Glaucoma is a multifactorial eye disorder that can cause vision loss and irreversible blindness, affecting individuals aged 40 to 80 years worldwide. Due to the aging population, it is expected that the number of people affected by glaucoma will surpass 111 million by 2040 as the disease becomes more prevalent. Glaucoma primarily contributes to optic nerve axon loss and the progressive degeneration of retinal ganglion cells (RGCs), subsequently leading to vision impairment. The onset of glaucoma is affected by various factors, including genetic predispositions, environmental influences, and physiological conditions. The major risk factors include elevated intraocular pressure (IOP), aging, genetic/epigenetic factors, immune system dysregulation, and vascular dysfunction. Elevated IOP is currently the only adjustable and treatable risk factor for glaucoma through medications, laser procedures, and surgical interventions. However, reducing IOP by these treatments is insufficient to manage the progression of glaucomatous optic nerve degeneration and RGC death. There is growing interest in elucidating the pathophysiological mechanisms underlying glaucoma pathogenesis, particularly regarding the involvement of glia-mediated neuroinflammation.
Cholesterol is essential for the structural and functional integrity of RGCs, playing a pivotal role in maintaining cell membrane integrity, signal transduction, synaptic function, and neuroprotection. However, excessive accumulation of cholesterol deteriorates cellular functions by enhancing toll-like receptor (TLR) signaling, activating inflammasomes, and inducing inflammatory responses (Tall and Yvan-Charvet, 2015). Recent evidence indicates that ATP-binding cassette transporter 1 (ABCA1) deficiency triggers retinal cholesterol accumulation, resulting in alterations in mitochondrial function and autophagy flux, and RGC death (Yang et al., 2023). Additionally, glia-specific ABCA1 deficiency induces cholesterol accumulation in the astrocytes, which is linked to RGC degeneration and inflammation (Shinozaki et al., 2022).
Recent studies from our group have demonstrated that downregulation of apolipoprotein A-I binding protein (AIBP) and ABCA1, which regulate cholesterol depletion from the plasma membrane, increases the TLR4-lipid raft formation and interleukin-1β production in the retinas of patients with primary open-angle glaucoma (POAG) and experimental mouse models of glaucoma (Choi et al., 2020, 2024; Ju et al., 2023). Epidemiological evidence suggests that hyperlipidemia and increased levels of blood lipids are linked to POAG, elevated IOP, and ocular hypertension. A recent study demonstrated a correlation between glaucoma and high total cholesterol levels, along with low high-density lipoprotein levels (Wang and Bao, 2019). However, conflicting findings are present regarding the relationship between POAG and serum lipid levels, suggesting there may not be significant differences in the association between POAG and blood lipids. In a glaucomatous mouse model, disrupted cholesterol homeostasis was associated with RGC death, supporting the notion that cholesterol accumulation may be a risk factor in glaucoma pathogenesis (Ju et al., 2023). Considering that the retina regulates cholesterol balance through a complex interaction involving biosynthesis, lipoprotein uptake, transport, and efflux, understanding the pathophysiological mechanisms of increased cholesterol levels in the glaucomatous retina could provide valuable insights for the treatment of glaucoma.
AIBP is a secreted protein that interacts with apolipoprotein A-I (Fang et al., 2013). Apolipoprotein A-I, a primary component of high-density lipoprotein, plays a crucial role in facilitating cholesterol efflux to regulate cellular cholesterol levels. Cholesterol, an essential element of cellular membranes, plays a critical role in maintaining the integrity of lipid rafts, which serve as pivotal platforms for the regulation of membrane protein trafficking, facilitating receptor-mediated signal transduction. Alteration of cholesterol homeostasis and its buildup in the membrane can lead to the formation of inflammarafts, characterized by enlarged lipid rafts containing inflammatory receptors, and adaptor proteins, thereby initiating inflammation in glial cells (Miller et al., 2020). Physiologically, cholesterol efflux relies on the membrane and endosomal ABCA1 stabilized by AIBP. Genome-wide association studies revealed that individuals experiencing the onset of POAG have susceptibility loci with genes such as ABCA1, TXNRD2, FOXC1, and ATXN2, all of which play roles in lipid metabolism (Gharahkhani et al., 2014; Bailey et al., 2016).
While several investigations have highlighted the role of AIBP in cholesterol efflux in endothelial cells and macrophages/microglia, the underlying mechanisms by which AIBP mitigates glaucomatous neuroinflammation and neurodegeneration remain to be fully elucidated. Thus, we hypothesized that AIBP deficiency would result in the activation of TLR4 and increased pro-inflammatory response; conversely, AIBP gain-of-function would inhibit TLR4-mediated retinal neuroinflammation and would be neuroprotective.
Glaucomatous neuroinflammation is linked to glial activation in the retina and results in the release of proinflammatory cytokines and chemokines, leading to RGC death (Choi et al., 2020, 2024; Ju et al., 2023). A recent study has revealed that IOP elevation led to a significant reduction in AIBP expression levels in RGCs, concurrently resulting in the increase of TLR4 and interleukin-1β expression in Müller glia (Choi et al., 2020). In Apoa1bp–/– mice, the AIBP deficiency triggered impairment of visual function, heightened TLR4 activity, compromised mitochondrial structure and function, elevated oxidative stress, and increased inflammatory responses in Müller glia (Choi et al., 2020). Furthermore, AIBP deficiency disrupted mitochondrial structure and function in RGCs and intensified RGC vulnerability in response to IOP elevation (Choi et al., 2020). In glaucoma, mitochondrial dysfunction is commonly observed in glaucomatous neurodegeneration, further linking it to oxidative stress and inflammation, leading to glaucomatous neuroinflammation. A recent study from our group has shown that AIBP expression in macrophages is crucial for effectively regulating reactive oxygen species generation and preventing cell death, as well as for maintaining mitochondria quality control in the context of atherosclerosis (Choi et al., 2021). Intracellular AIBP is localized to mitochondria and interacts with E3 ubiquitin-protein ligase PARK2 (Parkin), as well as with mitofusin 1 (MFN1), and MFN2, and regulates the ubiquitination of MFN1/2, which is the key process of mitophagy (Choi et al., 2021). AIBP deficiency significantly impairs oxidative phosphorylation (OXPHOS) in the retina. Additionally, it disrupts the mitochondrial network and reduces ATP generation in Müller glia and RGCs. These disturbances lead to extensive mitochondrial fragmentation, resulting in energy depletion and the potential formation of mitophagosomes. Thus, the data showing reduced AIBP expression and activated Müller glia in glaucomatous retina suggest that AIBP deficiency may not only impact the somas and axons of RGCs but also affect the dendrites and synapses of RGCs in the retina through an autocrine/paracrine mechanism during the process of glaucomatous neuroinflammation and neurodegeneration.
AIBP has been demonstrated to protect RGCs against both glaucomatous neuroinflammation and neurodegeneration (Choi et al., 2020, 2024; Ju et al., 2023). Our findings have shown that administering recombinant AIBP enhances the survival of RGCs, prevents apoptotic cell death, and inhibits inflammatory response in Müller glia when subjected to acute IOP elevation (Choi et al., 2020). More importantly, our emerging evidence utilizing adeno-associated virus serotype 2-AIBP transduction system suggests that restoring AIBP expression in the retina has neuroprotective effects in experimental glaucoma (Ju et al., 2023; Choi et al., 2024). Notably, this restoration of AIBP expression not only enhanced RGC survival and preserved RGC axons in glaucomatous DBA/2J mice but also rescued RGCs and reduced vision impairment in optic nerve crush, an experimental model of traumatic optic neuropathy (Ju et al., 2023). Moreover, restored AIBP expression notably reduced cholesterol deposition in the inner retina and suppressed the expression of TLR4 and interleukin-1 in glaucomatous Müller glia (Ju et al., 2023). Furthermore, restoring AIBP expression prevented the activation of mitogen-activated protein kinase and 5’AMP-activated protein kinase in glaucomatous retinal cells, including RGCs and glial cells (Ju et al., 2023). Taken together, our findings indicate that restoring AIBP expression can promote RGC survival and axon protection, as well as energy preservation in RGCs by modulating inflammatory pathways in activated Müller glia during glaucomatous neuroinflammation.
We here present evidence supporting the proposed functional role of AIBP in rescuing RGCs and inhibiting glia-mediated inflammatory responses against glaucomatous neuroinflammation and neurodegeneration. We highlight AIBP as a promising therapeutic candidate pivotal in controlling TLR4-associated lipid rafts, managing cholesterol accumulation, regulating mitochondrial network and function, and modulating inflammatory pathways in the retina during glaucomatous neuroinflammation and neurodegeneration (Figure 1). Hence, it would be valuable to further investigate the protective mechanisms of AIBP on RGCs and glial cells in glaucoma.
Figure 1.
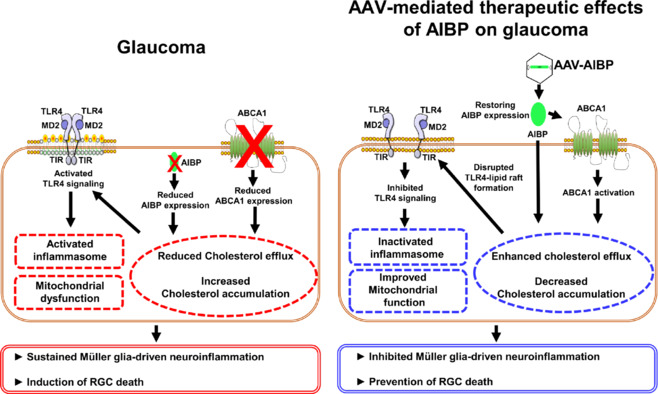
A model for the protective role of AIBP in glaucomatous neuroinflammation and neurodegeneration.
AIBP controls TLR4-associated lipid rafts, inhibits cholesterol accumulation, regulates mitochondrial network and function, and modulates inflammatory pathways in the retina during glaucomatous neuroinflammation and neurodegeneration. In glaucoma, reduced AIBP expression may deregulate retinal lipid metabolism, potentially lowering ABCA1 expression and resulting in cholesterol accumulation. Thus, cholesterol accumulation within lipid rafts activates TLR4 signaling, leading to mitochondrial dysfunction and inflammasome activation. Created with Microsoft PowerPoint. AAV: Adeno-associated virus; ABCA1: ATP-binding cassette transporter 1; AIBP: apolipoprotein A-I binding protein; MD2: an extracellular molecule; RGC: retinal ganglion cell; TIR: Toll/interleukin-1 receptor; TLR4: toll-like receptor 4.
This work was supported by the National Institutes of Health grants EY034116 (to WKJ, KYK, and SHC) and AG081037 (to YIM and WKJ).
SHC, YIM, and WKJ are co-inventors named on patents and patent applications by the University of California, San Diego, USA. YIM is a scientific co-founder of Raft Pharmaceuticals LLC. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. Other authors declare no conflict of interest exists.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Bailey JN, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet. 2016;48:189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Choi SH, Bastola T, Park Y, Oh J, Kim KY, Hwang S, Miller YI, Ju WK. AIBP: a new safeguard against Glaucomatous Neuroinflammation. Cells. 2024;13:198. doi: 10.3390/cells13020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim KY, Perkins GA, Phan S, Edwards G, Xia Y, Kim J, Skowronska-Krawczyk D, Weinreb RN, Ellisman MH, Miller YI, Ju WK. AIBP protects retinal ganglion cells against neuroinflammation and mitochondrial dysfunction in glaucomatous neurodegeneration. Redox Biol. 2020;37:101703. doi: 10.1016/j.redox.2020.101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Agatisa-Boyle C, Gonen A, Kim A, Kim J, Alekseeva E, Tsimikas S, Miller YI. Intracellular AIBP (apolipoprotein A-I binding protein) regulates oxidized LDL (low-density lipoprotein)-induced mitophagy in macrophages. Arterioscler Thromb Vasc Biol. 2021;41:e82–96. doi: 10.1161/ATVBAHA.120.315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, Wiesner P, Taleb A, Deer E, Pattison J, Torres-Vazquez J, Li AC, Miller YI. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharahkhani P, et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;46:1120–1125. doi: 10.1038/ng.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Ha Y, Choi S, Kim KY, Bastola T, Kim J, Weinreb RN, Zhang W, Miller YI, Choi SH. Restoring AIBP expression in the retina provides neuroprotection in glaucoma. bioRxiv. 2023 doi: 10.1101/2023.10.16.562633. [Google Scholar]
- Miller YI, Navia-Pelaez JM, Corr M, Yaksh TL. Lipid rafts in glial cells: role in neuroinflammation and pain processing. J Lipid Res. 2020;61:655–666. doi: 10.1194/jlr.TR119000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Leung A, Namekata K, Saitoh S, Nguyen HB, Takeda A, Danjo Y, Morizawa YM, Shigetomi E, Sano F, Yoshioka N, Takebayashi H, Ohno N, Segawa T, Miyake K, Kashiwagi K, Harada T, Ohnuma SI, Koizumi S. Astrocytic dysfunction induced by ABCA1 deficiency causes optic neuropathy. Sci Adv. 2022;8:eabq1081. doi: 10.1126/sciadv.abq1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bao X. Hyperlipidemia, blood lipid level, and the risk of glaucoma: a meta-analysis. Invest Ophthalmol Vis Sci. 2019;60:1028–1043. doi: 10.1167/iovs.18-25845. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Cholesterol homeostasis regulated by ABCA1 is critical for retinal ganglion cell survival. Sci China Life Sci. 2023;66:211–225. doi: 10.1007/s11427-021-2126-2. [DOI] [PubMed] [Google Scholar]


