Abstract
The quantitation of androgens is necessary to diagnose and monitor the development of diseases such as prostate cancer and polycystic ovary syndrome. Androgen measurements also support the laboratory-based study of androgen metabolism in cellular and animal models. The methods described in this chapter combine chemical derivatization of hydroxy- and keto-androgens with stable isotope dilution liquid chromatography mass spectrometry (SID-LC-MS). Chemical derivatization of hydroxy-androgens by picolinic acid and keto-androgens by Girard P enhances the ionization and detection sensitivity of androgens, while chromatographic separation and [13C]-labeled internal standards add specificity that allow for simultaneous quantitation of multiple androgens. This chapter describes the materials and protocols necessary for chemical derivatization, enzymatic synthesis of internal standards, and LC-MS detection of keto- and hydroxy-androgens.
1. Introduction
Androgens are essential male sex hormones required for the development and function of the male reproductive system (Rey, 2021). Androgen dysregulation drives endocrine diseases including prostate cancer (PC) (Huggins & Hodges, 1941) and polycystic ovary syndrome (PCOS) (Goodman et al., 2015). Consequently, the accurate measurement of androgens is needed to diagnose and monitor the development of endocrinopathies and to study the underlying principles of androgen metabolism in research laboratories. Traditional methodologies to measure androgens rely on immunoassays such as radioimmunoassays (RIAs) or enzyme-linked immunosorbent assays (ELISAs). However, these approaches can suffer from interference due to cross-reactivity with other androgens that share structural similarity and the existence of regio- and stereo-isomers. In addition, there can be interference from the biological matrix due to cross-reactivity of unknowns. Moreover, these immunochemical methods can only detect one analyte at a time. The methods described in this chapter combine chemical derivatization of hydroxy- and keto-androgens (Fig. 1) with stable isotope dilution liquid chromatography mass spectrometry (SID-LC-MS) to overcome these limitations of immunoassays (Frey et al., 2016; Tamae et al., 2013; Zang et al., 2017). Chemical derivatization of hydroxy-androgens by picolinic acid and keto-androgens by Girard P enhances the ionization and detection sensitivity of androgens, allowing MS detection down to 1 pg of androgen on the column (Tamae et al., 2013; Zang et al., 2017). Chromatographic separation and [13C]-labeled internal standards (ISTDs) adds specificity that allows for simultaneous identification of androgen isomers (Zang et al., 2017). This chapter describes derivatization protocols, enzymatic synthesis of internal standards, chromatographic separation, and MS operating parameters for the quantitation of keto- and hydroxy-androgens.
Fig. 1.
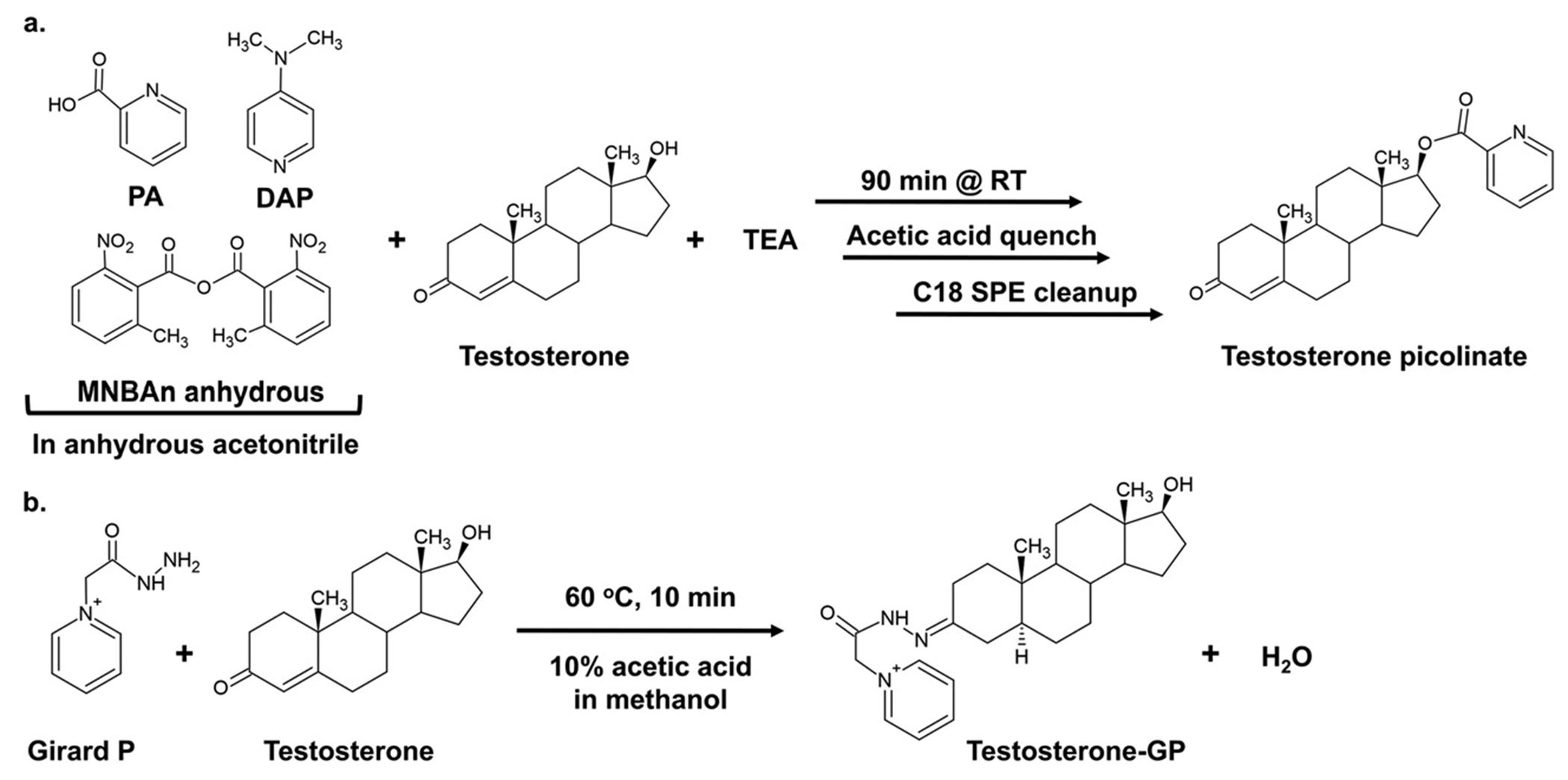
Schematics for derivatization of A) the 17β-hydroxy group on testosterone by picolinic acid and B) 3-keto group on testosterone by Girard P.
2. Instruments and equipment
Water bath at 60 °C, Isotemp 205, Fisher Scientific
VWR Shaking water bath at 37 °C
Analytical balance, Mettler Toledo AG245
IKA Vibrax VXR basic shaker
Thermo Scientific Savant SPD121P SpeedVac Concentrator
Millipore Sigma Supelco Visiprep SPE Vacuum Manifold
Nitrogen dryer, N-EVAP112 Nitrogen Evaporator, Organomation Associates, Inc
Beckman DU 640 spectrophotometer
Thermo Fisher Scientific Vantage UPLC and Thermo Fisher Scientific TSQ Atlas Plus triple quadrapole mass spectrometer (used for hydroxy-androgen detection)
Thermo Fisher Scientific Ultimate 3000 UPLC and Thermo Fisher Scientific Q Exactive-HF mass spectrometer (used for keto-androgen detection)
3. Materials and reagents
3.1. Androgen standards and internal standards
3.1.1. Androgen standards
Testosterone (T), Epitestosterone (EpiT), Dehydroepiandrosterone (DHEA), Dihydrotestosterone (DHT), Androsterone, Epiandrosterone, 5-Androstenediol (5-Adiol), 3α-Androstanediol (3α-Adiol), 3β-Androstanediol (3β-Adiol), 4-androstene-3,17-dione (4AD), 5α-Androstanedione (5AD), 11-keto-5α-androstane-3,17-dione (11K-5AD), 11-keto-5α-dihydrotestosterone (11K-DHT), and 11β-hydroxy-5α-androstane-3,17-dione (11β-OH-5AD) were purchased from Steraloids. 11β-hydroxy-DHT (11β-OH-DHT) was purchased from Isosciences. 11-keto-4-androstene-3,17-dione (11K-4AD), 11-keto-testosterone (11K-T), 11β-hydroxy-4-androstene-3,17-dione (11β-OH-4AD), and 11β-hydroxy-testosterone (11β-OH-T) were gifts provided by the Rainey laboratory (University of Michigan).
3.1.2. Androgen internal standards
[2,3,4-13C3]-4AD was purchased from Cerilliant. [2,3,4-13C3]-DHEA, [2,3,4-13C3]-T, and [2,3,4-13C3]-DHT were purchased from Cambridge Isotope Laboratories. [2,3,4-13C3]-3α-Androstanediol and [2,3,4-13C3]-3β-Androstanediol were synthesized from [2,3,4-13C3]-DHT as described in section 5.1. All steroid internal standards labeled at the 2, 3, and 4 positions.
3.1.3. [13C3]-3α-Adiol and [13C3]-3 β-Adiol enzymatic synthesis
Roche Diagnostics NADPH Grade I 10107824001
Roche Diagnostics NADH Grade II 10128023001
Fisher BioReagents Potassium Phosphate Dibasic BP363
Sigma Aldrich Potassium Phosphate Monobasic P-5655
Recombinant rat liver AKR1C9 (Schlegel, Jez, & Penning, 1998)
Recombinant human AKR1D1 E120H mutant (Chen, Drury, Christianson, & Penning, 2012)
3.2. Picolinic derivatization materials and reagents
Fisherbrand disposable culture tubes 12 × 75 mm borosilicate glass 14-957-22C
Fisherbrand TainerTop safety closure 14-376-74
Phenomenex Strata C18-E (55 μm, 70 Å) SPE columns 8B-S001-DAM
Sigma Aldrich 2-Picolinic acid (PA) ReagentPlus 99% P42800-100G
TCI Chemicals 2-methyl-6-nitrobenzoic anhydride (MNBAn) M1439
Sigma Aldrich 4-(Dimethylamino)pyridine (DAP) 107700-5G
Sigma Aldrich Triethylamine (TEA) 90340-25 mL
Sigma Aldrich Acros Organic Acetonitrile 99.9% Extra Dry Acroseal 326811000
Fisher Scientific Acetonitrile 4 L A998-4
Fisher Scientific Glacial Acetic Acid A38S-500
Fisher Scientific Methanol 4 L A452-4
3.3. Girard-P derivatization materials and reagents
Fisher Scientific Glacial Acetic Acid A38S-500
Fisher Scientific Methanol A452-4
TCI Chemicals Girard’s Reagent P (GP) G0030
Kimble screw cap disposable 10 mL centrifuge tube 73785-10
Kimble Black phenolic caps 15-415
3.4. MS materials and reagents
Fisher Scientific Methanol A452-4
Fisher Scientific Acetonitrile A998-4
Fisher Scientific Water Optima for HPLC, Fisher Chemical W7-4
Fished Scientific Formic Acid 99.0% Optima LC/MS Grade, Fisher Chemical A117-50
For Picolinic acid derivatized androgens: Phenomenex Kinetex C18 100 mm × 2.1 mm, 2.6 μm, 100 Å column 00D-4462-AN
For Girard-P derivatized androgens: Waters Cortecs Phenyl 2.1 × 100 mm 1.6 μm column 186008381
4. Stock solutions
4.1. Picolinic derivatization solutions
Timing: Picolinic derivatization reagent should be made directly before use. Picolinic resuspension solvent can be stored for up to three months at room temperature.
-
Picolinic derivatization solution
- Add 1 mL of extra dry acetonitrile to a glass vial.
- Measure 20 mg DAP and dissolve in acetonitrile by gently shaking.
- Measure 50 mg PA and dissolve by gently shaking.
- Measure 40 mg MNBAn and dissolve by gently shaking.
Note: Picolinic derivatization solution can be scaled up to accommodate more samples.
- 60% acetonitrile resuspension solvent
- Mix 60 mL acetonitrile with 40 mL MilliQ water.
4.2. Hydroxy-androgen standard curve stock solutions and internal standard mixture
Timing: Hydroxy-androgen stock solutions, working standards solutions, and internal standard mixtures can be stored at − 20 °C for up to one year.
4.2.1. Hydroxy-androgen standard curve stock solutions
Prepare a 1 mg/mL stock solution in ethanol for each hydroxy-androgen standard (EpiT, T, DHEA, androsterone, DHT, epiandrosterone, 5-Adiol, 3α-Adiol, 3β-Adiol).
Prepare 100,000 pg/μL dilution for each hydroxy-androgen standard by mixing 100 μL of the 1 mg/mL stock with 900 μL ethanol.
Prepare a hydroxy-androgens mixture stock solution at a concentration of 1000 pg/μL by adding 40 μL of each 100,000 pg/μL into a final total volume of 4 mL ethanol.
Using the 1000 pg/μL hydroxy-androgen stock, perform a serial dilution to make additional standard curve stock concentrations of 500, 250, 100, 50, 25, 10, 5, and 2.5 pg/μL in ethanol.
4.2.2. Hydroxy-androgen internal standard mixture
Note: Depending on which androgens are being measured in the study, it may not be necessary to include all listed ISTDs. Additionally, because [13C3]-3α-Adiol and [13C3]-3β-Adiol are enzymatically synthesized in house, they are not obtained in as large amounts as the purchased [13C3]-T, [13C3]-DHT, and [13C3]-DHEA. Their final concentration stocks will be 25 pg/μL and therefore will need to be added separately to hydroxy-androgen samples instead of being diluted in the mixture described below.
Prepare a 1 mg/mL solution in ethanol of each hydroxy-androgen [13C3] internal standard ([13C3]-T, [13C3]-DHT, [13C3]-DHEA).
Prepare a 100,000 pg/μL dilution for each internal standard by mixing 100 μL of the 1 mg/mL stock solution with 900 μL of ethanol.
Prepare a 10,000 pg/μL dilution for each internal standard by mixing 100 μL of the 100,000 pg/μL solution with 900 μL of ethanol.
Prepare a 25 pg/μL mixture of the hydroxy-androgen [13C3] internal standards by adding 15 μL of each 10,000 pg/μL stock into a final volume of 6 mL ethanol.
Note: Create a set of quality control (QC) samples by derivatizing 200 μL of internal standard mixture, 200 μL each of the [13C3]-3α-Adiol and [13C3]-3β-Adiol solutions, and 100 μL of a calibration standard in the lower range of the standard curve (ex: the 50 pg/μL stock). Divide into 100 μL aliquots and store at − 20 °C for up to one year. Inject 5 μL before each LC-MS/MS run to monitor instrument performance and LC separation.
4.2.3. [13C3]-3α-Adiol and [13C3]-3 β-Adiol enzymatic synthesis
- 1 M potassium phosphate pH 6.0 @25 °C
- Mix 66 mL 1 M potassium phosphate dibasic and 434 mL 1 M potassium phosphate monobasic.
- Adjust pH by addition of 1 M monobasic or dibasic stocks.
- 40.2 mM NADH
- Dissolve 28.3 mg of NADH in 1 mL water.
- Titrate the concentration using the molar extinction coefficient 6220 M−1cm−1 at absorbance at 340 nm. Adjust the total volume of the stock accordingly.
- 17.8 mM NADPH
- Dissolve 14.8 mg of NADPH in 1 mL water.
- Titrate the concentration using the molar extinction coefficient 6220 M−1cm−1 at absorbance at 340 nm. Adjust the total volume of the stock accordingly.
- 1.55 μg/μL AKR1C9
- Dilute enzyme to 1.55 μg/μL with 0.1 M potassium phosphate pH 6.0 buffer directly before use.
- 1.55 μg/μL AKR1D1 E120H
- Dilute enzyme to 1.55 μg/μL with 0.1 M potassium phosphate pH 6.0 buffer directly before use.
- 250 pg/μL [13C3]-DHT in acetonitrile
- Perform a serial dilution of a 10,000 pg/μL[13C3]-DHT stock solution in acetonitrile.
4.3. Girard P derivatization solutions
Timing: The Girard P derivatization and 10% acetic acid in methanol solutions can be stored for up to one year at room temperature. The 50% methanol resuspension solvent can be stored for up to one month at room temperature.
- Girard P derivatization solution
- Make a 1 mg/mL solution of Girard’s Reagent P in water.
- 10% Acetic acid in methanol
- Mix 10 mL glacial acetic acid with 90 mL methanol.
- 50% methanol resuspension solvent
- Mix 50 mL methanol with 50 mL water.
4.4. Keto-androgen standard curve stock solutions and internal standard mixture
Timing: Keto-androgen stock solutions, working standards solutions, and internal standard mixtures can be stored at − 20 °C for up to one year.
4.4.1. Keto-androgen standard curve stock solutions
Prepare a 1 mg/mL stock solution in ethanol for each keto-androgen standard (4AD, Testosterone, 5AD, DHT, DHEA, 11K-5AD, 11K-DHT, 11β-OH-5AD, 11K-4AD, 11K-T, 11β-OH-DHT, 11β-OH-4AD, and 11β-OH-T).
Prepare 100,000 pg/μL dilution for each keto-androgen standard by mixing 100 μL of the 1 mg/mL stock with 900 μL methanol.
Prepare a keto-androgens mixture stock solution at a concentration of 1000 pg/μL by adding 10 μL of each 100,000 pg/μL into a final total volume of 870 mL methanol to make a 1 mL solution.
Using the 1000 pg/μL keto-androgen stock, perform a serial dilution to make additional calibration stock concentrations of 500, 250, 100, 50, 25, 10, 5, 2.5 and 1 pg/μL in methanol.
4.4.2. Keto-androgen internal standard mixture
Prepare a 1 mg/mL solution in ethanol of each keto-androgen [13C3] internal standard ([13C3]-T, [13C3]-DHT, [13C3]-4AD, [13C3]-DHEA).
Preform 1 mL serial dilutions to obtain 1 mL stock solutions of 200 pg/μL [13C3]-T, 400 pg/μL [13C3]-DHEA, 1000 pg/μL [13C3]-4AD, and 4000 pg/μL [13C3]-DHT in methanol by diluting no more than 10-fold at a time.
Prepare an internal standard mixture containing 50 pg/μL [13C3]-T, 100 pg/μL [13C3]-DHEA, 250 pg/μL [13C3]-4AD, 1000 pg/μL [13C3]-DHT by combining 250 μL of the four internal standards to make a 1 mL solution.
Note: Create a set of quality control (QC) samples by derivatizing 200 μL of internal standard mixture plus 100 μL of a calibration standard in the low to mid range of the standard curve (ex: 50 pg/μL). Divide into 100 μL aliquots and store at − 20 °C for up to one year. Inject 5 μL before each LC-MS/MS run to monitor instrument performance.
4.5. LC-MS solvents
4.5.1. Hydroxy-androgen Picolinic LC-MS solvents
Timing: Both solvents can be stored for up to one week at room temperature.
- Solvent A: Water with 0.05% formic acid
- Add 500 μL formic acid to 1 L water, sonicate for 10 min.
- Solvent B: 4/6 Acetonitrile/Methanol with 0.05% formic acid
- Mix 400 mL acetonitrile with 600 mL methanol. Add 500 μL formic acid and sonicate for 10 min.
4.5.2. Keto-androgen Girard P LC-MS solvents
Timing: Both solvents can be stored for up to one week at room temperature.
- Solvent A: Water with 0.1% formic acid
- Add 1 mL formic acid to 1 L water, sonicate for 10 min.
- Solvent B: 4/6 Acetonitrile/Methanol with 0.05% formic acid
- Mix 400 mL acetonitrile with 600 mL methanol. Add 500 μL formic acid and sonicate for 10 min.
5. Procedure
5.1. Synthesis of [13C3]-hydroxy-androgen internal standards: 3α-Adiol and 3β-Adiol
[13C3]-3α-Adiol and [13C3]-3β-Adiol can be synthesized from [13C3]-DHT using rat liver AKR1C9 and human AKR1D1 E120H mutant, respectively, as shown in Fig. 2 (Chen et al., 2012; Schlegel et al., 1998; Zang et al., 2017). The E120H mutant in AKR1D1 eliminates 5β-reductase activity and results in an enzyme that has only 3β-HSD activity. After enzymatic synthesis, the resulting internal standards can be collected, and their respective concentrations can be estimated using standard curves of unlabeled 3α-Adiol and 3β-Adiol.
Fig. 2.

Schematic for the enzymatic synthesis of A) [13C3]-3β-Adiol with recombinant rat liver AKR1C9 and B) [13C3]-3β-Adiol with the recombinant AKR1D1 E120H mutant from [13C3]-DHT. 13C3 carbons are labeled with asterisks.
5.1.1. Synthesizing 3α-Adiol
Note: Dried samples can be store at − 20 °C.
Make up a 1 mL reaction containing 805 μL milliQ water, 100 μL potassium phosphate buffer pH 6.0, 40 μL 250 pg/μL [13C3]-DHT in acetonitrile, 40 μL 40.2 mM NADH, and 15 μL 1.55 μg/μL AKR1C9. Final concentration in the reaction are 100 mM potassium phosphate pH 6.0, 4% acetonitrile, 0.0345 μM [13C3]-DHT, 1.6 mM NADH, and 0.0233 μg/μL AKR1C9.
React in a shaking water back at 37 °C for 1 h.
Add 1.5 mL of cold ethyl acetate to quench the reaction, and vortex for 15 s
Centrifuge samples for 15 min to separate organic and aqueous fractions.
Transfer the top organic layer to a clean tube using a pasture pipette.
Repeat the extraction with an additional 1.5 mL of ethyl acetate.
Dry the combined organic fractions down using a vacuum centrifuge concentrator.
5.1.2. Synthesizing 3β-Adiol
Make up a 1 mL reaction containing 795 μL milliQ water, 100 μL potassium phosphate buffer pH 6.0, 40 μL 250 pg/μL [13C3]-DHT in acetonitrile, 56.5 μL 17.8 mM NADPH, and 10 μL 1.55 μg/μL AKR1D1 E120H. Final concentration in the reaction are 100 mM potassium phosphate pH 6.0, 4% acetonitrile, 0.0345 μM [13C3]-DHT, 1 mM NADPH, and 0.0155 μg/μL AKR1D1 E120H.
React in a shaking water back at 37 °C for 1 h.
Add 1.5 mL of cold ethyl acetate to quench the reaction, and vortex for 15 s
Centrifuge samples for 15 min to separate organic and aqueous fractions.
Transfer the top organic layer to a clean tube using a pasture pipette.
Repeat the extraction with an additional 1.5 mL of ethyl acetate.
Dry the combined organic fractions down using a vacuum centrifuge concentrator.
5.1.3. Validating concentrations
Reconstitute the products to ~50 pg/μL in ethanol.
Derivatize three aliquots of 5 μL of each product with picolinic acid as described in Section 5.3.
Make 3α-Adiol and 3β-Adiol standard curve stocks solutions as described in Section 4.2.1 and derivatize 10 μL of each stock to create separate standard curves of unlabeled 3α-Adiol and 3β-Adiol. Run the standard curves and derivatized ISTD aliquots using the picolinic MS method described in Section 5.4. Estimate the concentration of the synthesized [13C3]-3α-Adiol and [13C3]-3β-Adiol against the unlabeled standard curves via their peak areas.
Re-validate the concentrations by combining equal amounts of 3α-Adiol and [13C3]-3α-Adiol or 3β-Adiol and [13C3]-3β-Adiol for derivatization and analysis by MS. If the concentration was estimated correctly, the peak areas of the combined standard and ISTD should be roughly equal. Additionally, the peak area ratio of analyte to ISTD should be ~1.
After the concentration has been validated, dilute to 25 pg/μL and store at − 20 °C.
5.2. Hydroxy- and keto-androgen standard curves
Note: This protocol describes how to make a standard curve to quantify hydroxy-androgens in cell culture media (Figs. 3 and 4). To account for matrix effects, standard curve samples are extracted from the same type of cell media used in cell treatments. To measure androgens in other biological samples, extract the standard curve samples from a different type of matrix (ex: charcoal dextran stripped (CDS)-FBS if measuring androgens in serum).
Fig. 3.
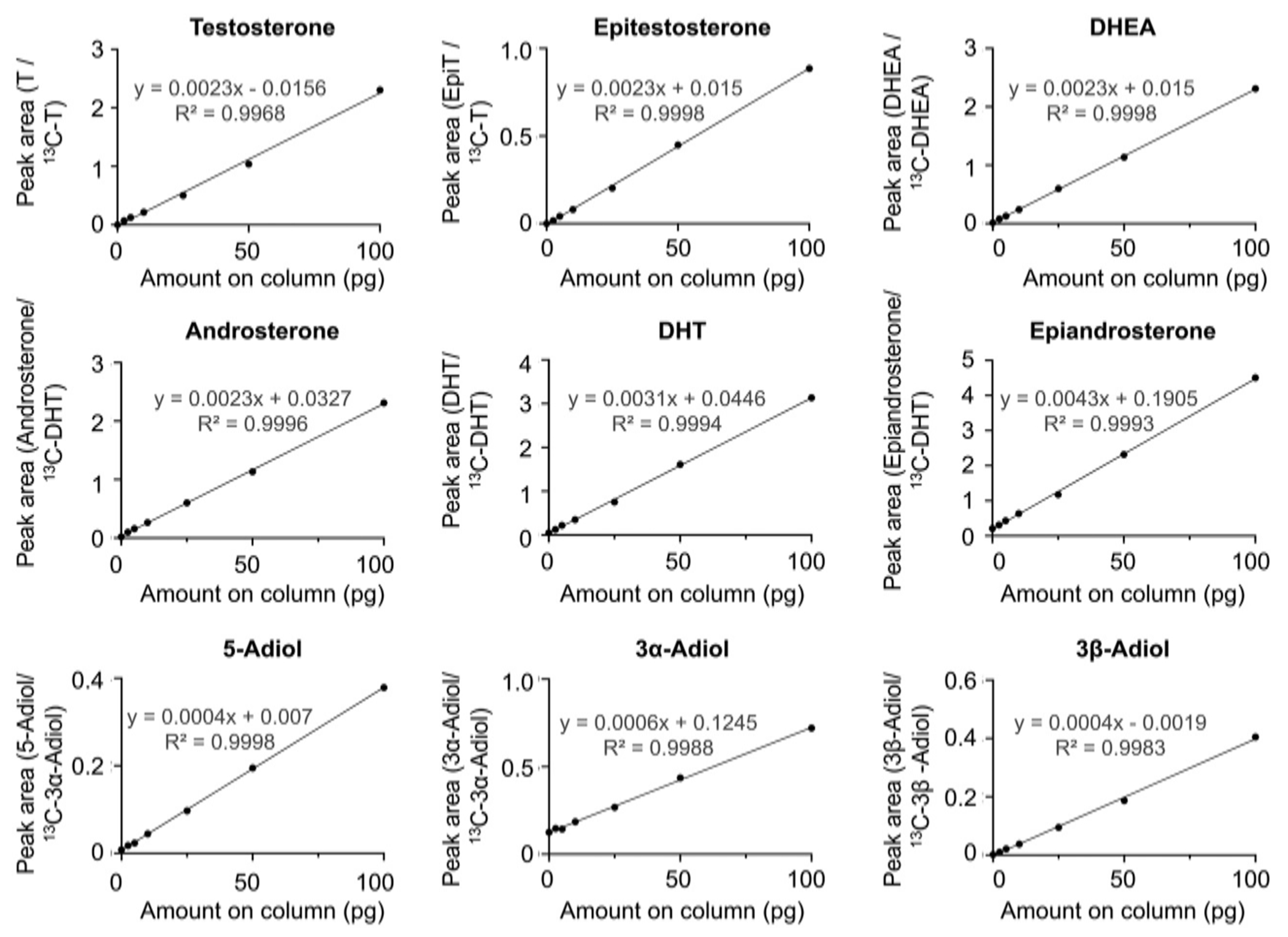
Picolinic acid derivatized hydroxy-androgen calibration curves.
Fig. 4.

Girard P derivatized keto-androgen calibration curves.
Spike 10 μL of hydroxy- or keto-androgen calibration stock and 20 μL of the hydroxy- or keto-androgen internal standard mixture (and 20 μL each of the [13C3]-3α-Adiol and [13C3]-3β-Adiol internal standard solutions if making hydroxy-androgen standard curve) to 2 mL cell media (phenol red free RPMI + 5% CDS-FBS, 1% P/S, 2 mMl-Glutamine) in glass screw cap vials.
Note: If using a different amount of ISTD in cell samples, make sure to adjust the amount used to make standard curves.
Add 2 mL of diethyl ether to media and vortex for 20 s
Centrifuge samples for 15 min to separate organic and aqueous phase.
Using a pasture pipette, transfer the organic (top) layer to a new tube.
Repeat the extraction with another 2 mL of diethyl ether and combine the organic fractions.
Dry down the combined organic fractions in a vacuum centrifuge concentrator.
Proceed with the protocol for picolinic or Girard P derivatization as described in section 5.3 and 5.6, respectively.
5.3. Picolinic acid derivatization
Add 100 μL of freshly made picolinic derivatization solution to tube with dried down androgens, then add 40 μL TEA.
Shake at 500 RPM for 90 min
After 90 min reaction, add 1 mL of quench solution to the tube and mix gently by pipetting.
Pause point: can store quenched samples overnight in 4 °C.
- Perform solid phase extraction (SPE) cleanup:
- Wash SPE column with 1 mL methanol, then 1 mL water.
- Load sample onto column, flow through to waste.
- Wash column with 2 × 1 mL water, flow through to waste.
- Wash column with 3 × 1 mL 30% acetonitrile.
- Place collection tubes into the vacuum manifold.
- Elute sample with 2 × 1 mL acetonitrile.
Pause point: can store eluted samples in acetonitrile at − 20 °C overnight.
Dry down samples using speed vac sample or under nitrogen.
Resuspend samples in 60% acetonitrile and transfer to HPLC vials.
Note: Dried samples can be stored for up to three months at − 20 °C, or resuspended samples for up to 1 month at − 20 °C.
5.4. LC-MS/MS for hydroxy-androgen picolinate derivatives
5.4.1. Picolinic LC operating conditions
Solvent A: Water with 0.1% formic acid
Solvent B: 4/6 Acetonitrile/Methanol with 0.05% formic acid
Washing solvent for the injection syringe: 50/50 Water/Methanol
Column: Phenomenex Kinetex C18 100 mm × 2.1 mm, 2.6 μm, 100 Å column 00D-4462-AN
Column temperature: 30 °C (ambient)
A linear gradient was initiated at a flow rate of 0.25 mL/min as follows (shown in Table 1): Initial conditions of 80% A and 20% B were held for 1 min, then increased to 60% B over 5 min and maintained at 50% B for 20 min. Then B was increased to 95% over 15 min and maintained at 95% B for 5 min, then decreased to 1% B over 1 min and maintained at 20% B for 15 min. Chromatographic separation of hydroxy-androgens and ISTDs is shown in Fig. 5.
Table 1.
Picolinic LC-MS/MS separation gradient.
| Time (min) | Flow (mL/min) | B |
|---|---|---|
| 0 | 0.25 | 20% |
| 1 | 0.25 | 20% |
| 6 | 0.25 | 60% |
| 26 | 0.25 | 60% |
| 41 | 0.25 | 95% |
| 46 | 0.25 | 95% |
| 47 | 0.25 | 20% |
| 62 | 0.25 | 20% |
Fig. 5.

LC-MS/MS ion-chromatogram showing separation of picolinic acid derivatized hydroxy-androgens using 5 ng of each hydroxyl androgen and 500 pg of each of the five hydroxyl-androgen-internal standards.
5.4.2. Picolinic mass spectrometry operating conditions
Spray voltage: 4400 V
Ion transfer capillary temperature: 350 °C
Collision gas, argon: 1.5 mTor
Ion polarity: positive
Scan type: selective reaction monitoring
Chrom filter peak width: 6 s
Q1 peak width (FWHM): 0.7
Q3 peak width (FWHM): 1.2
Dwell time:100 ms
The SRM transitions for all the analytes and internal standards are shown in Table 2.
Table 2.
Picolinic androgen MS parameters.
| Analyte | Parent (m/z) | Product (m/z) | Collision energy (V) | RF lens (V) |
|---|---|---|---|---|
| Epitestosterone | 394.1 | 124.0 | 15 | 54 |
| Testosterone | 394.1 | 124.0 | 15 | 54 |
| DHEA | 394.1 | 271.0 | 13 | 54 |
| Androsterone | 396.1 | 124.0 | 15 | 62 |
| DHT | 396.1 | 124.0 | 15 | 53 |
| Epiandrosterone | 396.1 | 124.0 | 18 | 50 |
| 5-Androstenediol | 501.1 | 255.1 | 15 | 66 |
| 3α-Androstanediol | 503.1 | 257.1 | 15 | 62 |
| 3β-Androstanediol | 503.1 | 257.1 | 16 | 62 |
| [13C3]-T | 397.1 | 124.0 | 15 | 54 |
| [13C3]-DHT | 399.1 | 124.0 | 15 | 53 |
| 13C-DHEA | 397.1 | 274.1 | 13 | 54 |
| [13C3]-3α-Androstanediol | 506.1 | 260.1 | 16 | 62 |
| [13C3]-3β-Androstanediol | 506.1 | 260.1 | 16 | 62 |
5.5. Keto-androgen Girard-P derivatization
Resuspend dried androgens in 200 μL 10% acetic acid in methanol, transfer to a 10 mL screw cap tube.
Add 20 μL of Girard P derivatization solution, mix by vortexing.
Incubate reaction in a 60 °C water bath for 10 min.
Remove from water bath, spin down, and dry under nitrogen.
Resuspend samples in 100 μL of 50% methanol.
Note: Store dried samples for at − 20 °C, or resuspended samples for up to 6 months at 4 °C.
5.6. LC-MS/MS for keto-androgen Girard P derivatives
5.6.1. Girard P LC operating conditions
Solvent A: Water with 0.1% formic acid
Solvent B: Methanol/acetonitrile 60/40 (v/v) with 0.05% formic acid
Washing solvent for the injection syringe: 50/50 Water/Methanol
Column: Waters Cortecs Phenyl 2.1 × 100 mm 1.6 μm column, 186008381
Column temperature: 30 °C
A stepwise gradient was initiated at a flow rate of 0.5 mL/min as follows (also shown in Table 3): Initial conditions of 85% A and 15% B, followed by stepwise increase of B to 25%, 28%, and 80% in 4-minute increments. Wash the column with 100% B for 5 min, then return to 15% B and re-equilibrate for 5 min. Chromatographic separation of keto-androgens is shown in Fig. 6.
Table 3.
Girard-P LC-MS/MS separation gradient.
| Time (min) | Flow (mL/min) | B |
|---|---|---|
| 0 | 0.5 | 15% |
| 4 | 0.5 | 25% |
| 8 | 0.5 | 28% |
| 12 | 0.5 | 80% |
| 13 | 0.5 | 100% |
| 18 | 0.5 | 100% |
| 19 | 0.5 | 15% |
| 24 | 0.5 | 15% |
Fig. 6.

LC-HRMS ion chromatogram showing separation of Girard P derivatized keto-androgens using 5 ng each of the standards and 500 pg of [13C3]-T used as internal standard.
5.6.2. Girard P mass spectrometry operating conditions HRMS)
Spray voltage: 4000 V
Ion transfer capillary temperature: 250 °C
Sheet gas and auxiliary gas, nitrogen: 50 and 15 arbitrary units
Ion polarity: positive
Scan type: full scan 120, 00 resolution
Probe temperature: 325 °C
S-lens: 55
AGC: 1e6
CID: 5 mTor
Peak width: 10 ppm from the theoretical mass, shown in Table 4.
Table 4.
Girard-P androgen MS parameters.
| Analyte | Parent (m/z) |
|---|---|
| 4AD | 420.2640 |
| DHEA | 422.2794 |
| T | 422.2794 |
| 5AD | 422.2794 |
| DHT | 424.2947 |
| 11K-4AD | 434.2431 |
| 11K-T | 436.2586 |
| 11K-5AD | 436.2586 |
| 11K-DHT | 438.2743 |
| 11β-OH-4AD | 436.2586 |
| 11β-OH-T | 438.2743 |
| 11β-OH-5AD | 438.2743 |
| 11β-OH-DHT | 440.2902 |
| [13C3]-4AD | 423.2640 |
| [13C3]-T | 425.2794 |
| [13C3]-DHEA | 425.2794 |
| [13C3]-DHT | 427.2947 |
5.7. Data analysis
Note: Xcalibur 2.6 software (Thermo Scientific) was used for data acquisition and processing.
- Identify hydroxy and keto-androgens by their retention time compared to independently run standards and internal standards.
- For hydroxy-androgens, peaks are identified by MS/MS data.
- For keto-androgens, peaks are identified by high resolution MS data.
Calculate peak area ratio of hydroxy-androgens by ratios of the peak area of the analyte to the peak area of the corresponding (or closest isomeric) internal standard.
Calibration curves for each androgen are constructed from the peak area ratio of each calibration standard to corresponding internal standard plotted by the total pg of the androgen in the vial with 1/x weighting in Xcalibur QuanBrowser.
6. Other considerations
6.1. Advantages and limitations
Advantage: Derivatization by either picolinic acid or Girard P increases ionization and enhances detection sensitivity for quantitative LC-MS analysis of androgens.
Advantage: Unlike deuterated internal standards, [13C3]-labeled internal standards elute at the same retention time as their unlabeled counter-parts.
Limitation: Picolinic acid and Girard P derivatizations target only hydroxy- or keto-androgens, respectively. Therefore, it may be necessary to split samples into two portions to derivatize with both reagents to detect the entire panel of androgens.
6.2. Optimization and troubleshooting
6.2.1. Picolinic acid optimization
When derivatizing -diol androgens with two hydroxy groups, derivatization goes to completion on both groups and bis-picolinate species are formed.
The picolinic acid derivatization reaction is sensitive to water. To prevent interference of water with the reaction, anhydrous acetonitrile is used as the solvent. Additionally, when derivatizing androgens that have been extracted from cell media or another matrix, ensure that the sample is completely dried down and that no solvent or water from an imprecise extraction remains. Derivatization reagents should also be stored in a desiccator.
Table 2 lists the most abundant MS/MS hydroxy-androgen transitions. In some cases, it was found that the most abundant daughter ion is the picolinic acid fragment, whereas in other cases it is the steroid base minus the picolinic acid. If the sensitivity of the recommended transition is low, try directly infusing a concentrated stock of the derivatized androgen into the MS to redetermine which daughter ion is most abundant and test for ideal fragmentation and transition conditions.
6.2.2. Girard P optimization
Girard P derivatization takes place at a high temperature (60 °C) and is therefore subject to evaporation. Perform this derivatization in screw cap tubes and make sure the cap is tightly sealed to prevent solvent evaporation.
Girard P oximes will form as both E and Z isomers. As a result, each analyte will have multiple isomeric peaks depending on number of derivatization positions. Note that the formation of each isomer is consistent, and that derivatization goes to completion each time. Therefore, it is not necessary to use all isomeric peaks to quantify Girard P androgens. Instead, identify the best separated and most abundant peak and use it to perform all quantifications for that analyte.
Drying down Girard P derivatized samples slowly overnight and storing under nitrogen will help to prevent degradation of oximes.
Funding
This work was supported by the National Institutes of Health grant P30ES013508.
References
- Chen M, Drury JE, Christianson DW, & Penning TM (2012). Conversion of human steroid 5beta-reductase (AKR1D1) into 3beta-hydroxysteroid dehydrogenase by single point mutation E120H: Example of perfect enzyme engineering. The Journal of Biological Chemistry. 287(20), 16609–16622. 10.1074/jbc.M111.338780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AJ, Wang Q, Busch C, Feldman D, Bottalico L, Mesaros CA, … Snyder NW (2016). Validation of highly sensitive simultaneous targeted and untargeted analysis of keto-steroids by Girard P derivatization and stable isotope dilution-liquid chromatography-high resolution mass spectrometry. Steroids. 116, 60–66. 10.1016/j.steroids.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, & Carmina E American Association of Clinical, E., American College of, E., Androgen, E., & Society, P.. (2015). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 1. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 21(11), 1291–1300. 10.4158/EP15748.DSC [DOI] [PubMed] [Google Scholar]
- Huggins C, & Hodges CV (1941). Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1. [DOI] [PubMed] [Google Scholar]
- Rey RA (2021). The role of androgen signaling in male sexual development at puberty. Endocrinology. 162(2), 10.1210/endocr/bqaa215 [DOI] [PubMed] [Google Scholar]
- Schlegel BP, Jez JM, & Penning TM (1998). Mutagenesis of 3 alpha-hydroxysteroid dehydrogenase reveals a “push-pull” mechanism for proton transfer in aldo-keto reductases. Biochemistry. 37(10), 3538–3548. 10.1021/bi9723055 [DOI] [PubMed] [Google Scholar]
- Tamae D, Byrns M, Marck B, Mostaghel EA, Nelson PS, Lange P, … Penning TM (2013). Development, validation and application of a stable isotope dilution liquid chromatography electrospray ionization/selected reaction monitoring/mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of keto-androgens in human serum. The Journal of Steroid Biochemistry and Molecular Biology. 138, 281–289. 10.1016/j.jsbmb.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang T, Tamae D, Mesaros C, Wang Q, Huang M, Blair IA, & Penning TM (2017). Simultaneous quantitation of nine hydroxy-androgens and their conjugates in human serum by stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry. The Journal of Steroid Biochemistry and Molecular Biology. 165(Pt B), 342–355. 10.1016/j.jsbmb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


