of microglia. T cells and microglia can achieve a suppressive effect on neuroinflammation after ischemic stroke through the IL-33/ST2 axis. Microglia overexpress IL-10, which promotes the activation of Treg. CD: Cluster of differentiation; IL: interleukin; INF-γ: interferon gamma; ITGβ1: integrin β1; MHC: major histocompatibility complex; NF-κB: nuclear factor kappa-B; NLR: nucleotide-binding domain and leucine-rich repeat; OPN: osteopontin; PD1: programmed cell death protein 1; PDL1: programmed cell death protein ligand 1; ST2: growth stimulation expressed gene 2; TGF-β: transforming growth factor-β; Th: helper T cell; TLR: Toll-like receptors; TNF-α: tumor necrosis factor alpha; Treg: regulatory T cells; TREM2: triggering receptor expressed on myeloid cells 2.
Table 2.
Interactions between microglia and T cells
| T cells | Interactions | References |
|---|---|---|
| Th1–M1 | Th1-derived factors trigger pro-inflammatory (M1) gene expression in microglia, causing microglia to differentiate toward the M1 phenotype and inducing a pro-inflammatory response. | Prajeeth et al., 2014 |
| The Th1-derived effector molecule IFN-γ upregulates the expression of the gene encoding MerTK, a functional regulator of myelin phagocytosis, in microglia. Thus Th1 may be able to regulate microglia phagocytosis. | Prajeeth et al., 2018 | |
| Th1 cell-derived IFNγ activates microglia to release IL-1β. | Watanabe et al., 2016 | |
| M1 microglia secrete TNF-α, IL-6, IL-12, and IL23 to recruit and induce the differentiation of Th1 cells. | Murray and Wynn, 2011 | |
| Th17–M1 | IL-17A secreted by Th17 cells can bind to IL-17AR on the surface of microglia, thereby promoting microglia activation. | Huo et al., 2019 |
| Th2–M2 | Excessive transfer of M2 microglia can change the local microenvironment from being dominated primarily by Th1 cells to being dominated by Th2 cells. | Ma et al., 2015 |
| IL-33 receptors are expressed on microglia, and IL-33 induces protection by promoting IL-4 secretion from T cells, driving Th2-type metastasis in the periphery. | Korhonen et al., 2015 | |
| Treg–M2 | Treg cell-derived osteopontin acts through integrin receptors on microglia to enhance microglia repair activity, promoting oligodendrocyte production and white matter repair. | Shi et al., 2021 |
| Microglia with high expression of IL-10 can promote the induction of Treg differentiation. | Ebner et al., 2013 | |
| The Treg cell-derived exosome miR-709 inhibits microglia death. | Xiong et al., 2022 |
IFN-γ: Interferon gamma; IL: interleukin; IL-17AR: interleukin 17A receptor; MerTK: tyrosine-protein kinase Mer; miR-709: mmu-miR-709; Th: helper T cell; TNF-α: tumor necrosis factor-α; Treg: regulatory T cells.
Conclusion
The mechanisms of ischemic stroke have been the subject of intense investigations in recent years. However, the complex pathogenesis of ischemic stroke makes this task particularly challenging. In this review, we have delved into the intricate role of the immune system in the pathophysiological changes that occur following the onset of ischemic stroke. The CNS and the immune system are intimately linked through a complex network of communication, with the immune system monitoring brain function and responding when homeostasis is disrupted by injury or disease. During the acute phase of ischemic stroke, there are interactions among the various immune cell types involving intricate mechanisms regulated by numerous factors. The triggering of inflammatory processes leads to the rupture of the BBB, allowing peripheral immune cells, particularly T cells, to enter the brain parenchyma and accumulate in the affected ischemic tissue. As these peripheral immune cells accumulate, microglia, pivotal immune cells of the brain, begin to activate in response to the ischemic event.
Upon activation, microglia release a range of pro-inflammatory cytokines that promote the expression of adhesion molecules on endothelial cells. This triggers the recruitment of additional leukocytes from the peripheral blood. T cells that infiltrate the brain release both pro- and anti-inflammatory factors, influencing microglia to differentiate into either the pro-inflammatory phenotype M1 or the anti-inflammatory phenotype M2. M1 microglia interact with Th1 and Th17 cells, secreting pro-inflammatory factors that amplify inflammatory responses. Conversely, M2 microglia interact with Th2 and Treg cells, secreting anti-inflammatory factors that mitigate the inflammatory response. A recent study has highlighted the crucial role of neuroinflammation in determining the prognosis of stroke (Tao et al., 2020). The research showed that suppressing the inflammatory response after acute stroke can prevent brain damage and improve long-term neurological outcomes. The interaction between microglia and T cells affects the direction of neuroinflammation progression, which in turn determines the prognosis of ischemic stroke. This suggests that exploring the mechanisms of microglia-T cell interactions may offer novel therapeutic targets for the treatment of ischemic stroke in the future.
The mechanisms of crosstalk between T cells and microglia are complex, and we have consulted both experimental and clinically validated articles to gain a deeper understanding. Currently, the most well-defined mechanism of interaction between T cells and microglia is the release of inflammatory cytokines. To some extent, there is a synergistic effect between T cells and microglia. Th1 cells secrete IFN-γ, which promotes the polarization of M1 microglia, while the TNF-α secreted by M1 microglia can induce the differentiation of Th1 cells. Together, Th1 and M1 microglia produce high levels of pro-inflammatory cytokines, iNOS, and neurotoxic substances that promote inflammation and brain injury following ischemic stroke. During cerebral ischemia, Th2 cells secrete IL-4 and IL-10, while Tregs secrete IL-10 and TGF-β. These cytokines promote the activation of M2 microglia and protect the brain from damage. M2 microglia can also secrete IL-10 and TGF-β, which induce the differentiation of Th2 and Treg cells. Th2, Treg, and M2 microglia work together to produce high levels of anti-inflammatory cytokines and growth factors, limiting secondary inflammation-mediated damage and promoting CNS repair. Therefore, in the treatment of ischemic stroke, the targeted regulation of inflammatory factors may play a moderating role in the interaction between the central and peripheral immune systems. This modulation can regulate the developmental direction of neuroinflammation and promote the repair of brain injury. In addition, T cells and microglia can also interact through certain signaling molecules. The surface receptor TLR4 on T cells triggers the downstream NF-κB signaling pathway, which is essential for the activation of M1 microglia. After cerebral ischemia, microglia activate the TLR17/IL-2/IL-23 signaling pathway, leading to the secretion of IL-17 and subsequent neuronal damage. IL-1β and TNF-α secreted by microglia activate the JAK2/STAT3 pathway, promoting Th17 cell differentiation and intensifying brain injury. Th2-derived IL-4 triggers the polarization of M2 microglia through the IL-4 Ra/JAK/STAT signaling pathway. In the future, exploring targeted drugs that specifically block these key signaling pathways could offer novel therapeutic approaches. In both clinical and experimental settings, various scholars have investigated drugs that can influence the immune roles played by microglia and T cells in ischemic stroke.
Fu et al. (2014) treated 11 patients with acute ischemic stroke using standard therapy plus fingolimod. By employing CET1-WI, they observed a significant reduction in infarct zones and microvascular permeability in patients who received fingolimod compared to those who received standard therapy alone. This clinical finding suggests that fingolimod, an immunosuppressant, effectively blocks T cell infiltration into the brain parenchyma. This attenuation of the inflammatory response in the nervous system may slow down the progression of secondary injury related to post-ischemic stroke. Consequently, fingolimod could serve as a potential therapeutic agent in the acute phase of ischemic stroke. In an animal model of middle cerebral artery occlusion, dimethyl fumarate administration was associated with the death of fewer neurons in the brain, reduced microglia activation, and the migration of fewer immune cells to the affected region. This led to lower systemic levels of immune cytokines, indicating that dimethyl fumarate may reduce brain inflammation through both direct and indirect mechanisms (Kunze et al., 2015). These findings suggest that administering dimethyl fumarate can lead to the reduced expression of various cytokines or chemokines. For instance, IL-17A, secreted by Th17 cells, promotes the production of inflammatory cytokines and chemokines, leading to microglia recruitment and activation (Lin et al., 2016). Additionally, dimethyl fumarate mitigates plasma levels of IL-12 and brain levels of IL-12p40. IL-12 is secreted by activated microglia and promotes inflammatory activity in ischemic stroke. Li et al. (2013) infused purified Treg cells intravenously into a mouse middle cerebral artery occlusion model of cerebral ischemia, and the number of Treg cells in the peripheral blood and peripheral immune organs increased. These cells, which survived for at least 12 days, significantly reduced the levels of inflammatory cytokines IL-6 and TNF-β induced by cerebral ischemia and improved cellular immune function after ischemic stroke.
Microglia, representative cells of central immune responses, and T cells, which typify peripheral immune responses, play pivotal roles in the prognosis of ischemic stroke. From a broad perspective, interactions between the central and peripheral immune systems hold the key to the effective treatment of ischemic stroke. These interactions, however, are complex, and while we have partially unraveled the mechanisms and verified their functions, a full explanation of the interplay between these two cell types remain elusive. At the clinical trial level, there are also gaps in our knowledge, as there are a lack of targeted treatment options. It is our hope that, in the future, we can identify practical and effective drug targets that can regulate the interactions between T cells and microglia. By promoting their anti-inflammatory functions, reducing neuroinflammation, and fostering nerve regeneration and repair following ischemic stroke, we can pave the way for more effective treatment options.
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 82104560 (to CL), U21A20400 (to QW); the Natural Science Foundation of Beijing, No. 7232279 (to XW); the Project of Beijing University of Chinese Medicine, No. 2022-JYB-JBZR-004 (to XW).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editor: Song LP; T-Editor: Jia Y
Data availability statement:
Not applicable.
References
- Abellanas MA, Zamarbide M, Basurco L, Luquin E, Garcia-Granero M, Clavero P, San Martin-Uriz P, Vilas A, Mengual E, Hervas-Stubbs S, Aymerich MS. Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflammation. 2019;16:233. doi: 10.1186/s12974-019-1628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Berchie K, Obuseh FO, Mooney DJ. T cell development and function. Rejuvenation Res. 2023;26:126–138. doi: 10.1089/rej.2023.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almolda B, González B, Castellano B. Activated microglial cells acquire an immature dendritic cell phenotype and may terminate the immune response in an acute model of EAE. J Neuroimmunol. 2010;223:39–54. doi: 10.1016/j.jneuroim.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci (Landmark edition) 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- Altin JA, Goodnow CC, Cook MC. IL-10+ CTLA–4+ Th2 inhibitory cells form in a Foxp3-independent, IL–2-dependent manner from Th2 effectors during chronic inflammation. J Immunol. 2012;188:5478–5488. doi: 10.4049/jimmunol.1102994. [DOI] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–1429. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktutan U. Endothelial progenitor cells: potential novel therapeutics for ischaemic stroke. Pharmacol Res. 2019;144:181–191. doi: 10.1016/j.phrs.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Llovera G, Liesz A. The meningeal and choroidal infiltration routes for leukocytes in stroke. Ther Adv Neurol Disord. 2018;11:1756286418783708. doi: 10.1177/1756286418783708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Simats A, Tritschler S, Heindl S, Besson-Girard S, Llovera G, Pinkham K, Kolz A, Ricci A, Theis FJ, Bittner S, Gökce Ö, Peters A, Liesz A. T cells modulate the microglial response to brain ischemia. Elife. 2022;11:e82031. doi: 10.7554/eLife.82031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu LY, Mooli RGR, Qu X, Marrero GJ, Finley CA, Fooks AN, Mullen ZP, Frias AB, Jr, Sipula I, Xie B, Helfrich KE, Watkins SC, Poholek AC, Ramakrishnan SK, Jurczak MJ, D’Cruz LM. Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight. 2021;6:e140644. doi: 10.1172/jci.insight.140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014. [DOI] [PubMed] [Google Scholar]
- Boscia F, Esposito CL, Casamassa A, de Franciscis V, Annunziato L, Cerchia L. The isolectin IB4 binds RET receptor tyrosine kinase in microglia. J Neurochem. 2013;126:428–436. doi: 10.1111/jnc.12209. [DOI] [PubMed] [Google Scholar]
- Butler CA, Popescu AS, Kitchener EJA, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158:621–639. doi: 10.1111/jnc.15327. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53:1473–1486. doi: 10.1161/STROKEAHA.122.036946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandina A, Favero C, Sacco RM, Hoxha M, Torgano G, Montano N, Bollati V, Tobaldini E. The role of extracellular vesicles in ischemic stroke severity. Biology (Basel) 2022;11:1489. doi: 10.3390/biology11101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M, Serpe C, Limatola C. Microglial extracellular vesicles as modulators of brain microenvironment in glioma. Int J Mol Sci. 2022;23:13165. doi: 10.3390/ijms232113165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Zhao Y, Song G, She K. Resveratrol protects hippocampal neurons against cerebral ischemia-reperfusion injury via modulating JAK/ERK/STAT signaling pathway in rats. J Neuroimmunol. 2018;315:9–14. doi: 10.1016/j.jneuroim.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Chao YY, Puhach A, Frieser D, Arunkumar M, Lehner L, Seeholzer T, Garcia-Lopez A, van der Wal M, Fibi-Smetana S, Dietschmann A, Sommermann T, Ćiković T, Taher L, Gresnigt MS, Vastert SJ, van Wijk F, Panagiotou G, Krappmann D, Groß O, Zielinski CE. Human T (H)17 cells engage gasdermin E pores to release IL-1α on NLRP3 inflammasome activation. Nat Immunol. 2023;24:295–308. doi: 10.1038/s41590-022-01386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatbar C, Prinz M. The roles of microglia in viral encephalitis: from sensome to therapeutic targeting. Cell Mol Immunol. 2021;18:250–258. doi: 10.1038/s41423-020-00620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Kim JY, Kim JY, Park J, Lee WT, Lee JE. M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol. 2017;26:33–41. doi: 10.5607/en.2017.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Ann Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa RAL. Reactive gliosis in Alzheimer’s disease: a crucial role for cognitive impairment and memory loss. Metab Brain Dis. 2022;37:851–857. doi: 10.1007/s11011-022-00953-2. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dodington DW, Desai HR, Woo M. JAK/STAT - emerging players in metabolism. Trends Endocrinol Metab. 2018;29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Dolati S, Ahmadi M, Khalili M, Taheraghdam AA, Siahmansouri H, Babaloo Z, Aghebati-Maleki L, Jadidi-Niaragh F, Younesi V, Yousefi M. Peripheral Th17/Treg imbalance in elderly patients with ischemic stroke. Neurol Sci. 2018;39:647–654. doi: 10.1007/s10072-018-3250-4. [DOI] [PubMed] [Google Scholar]
- Dong X, Zhang X, Li C, Chen J, Xia S, Bao X, Ge J, Cao X, Xu Y. γδ T cells aggravate blood-brain-barrier injury via IL-17A in experimental ischemic stroke. Neurosci Lett. 2022;776:136563. doi: 10.1016/j.neulet.2022.136563. [DOI] [PubMed] [Google Scholar]
- Ebner F, Brandt C, Thiele P, Richter D, Schliesser U, Siffrin V, Schueler J, Stubbe T, Ellinghaus A, Meisel C, Sawitzki B, Nitsch R. Microglial activation milieu controls regulatory T cell responses. J Immunol. 2013;191:5594–5602. doi: 10.4049/jimmunol.1203331. [DOI] [PubMed] [Google Scholar]
- Elmer BM, McAllister AK. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012;35:660–670. doi: 10.1016/j.tins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete R, et al. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. 2018;136:461–482. doi: 10.1007/s00401-018-1885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, He Y, Zhang H, Tan X, An T, Zheng J. Effect of fatigue loading on impact damage and buckling/post-buckling behaviors of stiffened composite panels under axial compression. Compos Struct. 2017;164:248–262. [Google Scholar]
- Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996;184:1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, Han W, Xue R, Liu Q, Hao J, Yu C, Shi FD. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire BP. Microglia as the critical regulators of neuroprotection and functional recovery in cerebral ischemia. Cell Mol Neurobiol. 2022;42:2505–2525. doi: 10.1007/s10571-021-01145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZS, Zhang CJ, Xia N, Tian H, Li DY, Lin JQ, Mei XF, Wu C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–223. doi: 10.1016/j.actbio.2021.03.018. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Hölscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Mosley RL. A perspective on roles played by innate and adaptive immunity in the pathobiology of neurodegenerative disorders. J Neuroimmune Pharmacol. 2015;10:645–650. doi: 10.1007/s11481-015-9639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. 2016;26:26–32. doi: 10.1016/j.coph.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Gocher AM, Workman CJ, Vignali DAA. Interferon-γ: teammate or opponent in the tumour microenvironment? Nat Rev Immunol. 2022;22:158–172. doi: 10.1038/s41577-021-00566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddery EN, Fain CE, Lipovsky CG, Ayasoufi K, Yokanovich LT, Malo CS, Khadka RH, Tritz ZP, Jin F, Hansen MJ, Johnson AJ. Microglia and perivascular macrophages act as antigen presenting cells to promote CD8 T cell infiltration of the brain. Front Immunol. 2021;12:726421. doi: 10.3389/fimmu.2021.726421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Prinz M. Role of microglia in CNS autoimmunity. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 2012;3:414. doi: 10.3389/fimmu.2012.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–127. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- Gu L, Jian Z, Stary C, Xiong X. T cells and cerebral ischemic stroke. Neurochem Res. 2015;40:1786–1791. doi: 10.1007/s11064-015-1676-0. [DOI] [PubMed] [Google Scholar]
- Gulyás B, Tóth M, Schain M, Airaksinen A, Vas A, Kostulas K, Lindström P, Hillert J, Halldin C. Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: a PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J Neurol Sci. 2012;320:110–117. doi: 10.1016/j.jns.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Guo S, Luo Y. Brain Foxp3 (+) regulatory T cells can be expanded by Interleukin-33 in mouse ischemic stroke. Int Immunopharmacol. 2020;81:106027. doi: 10.1016/j.intimp.2019.106027. [DOI] [PubMed] [Google Scholar]
- Halder SK, Sapkota A, Milner R. The importance of laminin at the blood-brain barrier. Neural Regen Res. 2023;18:2557–2563. doi: 10.4103/1673-5374.373677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka J, Hara H. Involvement of Toll-like receptors in ischemia-induced neuronal damage. Cent Nerv Syst Agents Med Chem. 2011;11:107–113. doi: 10.2174/187152411796011312. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harris NM, Roy-O’Reilly M, Ritzel RM, Holmes A, Sansing LH, O’Keefe LM, McCullough LD, Chauhan A. Depletion of CD4 T cells provides therapeutic benefits in aged mice after ischemic stroke. Exp Neurol. 2020;326:113202. doi: 10.1016/j.expneurol.2020.113202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM. T cell development: bottoms-up. Immunity. 2002;16:619–622. doi: 10.1016/s1074-7613(02)00316-3. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Aratake T, Shimizu S, Shimizu T, Nakamura K, Tsuda M, Yawata T, Ueba T, Saito M. Influence of extracellular zinc on M1 microglial activation. Sci Rep. 2017;7:43778. doi: 10.1038/srep43778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu T, Farzampour Z, Paz JT, Wang EH, Badgely C, Olson A, Micheva KD, Wang G, Lemmens R, Tran KV, Nishiyama Y, Liang X, Hamilton SA, O’Rourke N, Smith SJ, Huguenard JR, Bliss TM, Steinberg GK. Enhanced phasic GABA inhibition during the repair phase of stroke: a novel therapeutic target. Brain. 2016;139:468–480. doi: 10.1093/brain/awv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova M, Rocha-Ferreira E, Fontana X, Thei L, Buckle R, Christou M, Hompoonsup S, Gostelow N, Raivich G, Peebles D. Inhibition of Signal Transducer and Activator of Transcription 3 (STAT3) reduces neonatal hypoxic-ischaemic brain damage. J Neurochem. 2016;136:981–994. doi: 10.1111/jnc.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J, Shi Y, Gao Y, Zheng P, Chen J. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol. 2014;119-120:60–84. doi: 10.1016/j.pneurobio.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu Z, Cao BB, Qiu YH, Peng YP. Treg cells protect dopaminergic neurons against MPP+ neurotoxicity via CD47-SIRPA interaction. Cell Physiol Biochem. 2017;41:1240–1254. doi: 10.1159/000464388. [DOI] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T, Lu Z, Humayun MS, So KF, Pan Y, Li N, Yuan TF, Rao Y, Peng B. Author Correction: Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci. 2021;24:288. doi: 10.1038/s41593-020-00760-x. [DOI] [PubMed] [Google Scholar]
- Huang YC, Feng ZP. The good and bad of microglia/macrophages: new hope in stroke therapeutics. Acta Pharmacol Sin. 2013;34:6–7. doi: 10.1038/aps.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo W, Liu Y, Lei Y, Zhang Y, Huang Y, Mao Y, Wang C, Sun Y, Zhang W, Ma Z, Gu X. Imbalanced spinal infiltration of Th17/Treg cells contributes to bone cancer pain via promoting microglial activation. Brain Behav Immun. 2019;79:139–151. doi: 10.1016/j.bbi.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- Jiang CT, Wu WF, Deng YH, Ge JW. Modulators of microglia activation and polarization in ischemic stroke (Review) Mol Med Rep. 2020;21:2006–2018. doi: 10.3892/mmr.2020.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh cell differentiation. Front Immunol. 2016;7:520. doi: 10.3389/fimmu.2016.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Zouikr I, Patience M, Clarkson AN, Isgaard J, Johnson SJ, Spratt N, Nilsson M, Walker FR. Chronic stress exacerbates neuronal loss associated with secondary neurodegeneration and suppresses microglial-like cells following focal motor cortex ischemia in the mouse. Brain Behav Immun. 2015;48:57–67. doi: 10.1016/j.bbi.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Jones KA, Maltby S, Plank MW, Kluge M, Nilsson M, Foster PS, Walker FR. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun. 2018;67:299–307. doi: 10.1016/j.bbi.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ikegawa M, Kawai T. Antigen presentation in the lung. Front Immunol. 2022;13:860915. doi: 10.3389/fimmu.2022.860915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya T, Mattugini N, Liu L, Ji H, Cantuti-Castelvetri L, Wu J, Schifferer M, Groh J, Martini R, Besson-Girard S, Kaji S, Liesz A, Gokce O, Simons M. CD8 (+) T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat Neurosci. 2022;25:1446–1457. doi: 10.1038/s41593-022-01183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Khokhar M, Purohit P. The emerging role of T helper 9 (Th9) cells in immunopathophysiology: a comprehensive review of their effects and responsiveness in various disease states. Int Rev Immunol. 2024 doi: 10.1080/08830185.2024.2364586. doi: 10.1080/08830185.2024.2364586. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee W, Jo H, Sonn SK, Jeong SJ, Seo S, Suh J, Jin J, Kweon HY, Kim TK, Moon SH, Jeon S, Kim JW, Kim YR, Lee EW, Shin HK, Park SH, Oh GT. The antioxidant enzyme Peroxiredoxin-1 controls stroke-associated microglia against acute ischemic stroke. Redox Biol. 2022;54:102347. doi: 10.1016/j.redox.2022.102347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Kluge MG, Kracht L, Abdolhoseini M, Ong LK, Johnson SJ, Nilsson M, Walker FR. Impaired microglia process dynamics post-stroke are specific to sites of secondary neurodegeneration. Glia. 2017;65:1885–1899. doi: 10.1002/glia.23201. [DOI] [PubMed] [Google Scholar]
- Korhonen P, Kanninen KM, Lehtonen Š, Lemarchant S, Puttonen KA, Oksanen M, Dhungana H, Loppi S, Pollari E, Wojciechowski S, Kidin I, García-Berrocoso T, Giralt D, Montaner J, Koistinaho J, Malm T. Immunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav Immun. 2015;49:322–336. doi: 10.1016/j.bbi.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Kou L, Chi X, Sun Y, Han C, Wan F, Hu J, Yin S, Wu J, Li Y, Zhou Q, Zou W, Xiong N, Huang J, Xia Y, Wang T. The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson’s disease via the microglial NLRP3 inflammasome. J Neuroinflammation. 2022;19:133. doi: 10.1186/s12974-022-02494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M, Reischl S, Korff T, Marti HH. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Casili G, Campolo M, Paterniti I, Colarossi C, Mare M, Giuffrida R, Caffo M, Esposito E, Cuzzocrea S. Immunomodulatory effect of microglia-released cytokines in gliomas. Brain Sci. 2021;11:466. doi: 10.3390/brainsci11040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Understanding the mechanisms of sustained signaling and T cell activation. J Exp Med. 1997;185:1717–1719. doi: 10.1084/jem.185.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Keum S, Sheng H, Warner DS, Lo DC, Marchuk DA. Natural allelic variation of the IL-21 receptor modulates ischemic stroke infarct volume. J Clin Invest. 2016;126:2827–2838. doi: 10.1172/JCI84491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei TY, Ye YZ, Zhu XQ, Smerin D, Gu LJ, Xiong XX, Zhang HF, Jian ZH. The immune response of T cells and therapeutic targets related to regulating the levels of T helper cells after ischaemic stroke. J Neuroinflammation. 2021;18:25. doi: 10.1186/s12974-020-02057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang X, Gao L, Min J, Zhang Y, Zhang R, Yang Y. TNF-alpha is upregulated in subacute thyroiditis and stimulates expression of miR-155-5p in thyroid follicle cells. Discov Med. 2018;26:67–77. [PubMed] [Google Scholar]
- Li P, Mao L, Zhou G, Leak RK, Sun BL, Chen J, Hu X. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44:3509–3515. doi: 10.1161/STROKEAHA.113.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang YM, Han D, Hua R, Guo BN, Hu SQ, Yan XL, Xu T. Involvement of IL-17 in secondary brain injury after a traumatic brain injury in rats. Neuromolecular Med. 2017;19:541–554. doi: 10.1007/s12017-017-8468-4. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liesz A, Karcher S, Veltkamp R. Spectratype analysis of clonal T cell expansion in murine experimental stroke. J Neuroimmunol. 2013;257:46–52. doi: 10.1016/j.jneuroim.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Lin R, Cai J, Kostuk EW, Rosenwasser R, Iacovitti L. Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J Neuroinflammation. 2016;13:269. doi: 10.1186/s12974-016-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sorooshyari SK. Quantitative and correlational analysis of brain and spleen immune cellular responses following cerebral ischemia. Front Immunol. 2021;12:617032. doi: 10.3389/fimmu.2021.617032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hu R, Pei L, Si P, Wang C, Tian X, Wang X, Liu H, Wang B, Xia Z, Xu Y, Song B. Regulatory T cell is critical for interleukin-33-mediated neuroprotection against stroke. Exp Neurol. 2020;328:113233. doi: 10.1016/j.expneurol.2020.113233. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Schachtele SJ, Mutnal MB, Sheng WS, Prasad S, Hu S. Chronic reactive gliosis following regulatory T cell depletion during acute MCMV encephalitis. Glia. 2015;63:1982–1996. doi: 10.1002/glia.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Sun Y, Liu S, Yang S, Chen C, Zhang Z, Chu S, Yang Y, Pei G, Lin M, Yan Q, Yao J, Lin Y, Yi F, Meng L, Tan Y, Ai Q, Chen N. Targeting pyroptosis as a preventive and therapeutic approach for stroke. Cell Death Discov. 2023;9:155. doi: 10.1038/s41420-023-01440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY. T-cell subpopulation categorization and clinical association. Intermediate Med J. 1995;11:38–42. [Google Scholar]
- Luo Y, Zhou Y, Xiao W, Liang Z, Dai J, Weng X, Wu X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lü HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Mallat M, Marín-Teva JL, Chéret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mayassi T, Jabri B. Human intraepithelial lymphocytes. Mucosal immunol. 2018;11:1281–1289. doi: 10.1038/s41385-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró-Mur F, Urra X, Ruiz-Jaén F, Pedragosa J, Chamorro Á, Planas AM. Antigen-dependent t cell response to neural peptides after human ischemic stroke. Front Cell Neurosci. 2020;14:206. doi: 10.3389/fncel.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseman EA, Blanchard AC, Nayak D, McGavern DB. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci Immunol. 2020:5. doi: 10.1126/sciimmunol.abb1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Ferretti MT. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peine M, Marek RM, Löhning M. IL-33 in T cell differentiation, function, and immune homeostasis. Trends Immunol. 2016;37:321–333. doi: 10.1016/j.it.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Peterfalvi A, Molnar T, Banati M, Pusch G, Miko E, Bogar L, Pal J, Szereday L, Illes Z. Impaired function of innate T lymphocytes and NK cells in the acute phase of ischemic stroke. Cerebrovasc Dis. 2009;28:490–498. doi: 10.1159/000236527. [DOI] [PubMed] [Google Scholar]
- Ponsaerts L, Alders L, Schepers M, de Oliveira RMW, Prickaerts J, Vanmierlo T, Bronckaers A. Neuroinflammation in ischemic stroke: inhibition of cAMP-specific phosphodiesterases (PDEs) to the rescue. Biomedicines. 2021;9:703. doi: 10.3390/biomedicines9070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajeeth CK, Löhr K, Floess S, Zimmermann J, Ulrich R, Gudi V, Beineke A, Baumgärtner W, Müller M, Huehn J, Stangel M. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain Behav Immun. 2014;37:248–259. doi: 10.1016/j.bbi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Prajeeth CK, Dittrich-Breiholz O, Talbot SR, Robert PA, Huehn J, Stangel M. IFN-γ producing Th1 cells induce different transcriptional profiles in microglia and astrocytes. Front Cell Neurosci. 2018;12:352. doi: 10.3389/fncel.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad Singh N, Nagarkatti M, Nagarkatti P. From suppressor t cells to regulatory T cells: how the journey that began with the discovery of the toxic effects of TCDD led to better understanding of the role of AhR in immunoregulation. Int J Mol Sci. 2020;21:7849. doi: 10.3390/ijms21217849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Yuanshao L, Wensi H, Yulei Z, Xiaoli C, Brian W, Wanli Z, Zhengyi C, Jie X, Wenhui Z, Tieer Y, Hong W, Jincai H, Kunlin J, Bei S. Serum IL-33 is a novel diagnostic and prognostic biomarker in acute ischemic stroke. Aging Dis. 2016;7:614–622. doi: 10.14336/AD.2016.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Fan WH, Liu Q, Shang K, Murugan M, Wu LJ, Wang W, Tian DS. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48:3336–3346. doi: 10.1161/STROKEAHA.117.018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, Bosco DB, Wu LJ, Tian DS. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Akter F, Qin L, Cheng J, Guo M, Yao S, Jian Z, Liu R, Wu S. Adaptive immunity regulation and cerebral ischemia. Front Immunol. 2020;11:689. doi: 10.3389/fimmu.2020.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, Hu B. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol. 2021;12:678744. doi: 10.3389/fimmu.2021.678744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke. 2011;42:2578–2583. doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009;182:4137–4149. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch S, Rapp C, Dettling S, Herold-Mende C. When immune cells turn bad-tumor-associated microglia/macrophages in glioma. Int J Mol Sci. 2018;19:436. doi: 10.3390/ijms19020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Ruhnau J, Schulze J, Dressel A, Vogelgesang A. Thrombosis, neuroinflammation, and poststroke infection: the multifaceted role of neutrophils in stroke. J Immunol Res. 2017;2017:5140679. doi: 10.1155/2017/5140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, Carew JS, Nawrocki ST, Persky D, Anwer F. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32:480–489. doi: 10.1016/j.blre.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele SJ, Hu S, Sheng WS, Mutnal MB, Lokensgard JR. Glial cells suppress postencephalitic CD8+ T lymphocytes through PD-L1. Glia. 2014;62:1582–1594. doi: 10.1002/glia.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Schäbitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161:806–812. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol. 2014;9:679–689. doi: 10.1007/s11481-014-9560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj UM, Stowe AM. Long-term T cell responses in the brain after an ischemic stroke. Discov Med. 2017;24:323–333. [PMC free article] [PubMed] [Google Scholar]
- Sen MK, Mahns DA, Coorssen JR, Shortland PJ. The roles of microglia and astrocytes in phagocytosis and myelination: insights from the cuprizone model of multiple sclerosis. Glia. 2022;70:1215–1250. doi: 10.1002/glia.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Wang L, Sun J, Chang S, Di W, Lv H. Exercise preconditioning attenuates cerebral ischemia-induced neuronal apoptosis, Th17/Treg imbalance, and inflammation in rats by inhibiting the JAK2/STAT3 pathway. Brain Behav. 2023;13:e3030. doi: 10.1002/brb3.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM, Li S, Stolz DB, Chen K, Ding Y, Thomson AW, Leak RK, Chen J, Hu X. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. 2021;54:1527–1542.e1528. doi: 10.1016/j.immuni.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Shrivastava K, Llovera G, Recasens M, Chertoff M, Giménez-Llort L, Gonzalez B, Acarin L. Temporal expression of cytokines and signal transducer and activator of transcription factor 3 activation after neonatal hypoxia/ischemia in mice. Dev Neurosci. 2013;35:212–225. doi: 10.1159/000348432. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Das A, Khan MM, Pourmotabbed T. New insights into the therapeutic approaches for the treatment of tauopathies. Neural Regen Res. 2024;19:1020–1026. doi: 10.4103/1673-5374.385288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. 2017;9:139. doi: 10.3389/fnagi.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33:37–47. doi: 10.1038/jcbfm.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey SM, Ong LK, Collins-Praino LE, Turner RJ. Neuroinflammation as a key driver of secondary neurodegeneration following stroke? Int J Mol Sci. 2021;22:13101. doi: 10.3390/ijms222313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Liu M, Chen M, Luo Y, Wang C, Xu T, Jiang Y, Guo Y, Zhang JH. Natural medicine in neuroprotection for ischemic stroke: challenges and prospective. Pharmacol Ther. 2020;216:107695. doi: 10.1016/j.pharmthera.2020.107695. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, Pradervand S, Thimme R, Zehn D, Held W. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, van Beek EM, Bühring HJ, Colonna M, Hamaguchi M, Howard CJ, Kasuga M, Liu Y, Matozaki T, Neel BG, Parkos CA, Sano S, Vignery A, Vivier E, Wright M, Zawatzky R, Barclay AN. A nomenclature for signal regulatory protein family members. J Immunol. 2005;175:7788–7789. doi: 10.4049/jimmunol.175.12.7788. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Nabekura J. Functions of microglia in the central nervous system--beyond the immune response. Neuron Glia Biol. 2011;7:47–53. doi: 10.1017/S1740925X12000063. [DOI] [PubMed] [Google Scholar]
- Wang L, Yao C, Chen J, Ge Y, Wang C, Wang Y, Wang F, Sun Y, Dai M, Lin Y, Yao S. γδ T cell in cerebral ischemic stroke: characteristic, immunity-inflammatory role, and therapy. Front Neurol. 2022;13:842212. doi: 10.3389/fneur.2022.842212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Qu W, Shao ZH. Advance of researches on relation of Th17 cells with immuno-associatied hematologic diseases. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:1766–1770. doi: 10.7534/j.issn.1009-2137.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Xu Y. Crosstalk between microglia and T cells contributes to brain damage and recovery after ischemic stroke. Neurol Res. 2016;38:495–503. doi: 10.1080/01616412.2016.1188473. [DOI] [PubMed] [Google Scholar]
- Wang S, Yuan YH, Chen NH, Wang HB. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol. 2019;67:458–464. doi: 10.1016/j.intimp.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Wang YC, Lin S, Yang QW. Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflammation. 2011;8:134. doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YR, Cui WQ, Wu HY, Xu XD, Xu XQ. The role of T cells in acute ischemic stroke. Brain Res bull. 2023;196:20–33. doi: 10.1016/j.brainresbull.2023.03.005. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kawabori M, Houkin K. FTY720 (Fingolimod) ameliorates brain injury through multiple mechanisms and is a strong candidate for stroke treatment. Curr Med Chem. 2020;27:2979–2993. doi: 10.2174/0929867326666190308133732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Masaki K, Yamasaki R, Kawanokuchi J, Takeuchi H, Matsushita T, Suzumura A, Kira JI. Th1 cells downregulate connexin 43 gap junctions in astrocytes via microglial activation. Sci Rep. 2016;6:38387. doi: 10.1038/srep38387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Yamada Y, Murakami H. Expression of Th1/Th2 cell-related chemokine receptors on CD4(+) lymphocytes under physiological conditions. Int J Lab Hematol. 2020;42:68–76. doi: 10.1111/ijlh.13141. [DOI] [PubMed] [Google Scholar]
- Wei SS, Chen L, Yang FY, Wang SQ, Wang P. The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier. Neural Regen Res. 2023;18:2147–2155. doi: 10.4103/1673-5374.369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GM, Ducruet A. IL-21 receptor modulates ischemic severity in stroke. Neurosurgery. 2016;79:N14–N16. doi: 10.1227/01.neu.0000508602.53941.4e. [DOI] [PubMed] [Google Scholar]
- West PK, McCorkindale AN, Guennewig B, Ashhurst TM, Viengkhou B, Hayashida E, Jung SR, Butovsky O, Campbell IL, Hofer MJ. The cytokines interleukin-6 and interferon-α induce distinct microglia phenotypes. J Neuroinflammation. 2022;19:96. doi: 10.1186/s12974-022-02441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing committee of the report on cardiovascular health and diseases in china Report on cardiovascular health and diseases in China 2021: an updated summary. Biomed Environ Sci. 2022;35:573–603. [Google Scholar]
- Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18:258. doi: 10.1186/s12974-021-02309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Li C, Kong G, Zeng Q, Wang S, Yin G, Gu J, Fan J. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J Nanobiotechnology. 2022;20:529. doi: 10.1186/s12951-022-01724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. ScientificWorldJournal. 2013;2013:174373. doi: 10.1155/2013/174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Jin H, Pan C, Han X. Chronic microcystin-LR-induced α-synuclein promotes neuroinflammation through activation of the NLRP3 inflammasome in microglia. Mol Neurobiol. 2023;60:884–900. doi: 10.1007/s12035-022-03134-5. [DOI] [PubMed] [Google Scholar]
- Yang CN, Shiao YJ, Shie FS, Guo BS, Chen PH, Cho CY, Chen YJ, Huang FL, Tsay HJ. Mechanism mediating oligomeric Aβ clearance by naïve primary microglia. Neurobiol Dis. 2011;42:221–230. doi: 10.1016/j.nbd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Li JC, Lu FL, Wen AQ, Xiang J, Zhang LL, Huang ZY, Wang JZ. Upregulated expression of toll-like receptor 4 in monocytes correlates with severity of acute cerebral infarction. J Cereb Blood Flow Metab. 2008;28:1588–1596. doi: 10.1038/jcbfm.2008.50. [DOI] [PubMed] [Google Scholar]
- Ye Y, Jin T, Zhang X, Zeng Z, Ye B, Wang J, Zhong Y, Xiong X, Gu L. Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway. Front Cell Neurosci. 2019;13:553. doi: 10.3389/fncel.2019.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida TM, Wang A, Hafler DA. Basic principles of neuroimmunology. Semin Immunopathol. 2022;44:685–695. doi: 10.1007/s00281-022-00951-7. [DOI] [PubMed] [Google Scholar]
- Yu S, Cui W, Han J, Chen J, Tao W. Longitudinal change of Th1, Th2, and Th17 cells and their relationship between cognitive impairment, stroke recurrence, and mortality among acute ischemic stroke patients. J Clin Lab Anal. 2022;36:e24542. doi: 10.1002/jcla.24542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Hoi MPM. Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen Res. 2023;18:1890–1902. doi: 10.4103/1673-5374.367832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Bao T, Yang K, Zhu X, Wang S, Xiang W, Ge A, Zeng L, Ge J. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: a review. Front Immunol. 2022;13:1047550. doi: 10.3389/fimmu.2022.1047550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera KA, Buckwalter MS. T cells direct microglial repair of white matter after stroke. Trends Neurosci. 2021;44:769–770. doi: 10.1016/j.tins.2021.07.005. [DOI] [PubMed] [Google Scholar]
- Zhang A, Ren Z, Tseng KF, Liu X, Li H, Lu C, Cai Y, Minna JD, Fu YX. Dual targeting of CTLA-4 and CD47 on T (reg) cells promotes immunity against solid tumors. Sci Transl Med. 2021;13:eabg8693. doi: 10.1126/scitranslmed.abg8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Ren J, Luo Y, He Q, Zhao R, Chang J, Yang Y, Guo ZN. T cell response in ischemic stroke: from mechanisms to translational insights. Front Immunol. 2021;12:707972. doi: 10.3389/fimmu.2021.707972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Liu G, Wei C, Gao C, Hao J. Linc-MAF-4 regulates Th1/Th2 differentiation and is associated with the pathogenesis of multiple sclerosis by targeting MAF. FASEB J. 2017;31:519–525. doi: 10.1096/fj.201600838R. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang R, Chen H, Jin C, Jin Z, Lu J, Xu L, Lu Y, Zhang J, Shi L. Aged microglia promote peripheral T cell infiltration by reprogramming the microenvironment of neurogenic niches. Immun Ageing. 2022;19:34. doi: 10.1186/s12979-022-00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Taylor SE, Cao T, Harrison JL, Lifshitz J. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation. 2012;9:247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140:1900–1913. doi: 10.1093/brain/awx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


